Pyridoxine and folates during small and large scale brewing
Abstract
Beer contains the vitamins pyridoxine (B6) and folates (B9); their content is variable and depends on several factors. The aim of this study was to investigate the level of these vitamins during large and small scale brewing so as to identify the steps that can influence their levels in the final product. A Golden Ale and Scottish Ale were brewed at sales gravity in a 12 hL plant and a Gold Lager from a bottom fermented high gravity wort at a commercial scale. The levels of pyridoxine at mash-in differed between the brewing processes, due to the different malt used. Pyridoxine levels decreased during mashing while the pyridoxamine concentration remained constant. Although an increase of pyridoxine was observed after the fermentation, its level decreased in the final beers from the large scale process reflecting dilution and pasteurisation. With regard to folates, the levels increased during mashing, decreased after wort boiling and increased during fermentation. This study showed that both pyridoxine and folates were solubilised during the process and remained in the final product. © 2021 The Institute of Brewing & Distilling
Introduction
The quality of beer is evaluated by its sensorial profile, in particular by taste, aroma, colour and foam (1). Beer contains vitamins, minerals and antioxidants which can induce positive health effects if beer consumption is moderate (2-4). Significant levels of several B vitamins occur in beer and for some the range can be variable (5). Of these, changes to thiamine and riboflavin during malting and brewing have been reported (6-8); however, less is known about the other B-vitamins, such as nicotinic and pantothenic acid (9-11). With regard to pyridoxine and folates, a recent survey showed that their content varies in different beer samples, ranging from 15 to 376 μg/L for pyridoxine and from 15.5 to 104.8 μg/L for folates (12). The content of vitamins depends on several factors, including raw materials and the brewing method (13-16). The trend of pyridoxine during the fermentation of wort by bottom fermenting yeast was investigated several years ago (17, 18). More recently, an increase of folates (16) in the fermentation step was reported; however, this study did not consider the complete brewing process and was performed using laboratory or pilot scale brewing. Therefore, the aim of this study was to investigate the trend of pyridoxine and folates during small and large scale brewing, in order to evaluate the steps that most influence the levels in the final product.
Materials and methods
Brewing and sampling
Brewing processes were performed at both a small and large scale. Two different beer styles, Golden Ale (Beer 1) and Scottish Ale (Beer 2) were brewed on a 12 hL plant (small scale) by Ex Fabrica – Birrificio Artigianale (Milano, Italy). For the large scale beer, a bottom fermented gold lager of standard strength (International pale lager according to BJCP 2015) was brewed from a high gravity wort at a European commercial brewery producing 1-1.5 million hL per year. Samples were collected at different steps of the process and kept at -20°C until analysis.
Small scale brewing
Two small scale brews were performed at standard gravity in a 12 hL plant (Spadoni, Orvieto, Italy). In the first, the Golden Ale (Beer 1) wort was brewed with 225 kg of Pilsner Malt (Rhön-Malz GmbH, Mellrichstadt, Germany). The brewing steps were as follows: mashing-in at 60°C with a water/malt ratio of 3:1 (675 L water). The temperature was maintained at 63°C for 50 minutes (β-amylase rest). The mash out temperature was 78°C. The time between rests was as short as possible and the temperature raised at 1°C per minute. The mash was filtered using a thin bed membrane filter (Spadoni, Orvieto, Italy) and 700L of water was used as sparging water. The spent grain water was regulated by a final compression step at 1.5°P. The wort (1375 L) was boiled with 0.6 g/L hops (type 90 pellets) for 60 min. The wort was whirlpooled for 20 minutes and the trub removed. The wort (final volume: 1200 L) was filtered and cooled to 22°C, with an extract of 12° Plato (Densito 30PX manual oscillating densitometer, Mettler Toledo, Columbus, USA).
