Effects of magnesium ions on both VHG batch and continuous fruit wine fermentations
Abstract
In Poland, fruit wines or aromatized fruit wines are semi-sweet or sweet and contain approximately 15–16% (v/v) ethanol. Their production can be classified as a very high-gravity (VHG) fermentation. Magnesium has a beneficial effect on VHG fermentations as it protects the yeast cells against ethanol, osmotic and temperature stress. The effect of the magnesium concentration in an apple must, containing 32% sugars, on the fermentative parameters of batch and continuous fermentations was assessed. In the batch process, a magnesium concentration of ~8.5 mg/L resulted in decreased the ethanol production in comparison to a magnesium concentration of ~250, 490 and 970 mg Mg2+/L. The highest amount of Mg also caused a metallic taste. A continuous fermentation was carried out for 2.5 months in a four-column packed-bed fermentor. The medium contained ~50, 250 and 490 mg Mg2+/L and the yeast was immobilized on foam glass. During the continuous fermentation, no differences at p ≤ 0.05 in terms of fermentative parameters were seen with magnesium additions. The same beginning amount of magnesium ions in the medium led to a similar use of this element, both in batch and in continuous fermentation. The more Mg2+ that was present in the medium, the more Mg2+ was used by the yeast. The results suggest that the minimal dose of magnesium, under the described conditions, is 50 mg/L, corresponding to the amount of Mg in the medium prepared using concentrated apple juice and tap water. This finding has industrial significance, as Polish wine companies prepare their fruit musts using tap water. Copyright © 2014 The Institute of Brewing & Distilling
Introduction
Wine is generally obtained via the fermentation of grape juice or grapes. In Poland, there is very little historical tradition of grape wine production because environmental conditions are not favourable for viticulture. Therefore, other oenological products are obtained, for instance fruit wines, aromatized fruit wines, wine-like drinks or aromatized wine-like drinks. These beverages are classified, according to Polish law, as fermented wine products. In recent years the annual production of these beverages has been in the range of 80–90 million litres. The above-mentioned products are obtained by the alcoholic fermentation of juices or fruit musts containing juice, sugar and water. Aromatized beverages are produced from fruit wines by flavouring them with aromatic substances. During the production of aromatized drinks, commonly used technological operations include the following: sweetening, staining with colour additives and fortifying via the addition of a distillate. The alcohol concentration in fermented wine products is in the range 9–18% v/v. Furthermore, these beverages are semi-sweet (30–60 g sugars/L) or sweet (above 60 g/L). The desired strength is obtained by sweetening the juices or musts either before or during fermentation. The addition of sugar, as well as ethanol, to the fermented wine products can also be performed after the completion of the fermentation and this is permitted by Polish law.
Any alcohol that has been added in the form of spirits is more expensive than alcohol obtained by fermentation. Moreover, the addition of sucrose after the fermentation requires additional equipment in industrial settings – a stirred tank. Therefore, an economically efficient solution is a fermentation process that allows a product to be obtained with a high ethanol and sugar content. The industrial production of fruit wine containing ~16% v/v ethanol and 40 g of residual sugar per litre requires ~320 g per litre of sugar, because the efficiency is more often 90% of the theoretical value. Such a high level of sugar in the medium is defined as very high gravity (VHG) brewing technology 1-3. High-gravity or VHG fermentation is a widely used technology in both beer 4-7 and fuel ethanol production 2, 3, 8-11.
The process of continuous fermentation with immobilized cells can also be economically beneficial. The advantages of this system compared with free cell batch fermentation are substantial improvements in the efficiency of the process, an increase in ethanol productivity and lower operating costs 12-15. It should be noted that, during the continuous fermentation of high-gravity medium, cells are under constant osmotic and ethanol stress, which can lead to a negative impact on yeast growth, viability and fermentation performance.
Some studies have focused on finding agents to protect yeast cells under stressful environments. Walker 16 and Birch and Walker 17 have established that magnesium is a stress-protectant factor in the case of wine, brewing and distilling yeasts and can help to prevent cell death caused by temperature shock and ethanol toxicity. Walker et al. 18 showed a positive effect on ethanol production by yeasts by supplementing the medium with Mg2+. Rees and Stewart 6, 7 also found beneficial effects of magnesium on yeast fermentation performance in normal and high-gravity worts. In other research, magnesium ions have been found to influence the yeast's ability to undergo a dehydration–rehydration process 19. In the above-mentioned papers, the doses of magnesium were very different, ranging between ~20 and 1500 mg/L. Magnesium ions play a fundamental role in the growth and metabolism of yeast cells. Magnesium ions are required as a cofactor by over 300 enzymes, including those essential for the activity of glycolytic, alcohologenic and fatty acid biosynthesis. They stabilize biological membranes and are important for nucleic acids, ribosomes, polysaccharides and lipids 6, 16, 18.
