Optimization of fermentation conditions for Chinese bayberry wine by response surface methodology and its qualities
Abstract
Colour and flavour are both important challenges for bayberry wine production because of a shortage of selected strains. The yeast Saccharomyces cerevisiae strain YF152, separated from a natural Chinese bayberry fermentation mash [alcohol tolerance capacity (18.0%), SO2 (200 mg/L), sugar content (40%) and pH (2.50)] was employed to brew Chinese bayberry wine. Response surface methodology was used to simultaneously analyse the effects of the fermentation conditions on Chinese bayberry wine. The optimum conditions were found to be a temperature of 26.5 °C, an initial sugar content of 22.0°Brix, an inoculum size of 4.0% and an initial pH of 2.90. Under these conditions, the final alcohol content of the bayberry wine was 13.4%, the anthocyanin content was 77 mg/L and the residual sugar content was 1 g/L. These numbers agreed well with the predicted values. The major characteristic flavour components of the wine were 3-methyl-1-butanol, 2-phenylethanol, ethyl acetate, 2-methylbutyl acetate and acetic acid. Copyright © 2016 The Institute of Brewing & Distilling
Introduction
Chinese bayberry (Myrica rubra Sieb. & Zucc.), an important economic Asian fruit crop, belongs to the family Myricaceae (1). Chinese bayberry fruit, with a sweet/sour taste, an exquisite flavour and an attractive purple, red or dark red colour, is popular with local people (2). Unfortunately, the Chinese bayberry fruit is highly susceptible to mechanical injury and microbiological decay, which limits its postharvest life to 2 days at 20 °C or 5 days at 0 °C. The antioxidant capacity of the fruit decreases rapidly during storage (2, 3). Therefore, there is a demand for alternative processing methods to extend the storage time and achieve a longer consumption period (4).
Winemaking is a promising way of processing fruit. Increasingly, researchers have been directing their attention at fruity wines such as apple (5, 6), papaya (7), raspberry (8), strawberry (9), guava (10), pineapples (11), mango (12), bael (13), cagaita (14) and purple sweet potato (15). However, there is very little research about Chinese bayberry wine because of its short shelf-life and unstable quality.
Colour instability is one of the important challenges for bayberry products. The colour of Chinese bayberry is mainly related to the presence of anthocyanins (16). The major anthocyanin in bayberry fruit has been identified as a cyanidin 3-glucoside, which represents >95% of the total pigments (17); however, it is more unstable than other kinds of anthocyanins and usually decreases sharply during fermentation, leading to wine with a poor colour (15). The stabilities of anthocyanins of Chinese bayberry juice (2, 16, 18, 19) have been studied and the results can be used to improve the stability and content of anthocyanins of Chinese bayberry products.
The pH value of fresh bayberry ranges from 2.80 to 3.20, over which range the anthocyanin shows better stability and the colour appears more attractive. Saccharomyces cerevisiae is the core microorganism in fermentation, especially in winemaking (20). At present, there are only a few special yeast strains used for brewing Chinese bayberry wine. Currently, Chinese bayberry wine produced in China almost always uses commercial yeasts and wine active dry yeasts. However, the Chinese bayberry wine produced often fails to meet the needs of the consumer owing to the lower alcohol and poor flavour. In addition, these yeasts usually ferment at pH 3.30–3.60, leading to a Chinese bayberry wine with a faded colour. Thus, it was deemed necessary to develop a special yeast strain for brewing Chinese bayberry wine of good quality.
In this work, a wild yeast strain was selected after screening (YF152) and studied regarding the effect of fermentation conditions such as temperature, initial sugar, inoculum size and initial pH on alcohol content, anthocyanin content and the residual sugar content of the Chinese bayberry wine. The fermentation conditions were then optimized by response surface methodology (RSM) and the flavour analysed by gas chromatography–mass spectrometry (GC–MS). The study provides a reference for the industrial production of Chinese bayberry wine with a higher alcohol content, favourable colour and satisfactory flavour.
