Impact of the brewing process on the concentration of silicon in lager beer
Abstract
Silicon is an important trace element that is believed to be essential, especially for the proper functioning of connective tissues. Beer is claimed to be an excellent source of bioavailable silicon. However, little is known about the impact of the brewing process on the concentration of silicon in beer. To clarify this relationship, the concentration of silicon in various lager beer samples was determined by instrumental neutron activation analysis, and the mass balance of silicon during the brewing process was calculated. The concentration of silicon ranged from 13.7 to 44.2 mg/L and was highly dependent on the type and quantity of the raw materials used, as well as on the brewing technology. The concentration of silicon in beers of the same brands brewed by distinct breweries did not differ significantly. The silicon mass balance showed that the main silicon source in beer is the barley malt and that the concentration of silicon in solution increases significantly after mashing, whereas it decreases after fermentation. The majority of silicon remains in the spent grains and the resulting beer contains only about 10% of the silicon present in the raw materials. The results confirmed that beer is a rich source of silicon in the human diet. In addition, the important factors that determine the silicon concentration in beer were identified. Copyright © 2014 The Institute of Brewing & Distilling
Introduction
Silicon, the third most abundant trace element of the human body, is essential for the correct functioning of connective tissue components, such as glycosaminoglycans, collagen and elastin 1, 2. Because it also reduces the absorption of aluminium in humans 3, it also helps to protect the human body from the toxic effects of this metal and could be an important factor in preventing Alzheimer's disease 4. The recommended daily intake of silicon is about 10–25 mg 2 and its most readily absorbable form is orthosilicic acid H4SiO4 2, 5.
The main sources of dietary silicon are foods derived from plants, because certain plants, especially cereals, accumulate silicon, which is then deposited in the form of phytoliths 6, 7. In particular, high concentrations of silicon are found in beer, which is made from barley malt, whose silicon is released into the beer 5. In fact, beer is considered to be a major contributor to the overall silicon intake in the Western diet 7, 8. The content of silicon in beer is usually in the range of 20–50 mg/L 5, 9-11. In addition, silicon in beer is highly bioavailable as it is present in the form of orthosilicic acid 5, 9, 12. Thus, drinking one beer a day should be sufficient to cover the recommended daily intake of silicon.
However, the impact of the brewing process on the silicon concentration in beer has rarely been studied and is still rather unclear. In this research, the focus was on the relationship between the brewing process and the concentration of silicon in the beer. The concentration of silicon in various lager beer samples was determined using instrumental neutron activation analysis (INAA) and it was also calculated using the mass balance of silicon during the industrial scale brewing process.
Methods and materials
Beer brewing
Samples for the analysis were provided by brewery D, an industrial Czech brewery. The beer was brewed by the decoction method (double mash). Wort of original gravity 11.65°P, 24 EBC bitterness units and a colour of 11 EBC units was brewed. The raw materials used were barley malt (varieties for the production of Czech beer; Sladovny Soufflet ČR), hop pellets (Premiant and Saaz varieties; Bohemia Hop Co. Ltd) and CO2 hop extract (NATECO, Wolnzach 07DE, Germany). The cooled wort was fermented at 9.5 °C for 8 days in open vessels and then maturated at 1 °C for 25 days in maturation tanks. After maturation, the beer was filtered using kieselguhr filtration. The final concentration of ethanol was 4.4 % (v/v).
Mass balance of silicon during beer brewing
For this purpose, various materials originating from one industrial scale batch were sampled: brewing water, barley malt, sweet wort, spent grain, hopped wort, CO2 hop extract, hop pellets, hot break, yeast, unfiltered beer and filtered beer. These materials were provided by brewery D.
Sweet wort, hopped wort, spent grain and hot break were freeze-dried and then dry-ashed, while barley malt, hop pellets, hop extract and yeast were dry-ashed directly. From weighed ash aliquots (mass 150–200 mg), pellets of 14 mm diameter were made and the pellets were placed in 20 mm diameter acid-cleaned high-purity polyethylene (PE) disc capsules and heat-sealed.
Beers and brewing waters for analysis
Ten bottled lager-type beers together with corresponding brewing waters were provided by particular breweries. The original gravities of the beers were provided by the Research Institute of Brewing and Malting, plc. Individual beer types are specified in the subsection of the Results and Discussion entitled ‘Silicon content in beer samples’.