For the second brew, the Scottish Ale (Beer 2) wort was brewed with 100 kg of Pilsner Malt, 100 kg of Munich Malt and 50 kg of Cara Munich Malt (Rhön-Malz GmbH, Mellrichstadt, Germany). The brewing steps were as follows: mashing-in at 60 °C with a water/malt ratio of 3:1. The temperature of the mash was maintained at 63°C for 40 minutes (β-amylase rest), with a 15 minute rest at 72°C (α-amylase rest). The mash out temperature was 78°C. The time between rests was as short as possible and the temperature raised at 1°C per minute. The mash was filtered using a thin bed membrane filter (Spadoni, Orvieto, Italy) with 700 L of sparging liquor. The water content of the spent grain was regulated by a final compression step at 1.5°P. The wort was boiled with hops (0.5 g/L) for 60 min, the wort was whirlpooled for 20 minutes and the trub removed. The wort (final volume 1200 L) was filtered and cooled to a temperature of 22°C, with an extract of 14°Plato (Densito 30PX manual oscillating densitometer, Mettler Toledo, Columbus, USA).
Both worts (1200 L) were inoculated with 50 g/hL of top fermenting dry yeast, Safale US-04 (Fermentis, S.I. Lesaffre, USA) at 20°C. Fermentation was at 21°C until the apparent final attenuation of respectively 72 and 76% were achieved. After fermentation, both beers were chilled to 4°C for 20 days (maturation) after which 4 g/L of sucrose was added. The beers were bottled in 330 mL bottles with screwcaps with refermentation at 20°C for 10 days. Samples were collected at different steps: start of mashing, end of mashing, end of boiling, start of fermentation (after whirlpool and trub separation), end of fermentation, after maturation and the final product (after refermentation).
Large scale brewing
A 500 hL brewhouse consisting of a hammer mill, mashing kettle, mash filter, boiling kettle and whirlpool was used (Huppman, Kitzingen, Germany). For Beer 3, 10 tons of 80% pilsner malt and 20% unmalted barley were milled in a hammer mill. Infusion mashing was at 60°C with 240 hL water for a water/malt ratio of 2.4 with two amylolytic rests. The mash out temperature was 78°C. The mash was filtered using a thin bed membrane filter (Meura, Péruweltz, Belgium). Once the wort was recovered, the spent grains were washed with water to recover extract. Spent grain water content was regulated by a final compression step at 1.5°P. The wort was heated to 93°C using an external plate heat exchanger and boiled for 1 hour in a kettle with an internal boiler and dynamic low-pressure boiling technology. Different hop products (3.50 kg of α-acid) were added during boiling. The evaporation rate was approximately 4%. The wort was whirlpooled for 20 minutes and the trub removed. The clarified wort was cooled to 14°C, inoculated with 15x106 cells/mL of S. pastorianus and fermented at 14°C in cylindro-conical fermenters for eight days (real degree of fermentation > 69%). At the end of fermentation, the green beer was held at 0-2°C for 3 days. The beer was filtered, diluted with deaerated water to 12°P and the carbon dioxide content adjusted to 5 g/L. The beers were tunnel pasteurised (60°C, 20 minutes) and bottled in 330 mL glass bottles with screw caps. The samples were collected at different steps: start of mashing, end of mashing, end of boiling, start of fermentation (after whirlpool and trub separation), end of fermentation, after maturation and final product (after pasteurisation).
Reagents and standards
The chemicals and solvents used for the extraction and clean-up were ACS grade or equivalent (Carlo Erba, Milan, Italy); deionised water was purified through a Milli-Q treatment system (Millipore, Bedford, MA, USA). For LC-MS/MS analysis, water, methanol, acetonitrile and formic acid were HPLC grade (Merck, Darmstadt, Germany). Folic acid (FA) and 10-formyltetrahydrofolate (10-formyl-THF) were purchased from Schirck’s Laboratory (Jona, Switzerland). Pyridoxine and pyridoxamine were purchased from Sigma–Aldrich Co. (St. Louis, USA). Stock solutions of folate standards (0.5 mg/mL) were prepared in 10 mM ammonium acetate pH 6.8, containing 0.5% (w/v) ascorbic acid; stock solutions of pyridoxine, pyridoxamine and pyridoxal standards (0.5 mg/mL) were prepared in acetonitrile. The standard stock solutions were diluted appropriately in the LC-MS/MS mobile phase. The remaining stock solutions were flushed with nitrogen gas, and aliquots were stored at -80°C.