Materials and methods
Bayberry must
Chinese bayberry, hand-harvested at a mature stage from an orchard in Wuxi, Jiangsu province, China, was transported to our laboratory within 1 h and then frozen stored at −20 °C before use. The Chinese bayberry juice was obtained using a juice extractor and was filtered using four layers of sterile cheesecloth after first naturally thawing the juice at 20 °C. The composition of the fresh Chinese bayberry juice was analysed with contents as follows: total sugar of 92 g/L, titratable acidity of 0.86 g/100 mL (expressed as citric acid), pH of 2.95, total soluble solid content (TSS) of 11.2°Brix and an anthocyanin content of 290 g/L. Sucrose was added into the juice to ameliorate the TSS and the original pH was adjusted using calcium carbonate or citric acid. Equal volumes of 120 mL of juice, with different TSS and pH levels, were placed into separate 250 mL sterilized Erlenmeyer flasks, pasteurized at 100 °C for 5 min, and immediately cooled to 20 °C.
Yeast strain
The yeast strain used in this study was a wild strain, designated YF152, isolated from a natural bayberry fermentation mash and identified as Saccharomyces cerevisiae, cultivated by the China Centre for Type Culture Collection, Wuhan University, with accession number CCTCC M 2015627. The pure culture of the strain was conserved on a YPD agar slant (1.0% yeast extract, 2.0% peptone, 2.0% dextrose, adding 2.0% agar when required) at 4 °C. The inoculation steps were as follows. A tricyclic pure culture from the slant was placed into 50 mL of the liquid YPD medium and stirred at 150 rpm in an incubator shaker for 12 h, then inoculated as 10% (v/v) of the culture with 100 mL of the liquid YPD medium and incubated in the shaker under the same conditions for 12 h. The yeast concentration was determined with a haemocytometer and adjusted to 2.00 × 108 cell/mL with sterile distilled water.
The strains RV171, BV818 and RW (Angel Yeast Co. Ltd, China), which are commercial yeast strains, were employed to brew Chinese bayberry wine, and a comparison was made with the experimental yeast strain YF152. The fermentation conditions were as follows: initial sugar content of 20°Brix, inoculum of 0.2%, initial pH of 3.5 and fermentation at 28 °C for 8–14 days (until the weight loss was <0.2 g/day).
The tolerance capability of the yeast
The tolerance capacity of YF152 for alcohol, sugar, pH and SO2 was studied by determining the inflated conditions of the inverted tube (Durham) used for collecting the CO2 that was liberated through fermentation (21). The media were liquid YPD, modified with increasing concentrations of alcohol (10.0, 12.0, 14.0, 16.0, 18.0 and 20.0%), sugar (20, 30, 40 and 50%), pH (1.50, 2.00, 2.50 and 3.00) and SO2 (100, 150, 200 and 250 mg/L) (22). The yeasts were inoculated at 5.0% into 25 mL tubes containing 10 mL liquid YPD and were inoculated at 28 °C for 4 days. The Durham tubes were assessed every 24 h.
Single factor experiment
The following four main influencing factors were investigated in this study: temperature (16.0, 20.0, 24.0 and 28.0 °C), initial sugar concentration (20.0, 22.0, 24.0, 26.0 and 28.0°Brix), inoculum size (0.2, 1.0, 5.0 and 9.0%) and initial pH (2.50, 3.00, 3.50 and 4.00). The alcohol content, anthocyanin content and residual sugar content were measured after the fermentation. In addition to the studied factors, other conditions were fixed at a temperature of 24 °C, an initial sugar of 24.0°Brix, an inoculum size of 5.0% and an initial pH of 3.00. After being inoculated with varied inoculum sizes, all flasks were fitted with a fermentation bolt and fermentation took place under stationary conditions at different temperatures for 8–14 days until the weight loss was <0.2 g/day. Each treatment was performed in triplicate.
