Antioxidant activities of volatile and non-volatile fractions of selected traditionally brewed Korean rice wines
Abstract
Antioxidant activities of traditionally brewed Korean rice wines have recently been reported. The objective of this study was to identify the antioxidant activities of the volatile and non-volatile fractions of three Korean rice wines, and further to identify the active ingredients responsible for the antioxidant properties. Volatile and non-volatile components of three rice wines, Measilju, Kukwhaju and Gugijaju, were separated into seven fractions according to their polarities. Antioxidant activities were determined by an aldehyde–carboxylic acid assay for the volatile fractions and a lipid–malondialdehyde assay for the non-volatile fractions. Volatile constituents of each fraction were identified and quantified using gas chromatography–mass spectrometry. In addition, antioxidant activities of specific authentic chemicals from the volatile fractions of the wines were measured, and the antioxidant properties of the volatile fractions were determined. Identification of volatile compounds potentially showing high antioxidant activities were conducted in each fraction of the three wines. Among the volatile compounds identified from volatile fractions of the wines, benzeneethanol, 2-furanmethanol and 4-ethyl-2methoxyphenol showed the highest antioxidant activities, when added at higher concentrations (~100 µg/g). Identification of antioxidant properties, as well as the specific compounds responsible for these antioxidant properties, could aid Korean rice wine product developers, in terms of strategic planning for sales in the Korean market. Copyright © 2014 The Institute of Brewing & Distilling
Introduction
Health benefits associated with several types of alcoholic beverages have been reported, even though the general consumer perception of health and drinking alcoholic beverages is negative. For example, red wine has been known for decades to lower the risk of heart disease, arteriosclerosis and cancer 1-4. Japanese rice wine, commonly known as ‘sake’, is also known for its antimicrobial and anticancer properties 5. Similar to sake, Korean rice wine (KRW) has recently been reported to have beneficial health effects, with gastroprotective 6 and antioxidant properties 7. A traditional KRW production process involves ‘nuruk’ (Korean-style koji), which is composed of steamed rice and raw barley 8. Like sake, traditional KRW uses rice as a main ingredient, but medicinal plants and/or herbs are added during or after the fermentation to enhance the flavours of the final products, as well as to provide the health effects associated with medicinal plants and herbs.
Distinctive sensorial and health-related characteristics of traditional KRWs are derived from added medicinal plants and herbs to KRWs 8. Medicinal plants and herbs widely used in KRWs include Japanese apricot (Prunus mume), Indian dendranthema (Dendranthema indicum) and Chinese matrimony vine (Lycium chinense Mill.). The medicinal effects of these plants and herbs have been previously reported in several studies 9-15. Prunus mume is known for its antioxidant and anti-cancer activities 9, 12, D. indicum is known for its antimicrobial and antioxidant properties 10 and L. chinense Mill. is a known anti-febrile agent and protects against carbon tetrachloride-induced hepatotoxicity 9, 13, 15. Although beneficial effects associated with medicinal plants and herbs have been widely studied, limited research has been conducted to determine the positive health effects associated with herbal ingredients when added to alcoholic beverages, such as KRWs 7, 16, 17.
Recent studies identified the functional ingredients that exhibited the high antioxidant properties of traditionally brewed KRW 7. They reported on the antioxidant activities of volatile and non-volatile extracts in six traditionally brewed KRWs using different medicinal plants and herbs. Results indicated that polyphenols, polysaccharides and polysaccharide–peptide complexes in the non-volatile extracts of traditional KRWs contained the antioxidant properties 7. To further investigate the active ingredients responsible for the antioxidant properties of KRW concentrates, the current study focused on identifying the volatile compounds that might possess high antioxidant properties using gas chromatography–mass spectrometry (GC-MS) with a flame ionization detector (FID) and a nitrogen phosphorus detector (NPD). The antioxidant activities of selected compounds were measured by an aldehyde–carboxylic acid assay for volatile compounds and by a lipid–malondialdehyde (MA) assay for the non-volatile compounds. Three Korean rice wines – Measilju (MSJ), Kukwhaju (KHJ), and Gugijaju (GGJ) – were selected based on their high antioxidant activities reported in previous studies 7. The current study focused on the three KRWs that had shown the highest antioxidant properties in order to identify the key compounds responsible for these properties.