The available literature is lacking in information about the impact of magnesium on long-term high-gravity continuous production of fruit wines. Thus the effects of Mg2+ ions on the process of batch and on a 2.5 month continuous fermentation of a medium containing 32% sugars were investigated. In the continuous process, the yeasts cells were immobilized on foam glass. All fermentations were carried out using apple must. Apple juice or concentrated apple juice is the most popular raw material used in the Polish fruit wine industry.
Material and Methods
Yeast
In this study, an alcohol- and sugar-tolerant pure strain of Saccharomyces bayanus S.o./1 AD, from the culture collection of the Department of Food Biotechnology and Microbiology, Warsaw University of Life Sciences, was used. This particular yeast strain was a 5-year adaptation from our department from the original S. bayanus S.o.1 strain and is able to perform VHG fermentations 20. The stock culture was maintained on malt agar slants and stored at 4 °C.
Inoculum preparation
Before fermentation, the yeasts cells were acclimated to the fermentation parameters by four passages on media with increasing concentrations of sugar and SO2. At the beginning, the cells from a slant were transferred to a 500 mL flask containing 80 mL of sterile medium with a composition of 70% apple juice, 20% sucrose, 20 mg/L SO2, 0.3 g/L (NH4)2HPO4 and 0.2 g/L (NH4)2SO4, and maintained under aerobic conditions on a 200 rpm reciprocating shaker (Büchler SM-30 Control, Germany) at 28 °C for 48 h. Then, 10% of the inoculum was transferred to a second medium, containing a higher amount of sugar and SO2 – 24% and 40 mg/L, respectively. After growth (under the same conditions as in the first medium), 10% of the inoculum was placed in a third medium (28% sugar and 60 mg/L SO2) and it was incubated as previously described. The last propagation was performed in medium with the highest sugar and SO2 concentration – 32% and 80 mg/L. All of the media were sterilized at 117 °C for 10 min. After the sterilization, SO2 in the form of K2S2O5 was added.
Fruit must
The must was prepared from concentrated apple juice (sugar content 70°Brix) received from the Warwin Co., one of the largest producers of juice concentrates in Poland. This concentrated juice was diluted with deionized water (except for the control in the continuous fermentation, where tap water was used) to obtain an apple juice of 10°Brix. The juice content in the must was 70%. The minimal volume of apple juice in the must, according to Polish law, must be 60%. Sugar (in the form of sucrose) was added to obtain a total concentration of sugars of ~320 g/L. Apple juices contain a low concentration of nitrogenous compounds and therefore the must was enriched with ammonium salts [0.3 g/L (NH4)2HPO4 and 0.2 g/L (NH4)2SO4]. Magnesium was added in the form of MgSO4 · 7H2O, in the following quantities: 240, 480 and 960 mg Mg2+/L (batch fermentations) and 240 and 480 mg Mg2+/L (continuous fermentations). Total concentrations of Mg in the fruit medium prepared using tap water amounted to ~50 mg/L. The apple musts were used in the fermentations without any sterilization. In order to prevent wine spoilage, SO2 in the form of K2S2O5 was added in appropriate amounts to obtain a final total SO2 concentration of about 80 mg/L.
Batch fermentations
Batch fermentations were carried out in 2 L glass flasks filled 80% with apple must. After growth in the last medium (32% sugar and 80 mg/L SO2), the yeast inoculum was added in the amount of 6% of the total volume of the must. Fermentations were conducted for 35 days, at 22 ± 1 °C. During this time, the fermentation kinetics were monitored via measurement of the apparent extract level. Three series of fermentations (in duplicate) were conducted. After the fermentation, the young apple wines were subjected to analysis. All samples were tested in triplicate.
Continuous fermentation
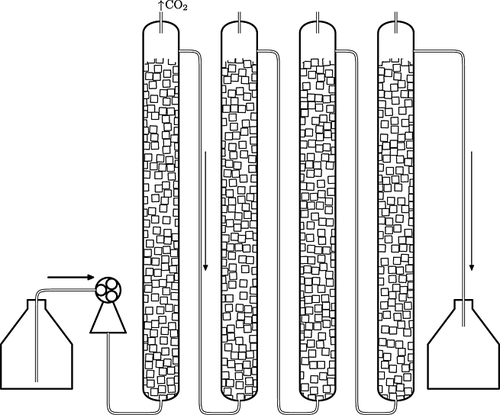
Continuous fermentation (two series) was performed using a four-column packed-bed bioreactor (Fig. 1). Each column (70 cm high and 5.5 cm internal diameter) was filled with 150 g of foam glass cubes. Before the experiment, the carrier was washed twice with 3% citric acid and deionized water to eliminate diffusion of calcium and magnesium from the foam glass to the medium. The yeast inoculum, after growth in medium containing 32% sugar and 80 mg/L SO2, was added every 2 days to each column of the bioreactor (beginning with the last one). This interval was necessary to allow the yeast to become immobilized on the support. After filling the first column, the flow was started. Apple must was fed into the bioreactor at a dilution rate of 0.022 L/h using a peristaltic pump (Zalimp PP1B-05, Poland) and this rate was the same throughout this stage. The system was operated continuously for 2.5 months at 22 ± 1 °C. During the operation of the fermentor, young fruit wine was sampled for analysis.