Optimization experimental design
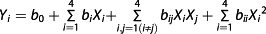 ()
()| Independent variable | Units | Symbol | Coded levels | ||||
|---|---|---|---|---|---|---|---|
| -2 | −1 | 0 | +1 | +2 | |||
| Temperature | °C | X1 | 20.0 | 22.0 | 24.0 | 26.0 | 28.0 |
| Initial sugar | °Brix | X2 | 22.0 | 23.0 | 24.0 | 25.0 | 26.0 |
| Inoculum size | % | X3 | 1.0 | 3.0 | 5.0 | 7.0 | 9.0 |
| Initial pH | X4 | 2.50 | 2.75 | 3.00 | 3.25 | 3.50 | |
| Standard order | Coded values of the Independent variables | Dependent variables | |||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Y1 | Y2 | Y3 | |
| 1 | −1 | −1 | −1 | −1 | 11.1 | 91 | 51 |
| 2 | 1 | −1 | −1 | −1 | 13.0 | 86 | 22 |
| 3 | −1 | 1 | −1 | −1 | 10.8 | 79 | 79 |
| 4 | 1 | 1 | −1 | −1 | 12.0 | 77 | 61 |
| 5 | −1 | −1 | 1 | −1 | 11.2 | 87 | 52 |
| 6 | 1 | −1 | 1 | −1 | 12.6 | 86 | 27 |
| 7 | −1 | 1 | 1 | −1 | 11.0 | 72 | 76 |
| 8 | 1 | 1 | 1 | −1 | 12.2 | 69 | 56 |
| 9 | −1 | −1 | −1 | 1 | 13.0 | 58 | 19 |
| 10 | 1 | −1 | −1 | 1 | 14.0 | 57 | 4 |
| 11 | −1 | 1 | −1 | 1 | 13.4 | 52 | 34 |
| 12 | 1 | 1 | −1 | 1 | 13.6 | 48 | 30 |
| 13 | −1 | −1 | 1 | 1 | 13.8 | 57 | 11 |
| 14 | 1 | −1 | 1 | 1 | 14.2 | 55 | 2 |
| 15 | −1 | 1 | 1 | 1 | 13.5 | 48 | 32 |
| 16 | 1 | 1 | 1 | 1 | 13.6 | 46 | 28 |
| 17 | −2 | 0 | 0 | 0 | 11.4 | 73 | 56 |
| 18 | 2 | 0 | 0 | 0 | 13.1 | 56 | 24 |
| 19 | 0 | −2 | 0 | 0 | 12.8 | 70 | 8 |
| 20 | 0 | 2 | 0 | 0 | 12.9 | 53 | 49 |
| 21 | 0 | 0 | −2 | 0 | 13.0 | 74 | 26 |
| 22 | 0 | 0 | 2 | 0 | 13.1 | 57 | 25 |
| 23 | 0 | 0 | 0 | −2 | 9.9 | 109 | 79 |
| 24 | 0 | 0 | 0 | 2 | 14.1 | 35 | 14 |
| 25 | 0 | 0 | 0 | 0 | 13.7 | 62 | 21 |
| 26 | 0 | 0 | 0 | 0 | 13.4 | 67 | 25 |
| 27 | 0 | 0 | 0 | 0 | 13.6 | 64 | 22 |
| 28 | 0 | 0 | 0 | 0 | 13.4 | 68 | 25 |
| 29 | 0 | 0 | 0 | 0 | 13.5 | 65 | 24 |
| 30 | 0 | 0 | 0 | 0 | 13.8 | 61 | 18 |
- X1, temperature (°C); X2, initial sugar (°Brix); X3, inoculum size (%); X4, initial pH; Y1, alcohol content (%); Y2, anthocyanin content (mg/L); Y3, residual sugar content (g/L).
The dependent variables (Y) were the alcohol content [Y1, % (v/v)], the anthocyanin content (Y2, mg/L) and the residual sugar content (Y3, g/L) of the Chinese bayberry wine; b0 is the constant coefficient; bi is the linear effects; bij is the interaction term; bii is the squared effects; and Xi and Xj are the coded values of variables i and j, respectively. The significance of all terms in the polynomial functions was evaluated statistically using an F-value at a probability (P) of 0.01 and 0.05. All samples were fermented using stationary conditions for ~8–14 days until the weight loss was <0.2 g/day.
Component analysis
The anthocyanin content of Chinese bayberry wine was determined using the differential pH method (23). The absorbance was measured at 510 and 700 nm, using a UV-1800 spectrophotometer [Mapada Instruments (Shanghai) Ltd, China]. The alcohol content was determined by the method described by Kumar (12). The pH was determined using a Toledo FE-20 K pH-meter [Mettler-Toledo Instruments (Shanghai) Ltd, China] (19). The total sugar was first hydrolysed into reducing sugars using 20 mL of 6 mol/L HCl in a 68 °C water bath for 15 min, was then neutralized with 200 g/L NaOH, and finally determined using the dinitrosalicylic acid method (25). The titratable acidity was estimated according to the official methods of AOAC (26). The TSS (°Brix) content was determined using a hand refractometer (Erma, Japan) (27).