Materials and methods
Materials
Three KRWs brewed using traditional methods were selected for this study and were obtained from a traditional market in Seoul. The alcoholic content of each sample ranged between 14 and 15% (w/v). The active herbal ingredients in MSJ, KHJ and GGJ are P. mume, D. indicum and L. chinense (Mill.), respectively.
Chemicals
Hydrogen peroxide, cod liver oil, Trizma hydrochloride and Trizma base were purchased from Sigma-Aldrich Chemical Co. (Milwaukee, WI, USA); dichloromethane, pentane and ethyl acetate were purchased from the Junsei Chemical Co. Ltd (Tokyo, Japan). Hexanal (99%), undecane (99%), α-tocopherol (vitamin E; 95%), N-methylhydrazine (NMH), 2-methylpyrazine, sodium dodecyl sulphate (SDS), and ferrous chloride were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). Silica gel 60 N (particle size 63–200 µm; 70–230 mesh ASTM) and anhydrous sodium sulphate were purchased from the Kanto Chemical Co. Inc. (Tokyo, Japan). Amberlite XAD-2 resin was purchased from Supelco (Bellefonte, PA, USA).
Isolation of volatile and non-volatile components of Korean rice wines using a solvent-assisted flavour evaporation apparatus
The volatile components of KRWs were isolated using a solvent-assisted flavour evaporation (SAFE) apparatus as suggested in previous studies 7, 18. Briefly, 100 mL of KRWs was poured into a distillation vessel in the SAFE apparatus. The volatiles were distilled at 45°C under reduced pressure (10−3 Pa) obtained using a rotary vacuum pump (ULVAC Technologies Inc., Methuen, MA, USA), and collected in a distillate flask cooled by liquid nitrogen. After distillation, the SAFE's distillate flask was thawed at room temperature and the distillate extracted with 50 mL dichloromethane from the aqueous solution (1.0 h). After extraction, the dichloromethane (lower layer) and aqueous solution (upper layer) were separated using a separatory funnel. The dichloromethane was concentrated to 1.0 mL with a Vigreux column after drying over anhydrous sodium sulphate. After concentration, the dichloromethane was removed using a nitrogen stream until the total volume was reduced to approximately 0.4 mL. The aqueous solution in the upper layer was poured into unextracted materials, which remained in the distillation vessel containing the non-volatile components, then concentrated to 1.0 mL using a rotary evaporator at 90°C (R-124, Buchi, Switzerland).
Fractionation of volatile and non-volatile components of Korean rice wines
In order to investigate the active ingredients responsible for the antioxidant properties of three KRWs, fractionations of the volatile and non-volatile components were performed in a similar manner, except for the column and solvent choices. Volatile components were fractionated using a glass column packed with silica gel, and pentane/ethyl acetate was used as the solvent mixture. For the non-volatile fractionation, a glass column packed with XAD-2 resin was selected and water/methanol was used as the solvent mixture. In addition, different GC-FID and GC-NPD were used for the volatile non-volatile fractions analyses, respectively.
The concentrated sample of the volatile fraction was placed in a glass column (15 × 1.0 cm) packed with 160- to 200-mesh silica gel and eluted sequentially with a 100 mL solvent mixture at different ratios of pentane–ethyl acetate: 100:0 (fraction i); 95:5 (fraction ii); 80:20 (fraction iii); 50:50 (fraction iv); 20:80 (fraction v); and 0:100 (fraction vi). In addition, 100 mL acetone (fraction vii) was used, as adopted from previous studies 19. Each volatile fraction was concentrated to 1.0 mL using a rotary evaporator and then concentrated to approximately 0.4 mL using a purified nitrogen stream.
The concentrated sample of the non-volatile fraction of the KRWs was placed in a glass column (15 × 1 cm) packed with 160- to 200-mesh XAD-2 resin and eluted sequentially with a 1000 mL solvent mixture of different ratios of water–methanol: 1000:0 (fraction i); 950:50 (fraction ii); 800:200 (fraction iii); 500:500 (fraction iv); 200:800 (fraction v); and 0:1000 (fraction vi) ; and 1000 mL acetone (fraction vii; Yanagimoto et al., 2004). Each fraction was concentrated to a final volume of 1.0 mL using a rotary evaporator.