Analysis of pyridoxine, pyridoxamine and pyridoxal
Pyridoxine and its vitamers were quantified using the method of Bertuzzi et al., (12) with some modifications. After dilution with 40 mL of water:acetonitrile (9+1 v/v), centrifugation and filtration (0.45 μm), 20 μL was injected into an LC-MS/MS system. After chromatographic separation on a XSelect HSS T3 column (RP 18 with low ligand density, 2.5 μm, 3.0x100 mm, Waters) using gradient elution water and methanol, both acidified with 0.2% formic acid, the detection was performed using selected reaction monitoring (SRM). For fragmentation of the [M + H]+ ion (170, 169 and 168 m/z for pyridoxine, pyridoxamine and pyridoxal, respectively), the detected and quantified fragment ions were: 152 m/z (collision energy 20 V), 134 m/z (20 V) and 77 m/z (35 V) for pyridoxine, 134 (13 V) and 152 (13 V) m/z for pyridoxamine, 94 (18 V), 122 (18 V) and 150 (13 V) m/z for pyridoxal. LOD e LOQ were 2 and 5 μg/L, respectively.
Analysis of folic acid and 10-formyltetrahydrofolate
Folates were quantified using the method of Bertuzzi et al. (19). After dilution with phosphate buffer (PBS) and clean-up through an immunoaffinity column for folic acid (R-Biopharm, Glasgow, UK), folates were determined by LC-MS/MS in ESI positive mode. LOD and LOQ were respectively 0.1 μg/L and 0.3 μg/L for both FA and 10-formyl-THF.
Statistical analysis
Using Microsoft Excel 2017, data presented in tables and graphs are reported as mean ± standard deviation (SD). Statistically significant differences between samples were tested using a post-hoc comparison test (Duncan’s test) at α < 0.05. Statistics were carried out by IBM SPSS Statistics 24.0 (SPSS Inc., Chicago, IL, USA).
Results and discussion
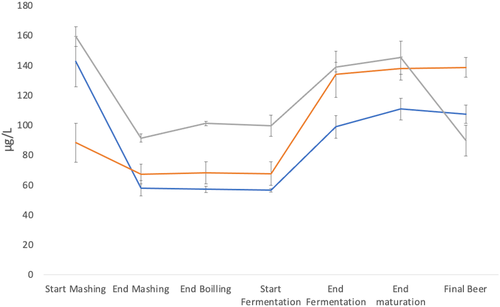
The concentration of pyridoxine, pyridoxamine and folate in samples collected during small and large scale brewing are shown in Table 1. Whilst pyridoxal was not detected, pyridoxine occurred at higher levels than pyridoxamine. Of the folates, 10-formyltetrahydrofolate (10-formyl-THF) was present at a markedly higher concentration than folic acid. In previous reports, beer contains primarily these two folate vitamers (19, 20). Comparing the three different brewing processes, the trends were comparable for both pyridoxine and folates. The initial level of pyridoxine (start of mashing) differed in the brewing processes, depending on the malt used (Beer 3>Beer 1>Beer 2). In particular, Munich and Cara Munich malts used in the Beer 2 wort; are specialty malts roasted at higher temperature than Pilsner malt. Accordingly, pyridoxine and folate levels in these specialty malts are lower (21). The pyridoxine level greatly decreased during mashing; Beer 1 had the most marked decrease (-69.1%, -44.5% for Beer 3 and -32.4% for Beer 2). The pyridoxamine concentration remained constant during mashing. From these results, the decrease was influenced more by the type of malt used rather than small differences in temperature and time. During the subsequent steps, the level of B6 vitamers was similar until the start of fermentation; a slight increase was observed during Beer 3 brewing. Probably, sparging extracted additional pyridoxine and the concentrations did not change after wort boiling. Indeed, these data suggest that the mash filter used in the production of Beer 3 achieved a better extraction compared to lauter tun used in Beer 1 and Beer 2, confirming previous studies (22, 23). As expected, a considerable increase of pyridoxine was observed after the fermentation step (Beer 1 +106.1%, Beer 2 +130.7% and Beer 3 +41.2%). The increase for Beer 1 and Beer 2 was similar, as this step used the same yeast and the same time and temperature. After fermentation, a slight increase was observed in the final product for small scale brewing (Beer 1 and 2), probably due to a complete solubilisation of B6 vitamers during the maturation caused by the lysis of yeast cells. On the contrary, pyridoxine levels decreased in the final beer obtained from large scale brewing because of dilution and pasteurisation which is known to decrease vitamin content in the food matrix (24).