Volatile organic compound analysis
Solid-phase microextraction (SPME)–GC–MS was used to analyse the volatile organic compounds (VOCs) of the Chinese bayberry wine. Aliquots of wine samples (8 mL) were placed in each SPME vial (15 mL). The extraction was performed using SPME fibre (carboxen/polydimethysiloxane fibre, Supelco, Belfonte, PA, USA) with an extraction temperature of 50 °C for 30 min, allowing the volatiles to reach equilibrium in the headspace. After the extraction, the fibre was inserted immediately into an injection port of a GC–MS (Scion SQ 456, Bruker, USA) and desorbed for 8 min at 250 °C. The chromatography was performed using a DB-Wax column (30 m × 0.25 mm × 0.25 μm) helium as a carrier gas at 0.8 mL/min (constant flow). The oven temperature programme was as follows: 40 °C for 3 min; 90 °C at 5 °C/min; 90–230 °C at 10 °C/min; and finally maintained at 230 °C for 7 min. The electron impact ionization mass spectrometer was operated as follows: ionization voltage, 70 eV; ion source temperature, 200 °C; and injection port temperature, 250 °C. Compounds were identified by comparison with the reference spectra of the National Institute for Standards and Technology (Gaithersburg, MD, USA), the Wiley MS library and the relative peak area (28).
Statistical analysis
All sample analyses were performed in triplicate. The data of the single factor experiment were expressed as the means ± standard deviation, subjected to a statistical analysis with the software SPSS/PC version 13.0 (SPSS Inc., Chicago, IL, USA) and graphically plotted using Origin Lab (Origin Pro, version 8.0). Significant differences among the mean values were analysed by a one-way analysis of variance (ANOVA) with the application of Duncan's test at a level of p < 0.05.
Results and discussion
The tolerance capacity of the yeast
The yeast YF152 showed an excellent tolerance capacity by rapidly inflating the Durham tubes within 24 h under the following conditions: 18.0% alcohol content, 200 mg/L of SO2, 40% sugar content and a pH of 2.50, which was very suitable to brew Chinese bayberry wine. Osho (29) studied the alcohol and sugar tolerance of wine yeasts isolated from fermenting cashew apple juice and found that the best four yeasts were able to grow in alcohol concentrations of 9.0, 10.0, 11.0 and 12.0% (29); however, their alcohol tolerances were below that of YF152.
Effect of temperature on fermentation of Chinese bayberry wine
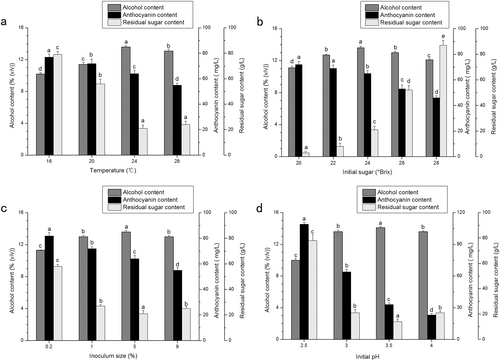
As shown in Fig. 1(a), the temperature significantly affected the alcohol content, anthocyanin content and residual sugar content (p < 0.05). It was found that, when the temperature was <24 °C, the alcohol content increased significantly with the rise in temperature but decreased obviously at 28 °C, which may be because the higher temperature leads to a rapid release of CO2, removing some of the alcohol at the same time. The alcohol content of the bayberry wine fermented at 24 °C was the highest (13.6%). The anthocyanin content decreased significantly from 77 to 55 mg/L when the temperature varied between 20 and 28 °C, illustrating that temperature is an important factor in destabilizing the molecular structure of anthocyanins (30), and higher temperatures will accelerate the destruction of anthocyanins (31, 32). The residual sugar showed an opposite trend compared with the alcohol content, and the lowest content was 23 g/L fermented at 24 °C. In summary, 24 °C was chosen as the optimum fermentation temperature in the following experiment.