Analytical determinations
Enological parameters (ethanol, total and reducing sugar content, titratable acidity, volatile acidity, dry extract, sugar free extract and free and total SO2) were determined according to validated standard methods 21. Because of sucrose addition to the must, in the case of total sugars, hydrolysis was performed before the analysis according to the method described in the literature 22. All samples were analysed in triplicate. The magnesium content in the wines was determined using an atomic absorption spectrometer (Shimadzu AA-600) at λ = 285.2 nm, after the mineralization of wine samples in a mixture of nitric acid and perchloric acid (Büchi Digestion Unit K-435).
Fermentation parameters
To evaluate fermentation performance, the following fermentative parameters were computed: efficiency, yield, sugar conversion and alcohol productivity. These parameters were calculated using the equations described by Liang et al. 15. Delle units were evaluated according to the following formula: DU = s + 4.5a, where s is the percentage of sugars (g/100 mL) and a is the volume percentage of alcohol (mL ethyl alcohol/100 mL). Delle units are a measure of the inhibitory activity of the mixture of sugars and ethanol on yeast cell metabolism 23.
Number of yeast cells
The total number of cells was determined by direct microscopic count using a hemocytometer. In the case of continuous fermentation, when the fermentor was stopped, two cubes of the carrier from three levels of each column (top, middle, bottom) were placed in Erlenmeyer flasks with 100 mL of sterile 0.8% NaCl solution. The flasks were shaken at 200 rpm at 28 °C for 2 h to remove the yeast from the foam glass. The number of cells was calculated as a mean for three levels and expressed as the amount present on 1 g of the carrier.
Carrier characteristics
The carrier structure, before and after the continuous fermentation, was observed using an environmental scanning electron microscope ESEM (FEI Quanta 200 ESEM) in a low-pressure gaseous environment, without any prior preparation. In order to determine the immobilization capacity of the yeast cells to the foam glass, a few cubes of foam glass were placed in Erlenmeyer flasks with 0.8% sterile solution of NaCl after the continuous process and were shaken at 200 rpm at 28 °C for 2 h to remove yeast cells from the carrier. Then, the foam glass was washed and dried. Density was measured using a volume presser (J. Engelsmann A.G., Germany). Particle density was measured using a gas stereopycnometer (Quantachrome Instruments, USA). A sample was placed in the sample cell and degassed by purging with a flow of dry gas (helium) over a series of pressurization cycles. Carrier porosity was calculated according to the relationship between the density and the particle density.
Statistical analysis
All analytical parameters determined were submitted to a statistical analysis using the Statgraphics Plus 5.1 program. An analysis of variance (Tukey's or Fisher's test) and a comparison of samples were carried out at a significance level of α = 0.05.
Results and discussion
Batch fermentation
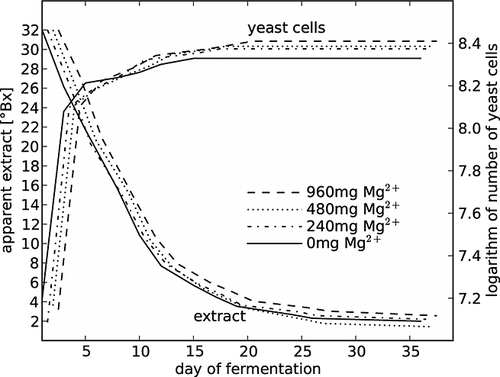
In the first part of this experiment, the effect of addition of 240, 480 and 960 mg Mg2+/L to the medium during batch fermentations was evaluated. The total level of magnesium ions was ~10 mg/L higher, because the basal medium contained ~10 mg/L of this element. These concentrations were used since other publications focusing on magnesium as a stress protectant factor used similar amounts of these elements for their studies [Rees and Stewart 6, 7, 500 mg/L; Walker 16, 480 mg/L; Birch and Walker 17, 240, 480 and 1200 mg/L]. As shown in Fig. 2, during fermentation, the decrease in the apparent extract level was statistically significant until day 17, then the density decreased more slowly until day 24. The number of yeast cells increased substantially for the first three days of the fermentation and then remained constant. Magnesium addition however did not cause any statistical differences, either in °Brix density or in the number of yeast cells. Otherwise, the ethanol concentration in all of the apple wines obtained during fermentation of the medium containing magnesium was about 17% v/v and this was 1% v/v higher than in the control process, when apple must prepared with deionized water was used. The effect on the fermentative parameters of the addition of Mg2+ to the must is presented in Table 1 and that on the enological parameters of apple wines is given in Table 2.