Antioxidation test on fractions from volatile extractions using an aldehyde–carboxylic acid assay
For measuring the antioxidant activities of the volatile fractions in the KRWs, extractions i–vi of volatile fractions were evaluated using an aldehyde–carboxylic acid assay. With the aldehyde–carboxylic acid assay, the antioxidative activities of the samples are measured by their inhibitory effect on the conversion of aldehyde to carboxylic acids with active oxygen species, such as a hydroxyl radical 20. A sample of 100 µg/mL of volatile extract was added to a 2.0 mL dichloromethane solution of hexanal (3.0 mg/mL) containing 0.2 mg/mL undecane as a GC internal standard. The oxidation of the sample solution was initiated by heating at 60°C for 10 min in a sealed vial. Sample solutions were stored at room temperature. The headspace of each vial was purged with pure air (1.5 L/min, 3 s) every 24 h for the first 10 days. A decrease in hexanal was monitored at 10 day intervals. As a positive control, α-tocopherol (100 µg/mL) was also examined for its antioxidant activity.
The quantitative analysis of hexanal was conducted according to the internal standard method previously reported 7. A Hewlett-Packard (HP) model 6890 GC equipped with a 30 m × 0.32 mm i.d. (df = 0.25 µm) HP-1 bonded-phase fused-silica capillary column (Agilent Technologies, Wilmington, DE, USA) and an FID were used for the analysis. The linear velocity of the helium carrier gas was 30 cm/s at a split ratio of 20:1. The injector and the detector temperatures were 300 and 280°C, respectively. The oven temperature was programmed to be from 40 to 180°C at 4°C/min and held for 10 min.
Antioxidation test on fractions from non-volatile extractions using a lipid/malondialdehyde assay
The antioxidant activity of the non-volatile fraction of the KRWs was determined using a lipid–MA assay that measured MA formed from cod liver oil upon oxidation after derivatizing to 1-methylparazine (MP) with NMH 21. An aqueous solution (5.0 mL) containing 500 μL tested sample, 30 μL cod liver oil, 0.25 mmol Trizma buffer (pH 7.4), 5 mmol ferrous chloride, 10 mmol hydrogen peroxide, 0.75 mmol potassium chloride and 0.2% surfactant SDS was incubated with various amounts of the aroma extracts for 17 h at 37°C in a 20 mL test tube. Sample oxidation was stopped by adding 50 μL of a 4% BHT solution. A known antioxidant, α-tocopherol, was used to compare antioxidant activity to that of the non-volatile extracts tested. NMH (30 μL) was added to the oxidized cod liver oil solutions, and the solutions were stirred for 1.0 h at room temperature. The extract was adjusted to exactly 10 mL by adding ethyl acetate and 20 μL 2-methylpyrazine solution as a GC internal standard. The solution was analysed for MP by GC with NPD. An HP model 6890 GC equipped with a 30 m × 0.32 mm i.d. (df = 0.25 µm) DB-WAX bonded-phase fused-silica capillary column (J&W Scientific, Folsom, CA, USA) and an NPD were used for analysis of MP.
Analysis of volatile compounds in the fractions from the dichloromethane extracts

To determine the total aroma compounds in the extracts, an HP model 6890 GC equipped with a 60 m × 0.32 mm i.d. (df = 0.25 µm) DB-WAX bonded-phase fused-silica capillary column (J&W Scientific, Folsom, CA, USA) and an FID were used. HP model 6890 GC interfaced to a 5975A mass selective detector (GC/MS) was used for mass spectral identification of the GC components at an MS ionization voltage of 70 eV, along with a 60 m × 0.25 mm i.d. (df = 0.25 µm) DB-WAX bonded-phase fused-silica capillary column (J & W Scientific, Folsom, CA, USA) . The linear velocity of the helium carrier gas was 30 cm/s at a split ratio of 20:1. The injector and detector temperatures were 250°C. The oven temperature was programmed from 50°C (2°C/min isothermal) to 200°C at 3°C/min and held for 10 min.
Analysis of antioxidant activities of selected chemicals found in the volatile fractions of three traditionally brewed Korean rice wines
Different concentrations (0, 10, 20, 50 and 100 µg/mL) of selected volatile compounds were added to a 2 mL dichloromethane solution of hexanal (3 mg/mL). The antioxidant activities of each authentic chemical compound were monitored by quantitative analysis of hexanal, as described in the aldehyde–carboxylic acid assay method earlier, with undecane as a GC internal standard. The following seven volatile compounds were selected for further analysis: 3-furaldehyde from fraction ii of KHJ; benzeneethanol from fraction iii of GGJ, fraction iii of MSJ and fraction ii of KHJ; ethylbenzene from fraction v of KHJ and fraction vi of MSJ; 2-furancarboxaldehyde from fraction ii of GGJ; 2(3H)-furanone from fraction iii of GGJ and fraction iii of MSJ; 2-furanmethanol from fraction ii of GGJ; and 4-ethyl-2-methoxyphenol from fraction ii of MSJ. These compounds were selected based on the structures of the chemical compounds as suggested by several previous studies; radical scavenging activities and antioxidant activities of these phenolic compounds and furan derivatives have been reported 7, 19, 23, 24.