Effect of initial sugar on fermentation of Chinese bayberry wine
Figure 1(b) shows that the initial sugar content had a significant influence on the alcohol content, the anthocyanin content and the residual sugar content (p < 0.05). The data illustrated that, when the initial sugar content reached 20.0–24.0°Brix, the alcohol content began to increase gradually from 11.1 to 13.6%; however, when the sugar content exceeded 24.0°Brix, the alcohol content began to decrease. It is posited that the biological activity of the yeast was inhibited by the higher sugar content (20), leading to a higher residual sugar content and a lower alcohol content in the Chinese bayberry wine. There was no difference in the anthocyanin content between the initial sugar contents of 20.0 and 22.0°Brix (p > 0.05); however, when the content exceeded 22.0°Brix, the anthocyanin content decreased significantly from 68 to 46 mg/L (p < 0.05); the higher sugar content possibly had a negative effect on the stability of the anthocyanins (33). The lowest amount of initial sugar produced the lowest residual sugar content, and the residual sugar content of the bayberry wine, fermented at 20.0 and 22.0°Brix, was <12 g/L. The anthocyanin content of bayberry wine at an initial sugar content of 24.0°Brix decreased by 6.0% compared with that of 22.0°Brix; however, the alcohol content increased by 7.1%. Therefore, the initial sugar content had a greater effect on the alcohol content than on the anthocyanin content, and 24.0°Brix was the desired initial sugar content.
Effect of inoculum size on fermentation of Chinese bayberry wine
According to Fig. 1(c), the inoculum size played an important role in the alcohol, anthocyanin and residual sugar contents. When the inoculum size was 0.2%, the alcohol content was <12%, and the residual sugar could not be fully utilized (34). Between the inoculum sizes of 0.2 and 5.0%, the alcohol content increased with increases in the inoculum size, and the residual sugar content decreased at the same time. However, the alcohol content of the Chinese bayberry wine with an inoculum size of 9.0% was lower than that with an inoculum size of 5.0%; considering that, when the inoculum size was too high, most of the nutrition of the bayberry juice was utilized for the growth and reproduction of yeast, the substrate of the metabolic reaction was greatly reduced (34) and the alcohol decreased with the concurrent rapid release of CO2. In addition, the inoculum size also significantly influenced the colour of the bayberry wine (p < 0.05) because the cell walls of the yeast strains adsorbed anthocyanin during the fermentation (35, 36). As the inoculum size increased, the anthocyanin content decreased. However, the inoculum size also influenced the period of fermentation; therefore, a higher inoculum size could shorten the fermentation time, but an inoculum size of 5.0% appeared satisfactory.
Effect of initial pH on fermentation of Chinese bayberry wine
As shown in Fig. 1(d), the initial pH influenced the alcohol content significantly in the pH range of 2.50–3.50 (p < 0.05), with an increase in the alcohol content from 9.9 to 14.1%; however, when the pH exceeded 3.50, the alcohol content decreased to 13.6% at a pH of 4.00; therefore, the optimum fermentation pH of YF152 was 3.50. Sevda and Rodrigues (10) compared the alcohol production of guava wine using two different strains at different pH levels with a sugar content of 22.0°Brix. For one type of yeast, the maximum alcohol production was 7.3% (v/v) at a pH of 3.50, which was 6.9% lower than YF152 at the same pH. For the other type of yeast, the maximum alcohol production was 8.4% (v/v) at a pH of 4.00 (10), which was lower than YF152 at a pH of 2.50 at 9.9%. Therefore, YF152 proved to be more suitable for brewing at a low pH. The initial pH had a greater effect on the anthocyanin content than temperature, initial sugar and inoculum size, with a dramatic decrease in the anthocyanin content from 109 to 23 mg/L, with an increase in pH from 2.50 to 4.00. A lower pH led to a higher content of anthocyanin, since anthocyanins are more stable at a lower pH (16), and they are easily destroyed at a high pH. The effect of the initial pH on the residual sugar content was the opposite of that of the alcohol content, and the least residual sugar content was obtained at a pH 3.50 at 14 g/L. The anthocyanin content at a pH of 2.50 was the highest at ~109 mg/L, but the alcohol content only reached 10%, and the residual sugar was more than 75 g/L. Although the alcohol content at a pH of 3.50 was the highest and the residual sugar content was the lowest, the anthocyanin content only reached 34 mg/L, making the Chinese bayberry wine appear shallow. Therefore, a pH of 3.00 was the desired value.