| Mg addition | Ethanol (% v/v) | Total sugars (g/L) | Sugar conversion (%) | Utilized sugars (g/L) | Efficiency (%) | Yield (g ethanol/g glucose) | Delle units (DU) |
|---|---|---|---|---|---|---|---|
| 960 mg | 17.0b A | 46.2 A | 85.4 A | 269.3 A | 97.2 A | 0.497 A | 81.3 A |
| 480 mg | 17.1 A | 37.8 B | 88.0 B | 277.7 B | 94.7 AB | 0.484 AB | 80.6 B |
| 240 mg | 16.9 A | 43.5 AB | 86.3 AB | 273.5 AB | 95.5 AB | 0.487 AB | 80.4 B |
| Control | 16.1 B | 50.5 A | 84.1 A | 266.7 A | 93.3 B | 0.476 B | 77.5 C |
| LSDa | 0.18 | 7.34 | 2.33 | 7.19 | 3.08 | 0.016 | 0.53 |
- a LSD, Fisher's least significant difference.
- b Means followed by different letters (A–C) within the same column are significantly different at p ≤ 0.05.
| Magnesium addition | Sucrose (g/L) | Dry extract (g/L) | Sugar-free extract (g/L) | Total acidity (g/L) | Volatile acidity (g/L) | SO2 free (mg/L) | SO2 total (mg/L) |
|---|---|---|---|---|---|---|---|
| 960 mg | 3.5a A | 83.6 A | 37.5 A | 5.1 A | 0.40 A | 18.9 A | 58.2 A |
| 480 mg | 3.3 A | 76.2 A | 38.6 A | 5.2 A | 0.35 B | 13.1 B | 50.1 B |
| 240 mg | 3.7 A | 80.1 A | 36.7 A | 5.1 A | 0.34 B | 14.8 C | 50.4 B |
| Control | 7.2 B | 84.3 A | 34.1 A | 5.1 A | 0.31 B | 14.9 C | 56.1 A |
| LSD | 1.31 | 11.53 | 8.86 | 0.13 | 0.05 | 1.02 | 4.38 |
- a Means followed by different letters (A–C) within the same column are significantly different at p ≤ 0.05.
Continuous fermentation
In the second part of the study, a 2.5 month continuous fermentation was performed. In the batch process, when the magnesium supplement amounted to 960 mg/L, the highest efficiency was found. However, this young wine was characterized by a poor sensory quality and had a metallic taste. Therefore, in the continuous process, a medium with added Mg2+ ions in the amount of 240 and 480 mg/L and as a control fermentation a must containing ~50 mg/ L magnesium (prepared from tap water) were used. The apple must was pumped into the four-column bioreactor (Fig. 1) at a flow rate of 500 mL/day; thus, the time of total flow through the fermentor was 5.5 days and it was equivalent to the time of the apple wine fermentation.
In the continuous process, yeast cells immobilized on foam glass were used. The white foam glass is an inorganic material made from powdered glass with the addition of foaming agents, mainly CaCO3. The ingredients are heated and annealed in a high-temperature tunnel furnace. During the process, gases produced from foaming substances cause the formation of numerous pores. This material has been reported to be a good carrier for yeast immobilization 22, 24.
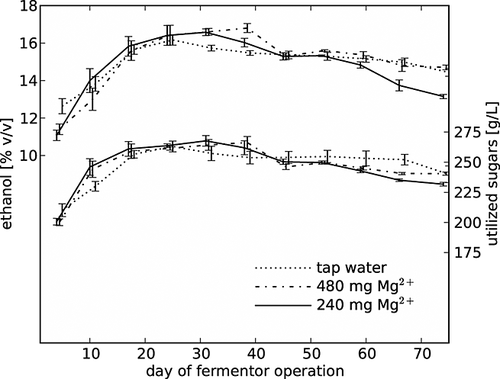
Figure 3 presents the effect of magnesium on ethanol concentrations in apple wines and on the amount of sugars utilized during 74 days of fermentor operation. Regardless of the magnesium content, the level of alcohol and sugar utilization increased for about 2 weeks and then remained at a steady state; finally, a slow decrease was observed. However, on day 74 of the continuous process, the alcohol strength of young apple wines was still ~14% v/v. The continuous fermentation resulted in an apple fruit wine with an average (calculated for all days of the fermentor operation) ethanol content of 15% v/v to be produced over a 5.5 day period. These results confirm the findings of others 12, 13, 25, 26, that is, that the continuous process is faster than batch fermentation. Bayrock and Ingledew 2 used a multistage continuous culture fermentation with the same sugar concentration as in the current described work. In the last (fifth) fermentor, a maximum ethanol amount of 16.73% v/v was achieved, but in contrast to this study, the fermentation was performed at 100 rpm agitation. According to Breisha 8, the addition of air during the first 12 h of fermentation led to the complete consumption of 35% sucrose and yielded 16% v/v ethanol production. Bai et al. 1 also found that the oxygen supply resulted in high yeast cell growth. They obtained the highest and average ethanol concentration, respectively 17% v/v and 15.8% v/v, in 2 months of continuous fermentation of a medium containing 280 g/L glucose.