Results and discussion
Antioxidant activities (%) of volatile and non-volatile fractions of three traditionally brewed Korean rice wines
The antioxidant activities of the volatile and non-volatile fractions were determined differently. From previous studies, the antioxidant activities of MSJ, GGJ and KHJ, as determined by lipid–MA assay, were 42, 43 and 42%, respectively, while the antioxidant activity of α-tocopherol (300 µg/mL) was 93% 7. To further investigate the non-volatile compounds showing antioxidant properties, antioxidant activity (%) of non-volatile fractions in an aqueous solution of KRWs was measured using a lipid–MA assay. The antioxidant activities of the non-volatile fractions are shown in Fig. 1. Fraction iv of KHJ showed the highest inhibition activity (69%). The inhibition activity of fraction v of MSJ was 46%. In fraction i, GGJ showed the highest antioxidant activity (65%), while the antioxidant activity of α-tocopherol (300 µg/mL) was 93%.
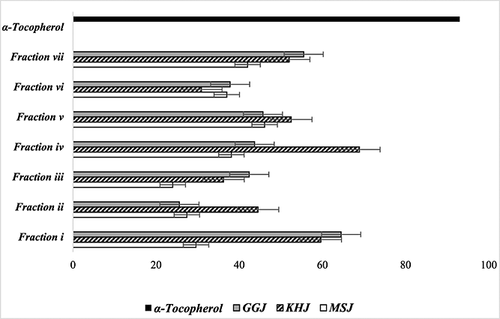
For the volatile fraction analysis, the overall antioxidant activities of MSJ, GGJ and KHJ were 90, 97 and 99%, respectively, in previous studies 7. This was expected in that the antioxidant activity of P. mume, L. chinense (Mill.) and D. indicum, raw materials of MSJ, GGJ and KHJ, have been well documented 6, 9, 10, 12, 13. Limited studies exist on antioxidant activity related to each material when used as an ingredient in KRWs. The current study further analysed the detailed antioxidant potentials of volatile extracts from the KRWs. Figure 2 represents antioxidant activity (%) of seven volatile fractions of KRWs measured by the aldehyde–carboxylic acid assay. The antioxidant activities of fractions i, iv, v and vii of KHJ were 78, 92, 87 and 97%, respectively. The antioxidant activities of fractions iv and vii of KHJ were higher than that of α-tocopherol. The antioxidant activities of fractions ii and iv of MSJ were 40 and 38%, respectively. The antioxidant activities of fractions i, iii and iv of GGJ were 81, 50 and 72%, respectively. Previous studies reported that ethyl acetate–butanol fractions from the extract of various Compositae plants showed about 90% antioxidant activity and were more polar than other fractions. The difference in antioxidant activities found in the studies amongst the fractions can be explained by the fact that the plants contain either polar or non-polar components. Strong antioxidant activity of fractions iv and vii of KHJ may have been derived from antioxidant properties of polar volatile compounds, such as 1,4-dimethylbenzene, 2-H pyran, ethylbenzene, thiocyanic acid, propanoic acid, 1,2-dimethylhydrazine and 3-pyrrolidinol, during the KRW fermentation process. In contrast, volatile extracts of GGJ showed strong antioxidant activity in fraction i (77%), indicating that GGJ had more non-polar compounds than other KRWs. GGJ is made with L. chinense (Mill.), which has higher concentrations of betaine, rutin, kukoamine A and β-sitosterol. These are known for their antioxidant, antimicrobial, anticancer and immune-modulatory properties 25.