Model prediction and optimization
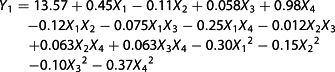 ()
()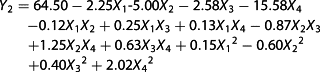 ()
() ()
()The experimental results and an ANOVA for the alcohol content, anthocyanin content and residual sugar content are presented in Tables 2 and 3, respectively. The model F-values of 49.51, 30.60 and 112.47 implied that the three models were significant. There was only a 0.01% chance that a ‘model F-value’ this large could occur owing to noise. The ‘lack of fit F-values’ of 2.38, 2.72 and 1.19 implied that the ‘lack of fit’ of the three models was not significant relative to the pure error. In addition, R2 values for all the response variables were >0.96, indicating that the regression model explained the reaction well.
| Source | d.f. | Y1 | Y2 | Y3 | |||
|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | ||
| Model | 14 | 49.51 | <0.0001** | 30.60 | <0.0001** | 112.47 | <0.0001** |
| X1 | 1 | 94.88 | <0.0001** | 7.55 | 0.0149* | 174.51 | <0.0001** |
| X2 | 1 | 5.50 | 0.0332* | 37.31 | <0.0001** | 415.24 | <0.0001** |
| X3 | 1 | 1.59 | 0.2260 | 9.96 | 0.0065** | 1.60 | 0.2252 |
| X4 | 1 | 453.06 | <0.0001** | 362.37 | <0.0001** | 766.47 | <0.0001** |
| X1X2 | 1 | 4.88 | 0.0431* | 0.016 | 0.9024 | 7.58 | 0.0148* |
| X1X3 | 1 | 1.76 | 0.2048 | 0.062 | 0.8065 | 0.47 | 0.5017 |
| X1X4 | 1 | 19.52 | 0.0005** | 0.016 | 0.9024 | 26.66 | 0.0001** |
| X2X3 | 1 | 0.049 | 0.8281 | 0.76 | 0.3966 | 0.47 | 0.5017 |
| X2X4 | 1 | 1.22 | 0.2867 | 1.55 | 0.2316 | 7.58 | 0.0148* |
| X3X4 | 1 | 1.22 | 0.2867 | 0.39 | 0.5424 | 1.07 | 0.3181 |
| X12 | 1 | 49.54 | <0.0001** | 0.036 | 0.8515 | 68.90 | <0.0001** |
| X22 | 1 | 12.73 | 0.0028** | 0.62 | 0.4424 | 9.72 | 0.0071** |
| X32 | 1 | 5.81 | 0.0292* | 0.27 | 0.6127 | 3.12 | 0.0979 |
| X42 | 1 | 71.99 | <0.0001** | 6.96 | 0.0186* | 126.12 | <0.0001** |
| Residual | 15 | ||||||
| Lack of fit | 10 | 2.38 | 0.1752 | 2.32 | 0.1408 | 1.19 | 0.4503 |
| Pure error | 5 | ||||||
| Total | 29 | ||||||
- * Significant at 0.05 level.
- ** Significant at 0.01 level.
- X1, temperature (°C); X2, initial sugar (°Brix); X3, inoculum size (%); X4, initial pH; Y1, alcohol content (%); Y2, anthocyanin content (mg/L); Y3, residual sugar content (g/L).
Alcohol content
Wine fermentation is the result of many interactions and depends not only on the strains but also on the physicochemical factors of the medium, sugars, acidity, temperature and others (37). Values of ‘Prob > F′ <0.05 indicated the model terms were significant. Among the model terms, X1, X4, X1X4, X12, X22 and X42 were significant with p < 0.01, and the model terms X2, X1X2, and X32 had significant effects with p < 0.05, whereas the X3, X1X3, X2X3, X2X4 and X3X4 model terms were not significant.