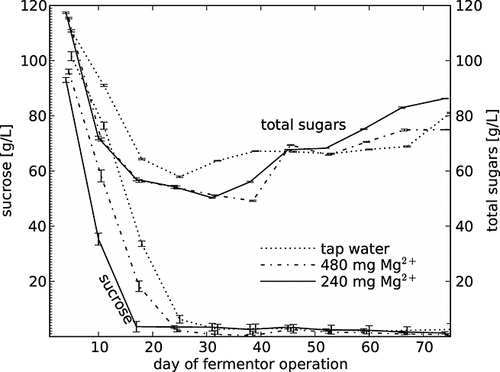
The curves of efficiency and sugar conversion (Fig. 4) demonstrate similar progress and also resemble those of ethanol. Sugar conversion was in the range 63–84%, depending on the time of fermentor operation. Many researchers have reported almost complete sugar conversion in continuous fermentation 9, 12, 15, 26. However, in the current study a higher sugar concentration in comparison to the cited authors was used, since the purpose of the process was to produce a sweet apple wine. This type of product is readily purchased in Poland. The total sugar concentration in the resultant apple wines was between ~50 and 115 g/L (Fig. 5) and with lower ethanol content. In other words, when more sugar was present in the final apple wine, less ethanol was present in the final product – according to the chemical conversion of sugars into ethanol. The level of sucrose decreased for the first 2–3 weeks of the operation of the fermentor and afterwards the content of this sugar was minimal, fluctuating between 0.5 and 3.5 g/L, although the concentration of total sugars was significantly higher. The yeast cells hydrolysed the disaccharide sucrose over the 5.5 days of flow through the bioreactor, although they were not able to convert all of the glucose and fructose to ethanol. The hydrolysis rate was much faster than the ethanol fermentation rate. It should be noted that the level of sucrose was the same during the time of continuous fermentation, ~245 g/L. Sucrose is hydrolysed by invertase, encoded in the Saccharomyces cerevisiae yeast by a family of genes comprising SUC1-SUC5 and SUC7. Production of invertase is highly variable, dependent on the strain and SUC gene present in the cell 27. Two ways of sucrose hydrolysis by S. cerevisiae have been described 28. According to Basso et al. 29, intracellular hydrolysis in anaerobic cultures theoretically enables a 9% higher ethanol yield than extracellular hydrolysis.

High concentrations of ethanol and sugar inhibit yeast metabolism and fermentation. However, a combination of both components acts synergistically on the yeast. In effect, a mixture of sugars and ethanol, less than those required separately, will inhibit growth and fermentation of the yeast. The Delle equation is based on the inhibitory activity of ethanol being 4.5 times higher that of sugar [Delle units (DU) = % sugar (w/v) + 4.5 (% alcohol, v/v)]. When DU values range from 75 to 85, depending on the yeast strain and the concentration of SO2, yeast growth and metabolism are inhibited 23. A Delle value equal to 80, for different concentrations of sugar and ethanol, corresponds to aw in the range 0.93–0.95, which is the limit for growth of S. cerevisiae 30. According to the results of the DU in the described continuous fermentations (Tables 3-5), the yeast cells were utilized for 2 months under very stressful conditions, and despite this, they were able to produce high levels of ethanol with high efficiency, as mentioned above.
| Day of fermentor operation | Yield (g ethanol/g glucose) | EtOH productivity (g/L/day) | Delle units | Total acidity (g/L) | Volatile acidity (g/L) |
|---|---|---|---|---|---|
| 4 | 0.450 ± 0.013 | 16.4 ± 0.4 | 62.8 ± 1.30 | 5.8 ± 0.09 | 0.24 ± 0.01 |