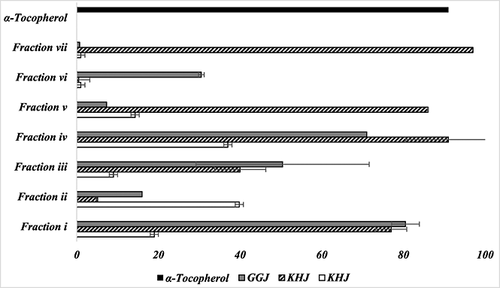
Although one cannot directly compare the antioxidant activities of the volatile and non-volatile fractions, the antioxidant activities determined from each of the volatile fractions were further investigated to focus on the identification of the key bioactive compounds responsible for the antioxidant properties of three KRWs. The determination of non-volatile compounds with antioxidant properties from KRWs is currently underway.
Major volatile compounds with relatively high antioxidant activities in the three selected Korean rice wines
Volatile compounds identified in each fraction of MSJ are listed in Table 1. In fractions ii and iv of MSJ, the antioxidant activities were 40 and 37%, respectively (Fig. 1). In fraction ii, 3-methybutanol was dominant (highest concentration; 34.3 ppm), followed by isobutyl alcohol, hexanoic acid, 3-difluromethylpyridine, ethyl 2-hydroxycaproate, octanoic acid and 4-ethyl-2-methoxyphenol. The major compounds of fraction iv were hexadecanoic acid (47.22 ppm) and butan-4-olide (9.54 ppm). The compound 3-methylbutanol (which was extracted using solvent mixtures of isopropanol and water at different ratios) is a major component of green coffee beans and beer 19, 26.
| Peak no. | Compounds | Kovats indexa | Concentration (ppm)b |
|---|---|---|---|
| Fraction i | |||
| 1 | 1,4-Dimethyl-undecane | 1320 | 0.01 |
| 2 | Docosane | 1521 | 0.09 |
| 3 | 1,2-Dithiacyclopentane | 1539 | 0.01 |
| Fraction ii | |||
| 4 | Isobutyl alcohol | 1048 | 2.86 |
| 5 | 3-Methylbutanol | 1219 | 34.3 |
| 6 | Ethyl hydroxycaproate | 1561 | 0.15 |
| 7 | 3-(Difluromethyl)pyridine | 1719 | 0.19 |
| 8 | Hexanoic acid | 2157 | 0.27 |
| 9 | 4-Ethyl-2-methoxyphenol | 2348 | 0.12 |
| 10 | Octanoic acid | 2372 | 0.14 |
| Fraction iii | |||
| 11 | 2(3H)-Furanone | 1671 | 1.48 |
| 12 | Pentanoic acid | 1715 | 0.26 |
| 13 | Methanol | 1864 | 0.46 |
| 14 | Benzeneethanol | 2131 | 7.32 |
| 15 | Ethyl 4-hydroxybutanoate | 2150 | 2.72 |
| Fraction iv | |||
| 16 | Butan-4-olide | 1672 | 9.54 |
| 17 | Hexadecanoic acid | 2483 | 47.22 |
| Fraction v | |||
| 18 | Acetic acid | 1048 | 2.53 |
| 19 | 2-Ethoxy-ethanol | 1312 | 1.32 |
| 20 | 2-Butoxy-ethanol | 1418 | 2.51 |
| 21 | Butanoic acid | 1652 | 0.46 |
| 22 | 2-Butenoic acid | 2022 | 1.05 |
| 23 | 1,2-Benzenedicarboxylic acid | 2482 | 20.6 |
| Fraction vi | |||
| 24 | Ethylbenzene | 1135 | 0.2 |
| 25 | Octadecanoic acid | 2481 | 27.49 |
| Fraction vii | |||
| 26 | 3-Hexen-2-one | 1144 | 0.06 |
| 27 | 2-Pentanone | 1386 | 127.15 |
| 28 | 3-Hexanol | 1543 | 0.24 |
| 29 | Propanoic acid | 1552 | 0.11 |
| 30 | 1-Butanamine | 1560 | 3.87 |
- a Kovats retention index on DB-WAX.
- b Solvent peak excluded.