Figure 2(a, b) shows the three-dimensional response surfaces that are constructed to show the effects of the variables of bayberry wine fermentation on the alcohol content (Y1). Figure 1(a) shows the effect of temperature and initial sugar on the alcohol content (inoculum size and initial pH were fixed at zero). The alcohol content increased with the increase in temperature in the range of 20–26 °C and initial sugar of 22.0–23.3°Brix, and it peaked with a temperature of 25–27 °C and the initial sugar content of 23.0–24.0°Brix. Figure 2(b) shows the effects of temperature and the initial pH on the alcohol content (initial sugar and inoculum size were fixed at zero), which demonstrated that the alcohol content peaked at a temperature of 23–26 °C and an initial pH of 3.20–3.40.
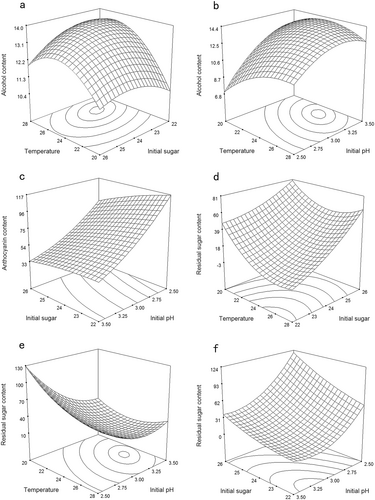
Anthocyanin content
The stability of anthocyanins, during the fermentation of purple sweet potato wine (15) and red wine (38), has been studied, but little research was related to the stability of anthocyanins in Chinese bayberry wine. The major anthocyanin was different from that in purple sweet potato and grape. Among the model terms, only X1, X2, X3, X4 and X42 had significant effects on the anthocyanin content (p < 0.05), especially the initial sugar (X2) and pH (X4), with p-values <0.0001.
Figure 2(c) shows the effect of the initial sugar and pH on the anthocyanin content (temperature and inoculum size were fixed at zero). The highest anthocyanin content was obtained when the variables were both at the minimum point within the range studied.
Residual sugar content
The residual sugar content reflects the ability of the yeast to utilize sugar and is responsible for imparting sweetness to the final wine (39). In this experiment, the desired residual sugar content was <4 g/L. As shown in Table 3, the model terms X1, X2, X4, X1X4, X12, X22 and X42 were significant at p < 0.01, and model terms X1X2, X1X4 had significant effects at p < 0.05, whereas the other model terms were not significant.
Figure 2(d) shows the effect of temperature and initial sugar on the residual sugar content (inoculum size and initial pH were fixed at zero). The lowest residual sugar content was obtained with a temperature of 24–28 °C and an initial sugar content of 22.0–23.0°Brix. The effects of temperature and initial pH on the residual sugar content (initial sugar and inoculum size were fixed at zero level) are shown in Fig. 2(e). The minimum response plot was obtained with a temperature of 24–26 °C and an initial pH of 3.20–3.40, which is opposite to the effect on the alcohol content. In contrast, Fig. 2(f) shows the effect of initial sugar and initial pH (temperature and inoculum size were fixed at zero) on the residual sugar content. The lowest residual sugar content was obtained with an initial sugar content of 22.0–23.0°Brix and an initial pH of 3.00–3.50.
The optimum fermentation conditions
The independent variables, namely fermentation temperature, initial sugar, inoculum size and initial pH, were optimized using the statistical software Design Expert 7.1.6. For convenient practical operation, the optimum combination of factors was set as follows: temperature (26.5 °C), initial sugar (22.0°Brix), inoculum size (4.0%) and initial pH (2.90). The responses predicted from the mode were 13.3%, 78 mg/L and 1 g/L. Repeated experiments were performed to verify the predicted model. The actual alcohol content, anthocyanin content and residual sugar content were 13.4%, 77 mg/L and 1 g/L, respectively. Verification of the model indicated no difference between the predicted and actual values.