| 10 | 0.420 ± 0.023 | 18.7 ± 0.9 | 65.6 ± 2.76 | 5.7 ± 0.1 | 0.28 ± 0.01 |
| 17 | 0.475 ± 0.019 | 22.4 ± 0.7 | 75.8 ± 2.42 | 5.5 ± 0.04 | 0.30 ± 0.03 |
| 24 | 0.496 ± 0.016 | 23.6 ± 0.8 | 79.3 ± 2.40 | 5.5 ± 0.06 | 0.32 ± 0.02 |
| 31 | 0.496 ± 0.010 | 23.8 ± 0.3 | 79.8 ± 0.94 | 5.6 ± 0.05 | 0.34 ± 0.03 |
| 38 | 0.498 ± 0.012 | 24.1 ± 0.3 | 80.5 ± 1.2 | 5.6 ± 0.05 | 0.32 ± 0.01 |
| 45 | 0.491 ± 0.007 | 22.0 ± 0.3 | 75.8 ± 0.88 | 5.5 ± 0.04 | 0.30 ± 0.01 |
| 52 | 0.493 ± 0.003 | 22.4 ± 0.1 | 76.8 ± 0.25 | 5.4 ± 0.04 | 0.30 ± 0.02 |
| 59 | 0.495 ± 0.006 | 22.1 ± 0.3 | 76.2 ± 0.8 | 5.4 ± 0.08 | 0.32 ± 0.01 |
| 66 | 0.486 ± 0.011 | 21.2 ± 0.5 | 74.1 ± 1.43 | 5.5 ± 0.05 | 0.34 ± 0.01 |
| 74 | 0.483 ± 0.005 | 21.1 ± 0.2 | 73.6 ± 0.59 | 5.5 ± 0.05 | 0.36 ± 0.02 |
| Day of fermentor operation | Yield (g ethanol/g glucose) | EtOH productivity (g/L/day) | Delle units | Total acidity (g/L) | Volatile acidity (g/L) |
|---|---|---|---|---|---|
| 4 | 0.436 ± 0.013 | 15.9 ± 0.4 | 61.6 ± 1.28 | 5.5 ± 0.08 | 0.34 ± 0.02 |
| 10 | 0.449 ± 0.024 | 20.1 ± 0.9 | 70.2 ± 2.95 | 5.6 ± 0.10 | 0.37 ± 0.03 |
| 17 | 0.479 ± 0.019 | 22.7 ± 0.8 | 76.9 ± 2.44 | 5.6 ± 0.05 | 0.39 ± 0.04 |
| 24 | 0.491 ± 0.016 | 23.5 ± 0.8 | 79.3 ± 2.40 | 5.6 ± 0.07 | 0.39 ± 0.02 |
| 31 | 0.489 ± 0.010 | 23.8 ± 0.3 | 79.6 ± 0.92 | 5.5 ± 0.04 | 0.40 ± 0.03 |
| 38 | 0.483 ± 0.013 | 23.0 ± 0.3 | 77.7 ± 1.18 | 5.5 ± 0.04 | 0.40 ± 0.02 |
| 45 | 0.482 ± 0.007 | 21.9 ± 0.3 | 75.6 ± 0.87 | 5.6 ± 0.04 | 0.39 ± 0.02 |
| 52 | 0.484 ± 0.003 | 22.0 ± 0.3 | 75.8 ± 0.24 | 5.6 ± 0.05 | 0.40 ± 0.01 |
| 59 | 0.482 ± 0.006 | 21.3 ± 0.2 | 74.3 ± 0.78 | 5.6 ± 0.07 | 0.41 ± 0.03 |
| 66 | 0.462 ± 0.010 | 19.7 ± 0.4 | 70.1 ± 1.32 | 5.5 ± 0.05 | 0.39 ± 0.02 |
| 74 | 0.448 ± 0.005 | 18.9 ± 0.2 | 67.8 ± 0.53 | 5.6 ± 0.05 | 0.39 ± 0.03 |
| Day of fermentor operation | Yield (g ethanol/g glucose) | EtOH productivity (g/L/day) | Delle units | Total acidity (g/L) | Volatile acidity (g/L) |
|---|---|---|---|---|---|
| 4 | 0.474 ± 0.019 | 18.1 ± 0.6 | 67.9 ± 1.88 | 5.5 ± 0.30 | 0.21 ± 0.06 |
| 10 | 0.474 ± 0.016 | 19.8 ± 0.6 | 67.9 ± 1.88 | 5.9 ± 0.28 | 0.24 ± 0.04 |
| 17 | 0.481 ± 0.010 | 22.5 ± 0.4 | 76.9 ± 1.18 | 5.9 ± 0.14 | 0.30 ± 0.02 |
| 24 | 0.485 ± 0.012 | 23.2 ± 0.4 | 78.5 ± 1.32 | 5.8 ± 0.26 | 0.31 ± 0.02 |
| 31 | 0.483 ± 0.012 | 22.6 ± 0.2 | 77.2 ± 0.92 | 5.7 ± 0.28 | 0.31 ± 0.04 |
| 38 | 0.481 ± 0.011 | 22.2 ± 0.2 | 76.4 ± 0.77 | 5.7 ± 0.29 | 0.32 ± 0.06 |
| 45 | 0.478 ± 0.011 | 22.1 ± 0.3 | 75.9 ± 1.05 | 5.7 ± 0.30 | 0.33 ± 0.07 |
| 52 | 0.474 ± 0.012 | 22.0 ± 0.3 | 75.5 ± 1.08 | 5.7 ± 0.26 | 0.34 ± 0.07 |
| 59 | 0.472 ± 0.013 | 21.7 ± 0.3 | 75.0 ± 1.00 | 5.7 ± 0.30 | 0.37 ± 0.07 |
| 66 | 0.469 ± 0.011 | 21.5 ± 0.3 | 74.3 ± 1.09 | 5.6 ± 0.34 | 0.40 ± 0.04 |
| 74 | 0.475 ± 0.015 | 20.7 ± 0.3 | 73.1 ± 1.15 | 5.7 ± 0.28 | 0.38 ± 0.08 |
Tables 3-5 show the ethanol productivity during the continuous process. With a constant flow rate during the 74 days of operation of the fermentor, the ethanol productivities under steady state varied between ~21 and 24 g/L/day regardless of the magnesium levels. Similar productivity has been reported in the literature 12, 31, 32 with the continuous fermentation of grape must, when a similar flow rate and temperature were used and the cited authors obtained wine with an ethanol concentration of 11–13% (v/v).