| Beer 1 | Beer 2 | Beer 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyridoxine | Pyridoxamine | 10-Formyl-THF | Folic acid | Pyridoxine | Pyridoxamine | 10-Formyl-THF | Folic acid | Pyridoxine | Pyridoxamine | 10-Formyl-THF | Folic acid | |
| Start mashing | 125.8 ± 11.7a | 16.7 ± 5.1c | 18.7 ± 0.8c | 0.8 ± 0.1d | 75.2 ± 12.7b | 13.1 ± 0.3a | 17.8 ± 0.6cd | 0.8 ± 0.1abc | 147.2 ± 6.4a | 1.18 ± 0.2e | 23.9 ± 0.9e | 3.0 ± 1.1cd |
| End mashing | 38.8 ± 1.9d | 19.0 ± 3.3bc | 24.6 ± 1.1b | 2.2 ± 0.1bc | 50.8 ± 5.5c | 16.4 ± 1.1a | 20.6 ± 0.9ab | 0.9 ± 0.2ab | 81.7 ± 2.2cd | 9.6 ± 0.6d | 45.4 ± 0.7b | 5.3 ± 0.2a |
| End boiling | 36.3 ± 1.5d | 20.8 ± 0.6abc | 15.3 ± 0.4d | 1.8 ± 0.1c | 48.9 ± 4.6c | 19.2 ± 2.8a | 11.7 ± 0.6e | 0.6 ± 0.1bc | 90.2 ± 0.8c | 10.9 ± 0.5cd | 27.7 ± 2.0d | 3.4 ± 0.8cd |
| Start fermentation | 35.9 ± 0.8d | 20.5 ± 0.4abc | 24.7 ± 1.0b | 2.3 ± 0.3b | 51.4 ± 4.3c | 16.2 ± 3.7a | 15.9 ± 1.2d | 0.5 ± 0.1c | 88.3 ± 7.0c | 11.2 ± 0.1cd | 34.3 ± 1.5c | 3.9 ± 0.7bc |
| End fermentation | 74.0 ± 3.5c | 24.8 ± 3.9ab | 35.3 ± 2.7a | 3.4 ± 0.5a | 118.6 ± 11.6a | 15.3 ± 3.8a | 21.5 ± 1.8a | 1.0 ± 0.2a | 124.7 ± 2.8b | 14.0 ± 0.3bc | 48.7 ± 1.6a | 4.8 ± 0.4ab |
| End maturation | 83.9 ± 2.6b | 26.8 ± 4.6a | 33.3 ± 2.5a | 2.1 ± 0.1bc | 122.0 ± 3.5a | 15.8 ± 4.1a | 18.0 ± 1.1cd | 0.8 ± 0.2abc | 121.5 ± 7.5b | 23.6 ± 3.6a | 47.9 ± 2.2ab | 4.5 ± 0.5ab |
| Final beer | 85.2 ± 2.4b | 22.0 ± 3.7abc | 33.8 ± 2.2a | 2.0 ± 0.1bc | 123.2 ± 3.2a | 15.5 ± 3.4a | 19.1 ± 1.6bc | 1.1 ± 0.2a | 74.1 ± 6.9d | 15.4 ± 3.4b | 31.6 ± 2.0c | 2.9 ± 0.4d |
- a, b, c, d : Different letters in the same column indicate statistically significant (p < 0.05) differences for each beer type.
There is limited information in the literature about the content of pyridoxine during brewing. In 1947, changes in this vitamin during brewing were evaluated by Hopkins et al., (17), who reported a decrease in pyridoxine comparable to this work during mashing, but a further decrease was found during fermentation, due to utilisation by yeast. Recently, broad variations of pyridoxine concentrations (from 15 to 376 μg/L) were reported in beer samples (12) from small and large breweries.