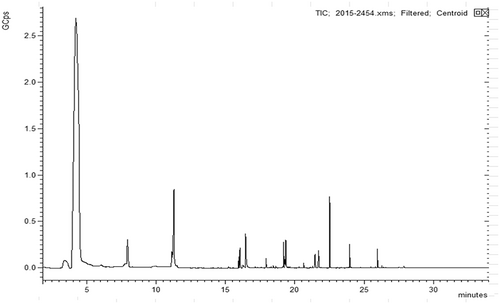
VOCs of bayberry wine
The VOCs of wines are a unique mixture of volatile compounds that originate from fruits (varietal aromas), secondary products formed during the wine fermentation (fermentative aromas) and aging (post-fermentative aromas) (40). The complexity of the VOCs can dramatically increase during alcoholic fermentation (41). Figure 3 displays the GC–MS photograph of VOCs in Chinese bayberry wine. Higher levels of alcohols, esters and acids are the main group of compounds that form the fermentation bouquet (42), taking up 34.3, 49.1 and 13.3%, respectively. The compounds 3-methyl-1-butanol (69.8%) and 2-phenylethanol (26.1%) were the major higher alcohols produced by YF152, contributing a fruity, apple brandy aroma (28) and a sweet, rose, floral flavour (43), respectively. The sum content of ethyl acetate and 2-methylbutyl acetate accounted for 59.4% of the total esters, mainly synthesized by the reaction between alcohols and acetyl-CoA during alcoholic fermentation, imparting a floral and fruity flavour (28). Acetic acid, which comprised 7.8% of the total VOCs, was the largest acid component. All of these substances are the major characteristic flavour components of Chinese bayberry wine.
Comparison with other wine yeast
The S. cerevisiae RV171, BV818 and RW strains were used to compare the fermentation efficiency of starters and the wine properties of Chinese bayberry wine with YF152. As shown in Table 4, the three dry-yeasts were not suitable for brewing bayberry wine because their alcohol contents were <10.0%, the anthocyanin content could not reach half of the concentration of the YF152 and the residual sugar content was >30 g/L, leading to a wine with a lower alcohol content and fading colour. Compared with the optimum fermentation conditions of mango wine (12) and mulberry wine (44), the optimum temperature of YF152 was between S. cerevisiae (var. bayanus) and CFTRI 1013, the optimum initial sugar was the same as CFTRI 101, but 2.0°Brix higher than that of S. cerevisiae (var. bayanus). It is worth noting that the optimum pH of YF152 was lower than that of S. cerevisiae (var. bayanus) and CFTRI 1013. Compared with S. cerevisiae (var. bayanus) and CFTRI 1013, the alcohol content of the wine that used YF152 as the starter increased by 34.0% and 7.5%, respectively. Therefore, YF152 is considered to be a good yeast strain for brewing Chinese bayberry wine with a better fermentation performance than many other yeast strains, especially at a lower pH.
| Yeast strains | Fermentation conditions | Fermentation indicators | |||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Y1 | Y2 | Y3 | |
| Saccharomyces cerevisiae YF152a | 26.5 | 22.0 | 4.0 | 2.90 | 13.4 | 77 | 1 |
| S. cerevisiae RV171a | 28.0 | 20.0 | 0.2 | 3.50 | 6.3 | 23 | 90 |
| S. cerevisiae BV818a | 28.0 | 20.0 | 0.2 | 3.50 | 7.1 | 30 | 76 |
| S. cerevisiae RWa | 28.0 | 20.0 | 0.2 | 3.50 | 10.0 | 21 | 32 |
| S. cerevisiae (var. bayanus)b | 22.5 | 20.0 | 11.9 | 3.80 | 10.0 | — | — |
| S. cerevisiae CFTRI 101b | 31.4 | 22.0 | 0.5 | 3.20 | 12.46 | — | — |
- a Used for brewing Chinese bayberry wine.
- b Used for brewing mango wine.
- c Used for brewing mulberry fruit wine.
- —, Not detected.
- X1, temperature (°C); X2, initial sugar (°Brix); X3, inoculum size (%); X4, initial pH; Y1, alcohol content (%); Y2, anthocyanin content (mg/L); Y3, residual sugar content (g/L).
Acknowledgements
The authors acknowledge the financial support of the Special Fund for Agro-scientific Research in the Public Interest (201303073-01), Grain Research in the Public Interest (201313011-6-4), the Fundamental Research Funds for the Central Universities (JUSRP51501), Jiangsu Province Science and Technology Infrastructure Construction plan (BM2014051/004), China National Natural Science Foundation (31371812, 31871878), National Key Research and Development Program (2016YFD0401404), Jiangsu Province Science and Technology Project (SBN2014010290, BN2014058), and Qing Lan Project which has enabled us to carry out this study.