In the continuous study experiment, the total acidity of the apple wines, expressed as malic acid, was at a similar level (i.e. from 5.4 to 5.9 g/L), regardless of the magnesium concentration and the period of fermentor operation, whereas the total acidity obtained in the batch process was lower (5.1–5.2 g/L). However, the volatile acidity of the apple wine produced by the periodic and continuous process was comparable (Tables 3-5). Using continuous fermentation of grape must, Sipsas et al. 26 observed a total acidity similar to that in the current continuous process, whereas other researchers 25, 31-33 have found ~1 g lower acidity. All of the above-mentioned authors obtained wines with a lower volatile acidity than our apple wines. This was due to the observation that the fermentation of must containing high concentration of sugars results in elevated levels of volatile acidity 23. However, the total and volatile acidities of the apple wines were within the legal Polish limits for the ranges in fermented beverages, including fruit wines. Total acidity should be between 4 and 9 g/L, while volatile acidity can be up to 1.3 g/L.
Comparing the results of ethanol, efficiency and sugar utilization obtained during the operation of the fermentor, no statistically significant differences (p ≤ 0.05%) were found between the average values dependent on the magnesium concentration in the medium. Therefore, the use of apple must containing ~50 mg Mg2+/L (prepared from tap water, without additional Mg addition) resulted in a similar fermentation performance to the utilization of apple medium with added magnesium. This has industrial importance as Polish wine companies prepare their fruit musts using tap water. Summarizing the results on periodic and continuous fermentations, it can be concluded that a very low magnesium concentration (8.5 mg/L) leads to a decrease in ethanol production in contrast to a medium containing 240 mg/L or more of Mg ions. The use of even larger quantities of magnesium than 240 mg/L had no additional effect on ethanol production.
Magnesium concentrations
The magnesium content in the control apple must, prepared from deionized water, amounted to 8.5 mg/L and the source of these ions was from the apple concentrate. Therefore, Mg levels in the supplemented musts were always 8–9 mg/L higher than the magnesium addition. In the case of the control batch fermentation, the amount of magnesium was the same both before and after fermentation (about 8 mg/L). This is in accordance with the findings of Walker and Maynard 34, who reported that, during yeast growth under Mg2+ limiting conditions (up to around 12 mg/L), progressive removal of magnesium from the medium followed by a gradual release, was observed. In the supplemented samples, the amount of Mg2+ ions after fermentation was between 65 and 360 mg/L, depending on the initial addition. The apple wines obtained from the continuous fermentations of supplemented media contained similar magnesium levels to the wines produced using the batch process (Table 6). During the operation of the fermentor, the amount of Mg2+ ions in the apple wines was 127–148 mg/L in the case of 480 mg added magnesium and 59–66 mg/L in the case of a 240 mg supplement. Different results were obtained when the must was prepared from tap water as this contained 50 mg Mg2+/L, and the yeast cells used only 19–32% of the magnesium (9–16 mg/L).
| Magnesium in the must (mg/L) | Magnesium in the apple wine (mg/L) | Difference – Mg used (mg/L) | Difference – Mg used (%) | |
|---|---|---|---|---|
| (A) Batch fermentation (the means are from three independent series) | ||||
| Magnesium addition | ||||
| 960 mg | 969 | 360 | 609 | 63 |
| 480 mg | 499 | 131 | 358 | 73 |
| 240 mg | 248 | 65 | 185 | 75 |
| Control – deionized water | 8.5 | 8.0 | 0.5 | 6 |
| (B) Continuous fermentation (the means are from two independent series) | ||||
| Day of fermentor operation | ||||
| 10 | 489 | 132 | 357 | 73 |
| 26 | 488 | 128 | 360 | 74 |
| 42 | 489 | 133 | 356 | 73 |
| 59 | 489 | 142 | 347 | 71 |
| 71 | 488 | 148 | 341 | 70 |
| 7 | 248 | 66 | 182 | 73 |
| 20 | 248 | 59 | 189 | 76 |
| 31 | 248 | 66 | 182 | 73 |
| 42 | 249 | 65 | 184 | 74 |
| 56 | 249 | 62 | 187 | 75 |
| 69 | 248 | 64 | 184 | 74 |
| 6 | 47 | 38 | 9 | 19 |
| 15 | 46 | 34 | 12 | 26 |
| 28 | 47 | 32 | 15 | 32 |
| 40 | 47 | 34 | 13 | 28 |
| 54 | 48 | 32 | 16 | 33 |
| 72 | 47 | 32 | 15 | 32 |
In relation to the above observations, it was difficult to determine a minimal dose of magnesium. Walker and Maynard 34 stated ~12 mg Mg2+ /L as a limit of the quantity of this element for ethanol production and glucose consumption. According to Rees and Stewart 6, yeast cells have a minimal requirement for magnesium of 40 mg/L, while the inhibition of growth occurred above 24 g/L. On the other hand, Rees and Stewart 7 found higher ethanol production, sugar uptake, yeast number and vitality when 500 mg/L magnesium was added to high-gravity worts. Also Wang et al. 10 reported that the ethanol concentration after batch fermentation of corn mash supplemented with 1200 mg/L magnesium and 1.5% peptone increased from 14.2 to 17% v/v, but the cited authors did not perform any single-factor experiments. The second important point is the bioaccumulation of metals by yeast cells. This process occurs in two stages. The first involves the accumulation of metal ions on the outer surface of cells and the second stage involves induction of the active transport of metal ions through the cytoplasmic membrane to the cell interior 35. Gniewosz et al. 36 found almost five times more magnesium in the cell wall of S. cerevisiae cultivated on a medium with 1250 mg Mg2+/L, in comparison to cells from a medium without supplementation, and the amount of this element in the cytosol was nearly double. Therefore, in this current study, the yeast cells probably adsorbed magnesium onto the surface or into the cell wall and the more magnesium there was in the medium, the more Mg2+ ions were bound.