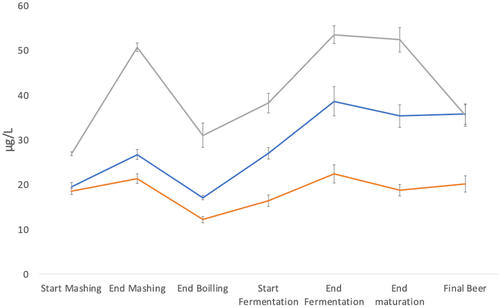
With regard to folates, wide variations of their content were found in beer with 10-formyl-THF the main folate detected in beer, confirming that formylate forms were predominant (14-16). The levels of FA and 10-formyl-THF increased during mashing, probably due to hydrolysis of polyglutamate forms and the following oxidation of reduced forms. For Beers 1-3, the increase was respectively +37.4%, +15.6% and +88.5%. A decrease was observed at the end of boiling in the three processes (-36.2% to -42.8%), probably due to dilution with sparge liquor and wort boiling. An increase in respect to hopped wort was observed during fermentation, with an increase of +126.3% for Beer 1, +82.9% for Beer 2, +72.0% for Beer 3. This is similar to the observations with pyridoxine and in agreement with the report of Pietercelie et al. (16). Finally, the concentration was constant after maturation and in final beer for small scale brewing; a decrease was observed for Beer 3, because of dilution, filtration and pasteurisation. The levels found in the final beer samples were in accordance with those of Bertuzzi et al. (12).
Figures 1 and 2 show the trend of B6 (pyridoxine + pyridoxamine) and B9 vitamers (10-formyl-THF and folic acid) during the brewing processes. During mashing and boiling, the trends of B6 and B9 vitamers were different. Where pyridoxine decreased in mashing and remained constant after sparging and boiling, folates increased in mashing, but lowered in boiling. Moreover, it is possible to observe a different trend for pyridoxine in the final beer with respect to the level at the start of mashing. In Beer 1 and Beer 3, the concentration decreased (-24.8% and -43.8%, respectively), while it increased in Beer 2 brewing (+57.1%). For folates, an increase was always found, even with wide variations (Beer 1 +83.6%, Beer 2 +8.6% and Beer 3 +28.2%).
Conclusions
This study showed that pyridoxine and folates dissolved easily during the brewing process and remained in the bottled beer. Despite different starting levels of these vitamins in the three brews due to different malts used, comparable trends were observed during small and large scale brewing processes because both plants used similar unit operations to obtain the final products (25). Fermentation increased vitamin content more significantly than the other brewing steps; moreover, comparable trends were shown for all the three beers studied, independently of the fermentation systems (top and bottom). Large scale brewing showed a decrease in vitamins between the end of maturation and the final bottled beer because of filtration, pasteurisation, and dilution to the desired gravity with deoxygenated water. These operations did not occur in small scale Beer 1 and 2 which were brewed from standard gravity worts, were unpasteurised but re-fermented. Unlike primary fermentation, bottle refermentation did not increase vitamin content. Future research would be of interest, particularly on the role of different yeast strains and the effect of ageing of re-fermented beer on pyridoxine and folate content.
Author contributions
Elia Romanini wrote the original draft of the manuscript.
Silvia Rastelli performed the analysis.
Gianluca Donadini was involved in review and editing the manuscript.
Milena Lambri was involved in review and editing the manuscript.
Terenzio Bertuzzi wrote the original draft of the manuscript.
All authors approved the final manuscript.
Conflict of interest
The authors declare there are no conflicts of interest.
Acknowledgements
The present work was supported by the European Foundation for Alcohol Research (ERAB, Project EA 16 37). The authors would like to thank Ex Fabrica – Birrificio Artigianale and Davide Pessina for supplying beer samples (B1 and 2). Elia Romanini is recipient of a fellowship from the Doctoral School on the Agro-Food System (Agrisystem) of the Università Cattolica del Sacro Cuore (UCSC, Piacenza, Italy).