The volatile components identified in the extracts of KHJ are listed in Table 2. In fractions i, iv, v and vii of KHJ, the antioxidant activities were 78, 91, 87 and 97%, respectively (Fig. 1). Fraction vii of KHJ showed the highest antioxidant activity (97%), containing 4-hydroxy-4-methyl-2-pentanone and 2-pentene as the major volatile compounds. In fraction iv, trans-4-hydroxymethyl-2-methyl-1,3-ethane, 1,4-dimethylbenzene and 2H-pyran were identified. In fraction v, 3-pyrrolidinol had the highest concentration; in fraction i, undecane had the highest concentration. Undecane, a major component of the aroma extracts in Tagetes erecta (L.). in Compositae plants, has also shown strong antioxidant activity 27.
| Peak no. | Compounds | Kovats indexa | Concentration (ppm)b |
|---|---|---|---|
| Fraction i | |||
| 1 | Undecane | 1250 | 0.23 |
| Fraction ii | |||
| 2 | Isoamyl alcohol | 1221 | 32.74 |
| 3 | 3-Furaldehyde | 1480 | 0.31 |
| 4 | 2-Methyl-4-octanol | 1561 | 0.16 |
| 5 | Pentanoic acid | 1720 | 0.35 |
| 6 | Hexanoic acid | 2158 | 0.31 |
| 7 | Benzeneethanol | 2233 | 15.54 |
| Fraction iii | |||
| 8 | Methanol | 2137 | 22.03 |
| 9 | Ethyl 4-hydroxybutanoate | 2234 | 18.35 |
| Fraction iv | |||
| 10 | 1,4-Dimethylbenzene | 1150 | 1.32 |
| 11 | Trans-4-hydroxymethyl-2-methyl-1,3-ethane | 1664 | 1.39 |
| 12 | 2H-Pyran | 1669 | 0.05 |
| Fraction v | |||
| 13 | Ethylbenzene | 1152 | 0.19 |
| 14 | Thiocyanic acid | 1313 | 0.82 |
| 15 | Propanoic acid | 1421 | 1.54 |
| 16 | 1,2-Dimethylhydrazine | 1464 | 0.25 |
| 17 | 3-Pyrrolidinol | 2377 | 10.31 |
| Fraction vi | |||
| 18 | Butyl acetate | 1050 | 0.77 |
| 19 | 2-Ethoxyethanol | 1313 | 0.66 |
| 20 | 2-Butoxyethanol | 1421 | 1.29 |
| Fraction vii | |||
| 21 | 2-Pentene | 1144 | 0.06 |
| 22 | 4-Hydroxy-4-methyl-2-pentanone | 1386 | 127.15 |
- a Kovats retention index on DB-WAX.
- b Solvent peak excluded.
Volatile components identified in the extracts of GGJ are listed in Table 3. In fractions i, iii and iv, the antioxidant activities were 80, 50 and 70%, respectively (Fig. 1). In fraction i of GGJ, benzene derivatives, such as 1, 4-dimethylbenzene and 1, 3-dimethylbenzene, were the major volatile compounds. In fraction iii, benzeneethanol, 2(3H)-furanone, 2-furanmethanol, isobutyl alcohol, 3-methyl-1-butanol, 1,2-dimethylbenzene and 1-(1H-pyrrol-2-yl)-ethanone were identified. In fraction iv, α-ketoglutaric acid, butanoic acid and ethyl 4-hydroxybutanoate were identified.
| Peak no. | Compounds | Kovats indexa | Concentration (ppm)b |
|---|---|---|---|
| Fraction i | |||
| 1 | 1,4-Dimethylbenzene | 1152 | 0.12 |
| 2 | 1,3-Dimethylbenzene | 1195 | 0.07 |
| Fraction ii | |||
| 4 | 2-Methyl-1-propanol | 1050 | 1.07 |
| 5 | 1-Pentanol | 1219 | 17.37 |
| 6 | 2-Furancarboxaldehyde | 1483 | 0.06 |
| 7 | 2,6-Dimethyl-4-heptanol | 1564 | 0.16 |
| 8 | Butanedioic acid | 1804 | 0.64 |
| 9 | 4-Propylbutan-4-olide | 2374 | 6.96 |
| Fraction iii | |||
| 11 | Isobutyl alcohol | 1035 | 1.61 |
| 12 | 1,2-Dimethylbenzene | 1085 | 0.11 |
| 13 | 3-Methyl-1-butanol | 1156 | 0.17 |
| 14 | 2(3H)-Furanone | 1686 | 4.96 |
| 15 | 2-Furanmethanol | 1732 | 1.85 |
| 16 | Benzeneethanol | 2238 | 30.95 |
| 17 | 1-(1H-Pyrrol-2-yl)-ethanone | 2282 | 0.09 |
| Fraction iv | |||
| 18 | Butanoic acid | 1686 | 4.46 |
| 19 | Ethyl 4-hydroxybutanoate | 2134 | 0.78 |
| 20 | α-Ketoglutaric acid | 2373 | 4.49 |
| Fraction v | |||
| 21 | Acetic acid | 1057 | 0.56 |
| Fraction vi | |||
| 22 | Butyl acetate | 1050 | 0.99 |
| 23 | 2-Ethoxyethanol | 1313 | 0.75 |
| 24 | 2-Butoxyethanol | 1421 | 1.44 |
| Fraction vii | |||
| 25 | 3-Methyl-2-pentanone | 1151 | 1.64 |
| 26 | 2,4-Dimethyl-2-pentanol | 1388 | 52.88 |
| 27 | Propanoic acid | 1566 | 0.94 |
- a Kovats retention index on DB-WAX.