Yeast number and carrier characteristics
After the continuous fermentations were completed, the yeast cells were isolated from the carrier to determine the total number of cells in each column per 1 g of foam glass. The results, as a mean for three levels, are shown in Table 7. Regardless of the magnesium concentration in the apple must, a reduction in the number of cells in subsequent columns was observed. Additionally, more cells were found when the must enriched with 240 mg Mg2+/L was used. Similarly, Ji et al. 9 observed a decrease in immobilized cell concentrations in the following columns of the reactor and an increase in ethanol concentration. However, the actual number of cells in each column was higher, because electron microscopic examination of the foam glass after fermentation still showed attached cells, despite 2 h of shaking to remove the yeast cells from the carrier (Fig. 6). Furthermore, when the cells were immobilized by physical adsorption and there were no barriers between the cells and the solution, cell detachment and relocation was possible with the potential occurrence of equilibrium between adsorbed and freely suspended cells 13, 14. The foam glass used in this experiment was characterized by a 94% porosity and the pore diameters were very large – often 1–2 mm. Inside of these holes were far smaller pores (Fig. 7). The density of the foam glass was 0.12 g/mL and the particle density was 0.21 g/mL. Owing to this physical property, the cells could form a three-dimensional structure that was observable under the microscope.
| Column number | 480 mg magnesium | 240 mg magnesium | Tap water |
|---|---|---|---|
| I | 3.00 × 109 | 3.36 × 109 | 1.92 × 109 |
| II | 1.56 × 109 | 2.64 × 109 | 1.86 × 109 |
| III | 1.56 × 109 | 2.04 × 109 | 1.56 × 109 |
| IV | 1.15 × 109 | 1.44 × 109 | 1.06 × 109 |


Sensory evaluation
The sensory analysis showed that acceptable and good quality young apple wine was obtained in the batch and in the continuous process (data not shown). The only exception was the apple wine produced from the fermentation with 960 mg/L magnesium addition, because of a resultant metallic taste. Summarizing, continuous fermentation of a 32% sugar apple medium resulted in a good base apple wine product. In the Polish industry, fruit wines obtained via fermentation of concentrated juices and water are flavoured. The reason is the de-aromatization of the juice before the heat treatment (pasteurization) during the concentrate juice production process.
Conclusions
A magnesium concentration of ~8.5 mg/L led to a decrease in ethanol production. The use of magnesium in a quantity of ~250 or 490 mg/L caused no effect on ethanol production, fermentative parameters and yeast cell growth in either batch or continuous fermentation, while 970 mg Mg2+ ions had a negative impact on sensory quality. It can be assumed that the high level of magnesium caused the accumulation of this element on the surface of the cell or in the cell wall. The minimal dose of magnesium under the described conditions is 50 mg/L (i.e. similar to that in the must prepared from tap water). This finding has industrial significance as Polish wine companies always prepare their fruit musts from tap water and magnesium addition is permitted by law. Further study is needed to evaluate the effect of magnesium levels between 2 and 10 mg/L.
The white foam glass was a suitable carrier for the immobilization of S. bayanus S.o.1/AD yeast cells and a 2.5 month continuous fermentation of fruit must, containing about 320 g/L sugar, with this biocatalyst is possible. The fermentation produced a sweet apple wine with an average 15% v/v ethanol concentration, over a period of almost 2 months. This product can be the basis for aromatized fruit wine-making.
Acknowledgements
Sincere thanks are expressed to Karolina Szulc from the Department of Food Engineering and Process Management for help with the physical analysis of foam glass and thanks to Edward Królak from the Analytical Centre of Warsaw Life Sciences University for assistance in the experimental part of the electron microscope study. Also thanks are due to Barbara Stanisławczyk and Joanna Skwira for technical assistance. Appreciation is also expressed to Warka Co. for the concentrated apple juice.