- b Solvent peak excluded.
Confirmation of antioxidant properties from selected authentic chemicals identified from three traditionally brewed Korean rice wines
From the identified volatile compounds with potentially high antioxidant activities, the current study further investigated the antioxidant properties of selected authentic chemicals. Different concentrations of each selected chemical compound were added to a hexanal solution, and an aldehyde–carboxylic acid assay was performed. The results are listed in Table 4.
| Compounds | Inhibitory effect (%) | ||||
|---|---|---|---|---|---|
| 0 µg/mL | 10 µg/mL | 20 µg/mL | 50 µg/mL | 100 µg/mL | |
| 3-Furaldehyde | 6 ± 2.7 | 10 ± 2.9 | 14 ± 9.0 | 23 ± 6.9 | 55 ± 4.9 |
| Benzeneethanol | 5 ± 0.4 | 32 ± 5.4 | 43 ± 2.9 | 59 ± 9.9 | 73 ± 7.8 |
| Ethylbenzene | 2 ± 1.6 | 4 ± 3.8 | 9 ± 3.5 | 8 ± 3.7 | 10 ± 2.1 |
| 2-Furancarboxaldehyde | 3 ± 1.6 | 15 ± 5.8 | 23 ± 9.4 | 34 ± 3.9 | 45 ± 6.8 |
| 2(3H)-Furanone | 5 ± 0.4 | 4 ±4.8 | 4 ± 2.5 | 8 ± 2.7 | 15 ± 3.9 |
| 2-Furanmethanol | 4 ± 0.7 | 15 ± 2.9 | 23 ± 9.3 | 34 ± 3.9 | 61 ± 4.0 |
| 4-Ethyl-2-methoxyphenol | 5 ± 5.2 | 19 ± 8.3 | 25 ± 3.9 | 45 ± 3.8 | 70 ± 2.9 |
At 100 µg/mL, benzeneethanol displayed the highest antioxidant activity (73%). Benzeneethanol was detected at 7.32, 15.54 and 30.95 µg/g in MSJ (fraction iii), KHJ (fraction ii) and GGJ (fraction iii), respectively. 4-Ethyl-2-methoxyphenol and 2-furanmethanol at 100 µg/mL showed relatively higher antioxidant activities than the other chemicals (71 and 61%, respectively). The compound 4-ethyl-2-methoxyphenol was identified in the MSJ (fraction ii) at 0.12 µg/g. The compound 2-furanmethanol was quantified in fraction iii of GGJ at 1.85 µg/g and was previously reported to have antioxidant properties when extracted from beer 26; however, this same study reported that benzeneethanol did not display antioxidative activity, which was not in agreement with the current study. Overall, the antioxidant activities of the selected chemical compounds were not as high as expected in concentrations up to 50 µg/g; however, three compounds (benzeneethanol, 2-furanmethanol and 4-ethyl-2-methoxyphenol) displayed significantly higher antioxidant activities when added at higher concentrations (~100 µg/g).
Conclusions
The current study demonstrated the antioxidant properties of seven fractions of each of three traditionally brewed Korean rice wines (Measilju, Kukwhaju, and Gugijaju). The degree of antioxidant activity was different among the volatile and non-volatile fractions. The volatile fractions of the wines displayed high antioxidant activity, and among the volatile compounds identified from the volatile fractions, benzeneethanol, 2-furanmethanol and 4-ethyl-2methoxyphenol displayed the highest antioxidant activities, when added at higher concentrations (~100 µg/g).
Acknowledgements
This study was supported by a grant (106018–2) of the IPET and R&D Convergence Centre Support Program of the Ministry for Agriculture, Food, and Rural Affairs, Republic of Korea.