Beer samples (250 mL) were freeze-dried. The mass of the lyophilized beer was 2–4% of the original mass. The lyophilized beer was then dry-ashed in air in a nickel crucible at 600 °C for several hours. The ash mass was 4–6% of that of the lyophilized beer. Ash aliquots, of a mass of 150–200 mg, were pelletized (14 mm diameter pellets) and heat-sealed in 20 mm-diameter PE capsules.
Brewing waters (1–1.5 L) were concentrated at least 20 times by boiling, and acidified with high-purity nitric acid to pH 2–3 to prevent the precipitation of calcium and magnesium carbonates and iron oxides. The samples were then placed in an ultrasonic bath at room temperature (about 22 °C) for several hours and then boiled briefly to remove dissolved chlorine, which would interfere in the subsequent analysis (by forming a high background in the γ-ray spectra). Next, the samples were slowly evaporated at 90 °C until a volume of 50 mL was obtained. From the concentrated samples, 2 mL aliquots were pipetted onto 14 mm discs of Whatman 1 chromatographic paper, dried with an infrared bulb and heat-sealed in 20 mm-diameter PE capsules.
Quality control samples NIST SRM 2704 Buffalo River Sediment and NIST SRM 1633b Constituent Elements in Coal Fly Ash were analysed in the ‘as received’ state. Masses of about 150 mg were pelletized and heat-sealed in PE capsules.
Instrumental neutron activation analysis
The nuclear reaction 29Si(n,p)29Al with fast neutrons was used for the determination of silicon by INAA. The activation cross section, σf , is 0.12 ± 0.02 × 10−24/cm2 (barn). The 29Al radionuclide has a half-life T1/2 = 6.56 min and emits well measurable γ-rays with energy Eγ = 1273.4 keV (91.3% abundance). This nuclear reaction enables the detection of much lower quantities of silicon compared with the nuclear reaction 30Si(n, γ)31Si with thermal neutrons (σth = 0.107 ± 0.002 barn, T1/2 of the product = 2.62 h, Eγ of the product = 1266.2 keV with only 0.07% abundance). Other details and possibilities of silicon determination by neutron and photon activation analysis have already been given elsewhere 13. The prepared ash and evaporated residues in PE capsules were irradiated for 45 s in the LVR-15 reactor of the Research Centre Řež Ltd at neutron fluence rates of 3.4 × 1013, 1.5 × 1013 and 1.1 × 1013/cm2/s of thermal, epithermal and fast neutrons, respectively. The PE capsules were placed in a Cd cylindrical box (diameter 34 mm, height 7 mm, wall thickness 1 mm) to shield against thermal neutrons. After a decay time of 10 min, the samples were counted for 20 min with a coaxial HPGe semiconductor detector having a relative efficiency of 23% and full width at half maximum (FWHM) resolution of 1.8 keV, both for the 1332.5 keV photons of 60Co. The distance between the sample and the detector cap (counting geometry) was 2 cm. The detector was coupled to the computer controlled γ-ray spectrometric system Canberra Genie 2000 (Canberra, USA) through a chain of linear electronics, which contained the Nuclear Data 699 Loss Free Counting module for the dynamic correction of dead-time and pile-up losses. For the quantification of silicon concentrations in the samples, a relative standard comprising a silicon disc of semiconductor purity was used, of a mass of about 10 mg, which was heat-sealed in PE capsules (20 mm diameter), irradiated and counted in the same way as the samples.
Results and discussion
Accuracy of INAA
Neutron activation analysis has recently been recognized as a primary ratio measurement method 14, that is, the measurement method with the highest metrological properties. This analytical technique is mostly recognized for its highly accurate determination of elements at trace and ultratrace levels. It is also valued for its accurate determination of elements at much higher levels, especially for those such as silicon that cannot be assayed with certainty using other analytical methods 15. For quality control purposes, NIST SRM 2704 Buffalo River Sediment and NIST SRM 1633b Constituent Elements in Coal Fly Ash were analysed in this work. Table 1 shows that the results with the associated expanded uncertainty (coverage factor k = 2) agree with the NIST certified values within the uncertainty margins, thus proving the accuracy of the INAA results.
| NIST SRM | This worka | NIST valueb |
|---|---|---|
| NIST SRM 2704 | 28.98 ± 0.35 | 29.08 ± 0.13 |
| NIST SRM 1633b | 22.94 ± 0.23 | 23.02 ± 0.08 |
- a Value ± expanded uncertainty (coverage factor k = 2).
- b Certified value ± the half-width of a 95% expected tolerance interval (2704) and/or the half-width of a 95% prediction interval (1633b).
Silicon balance during the brewing process
The results of duplicate analyses of raw materials, semiproducts, waste products and the final product from brewery D, together with combined uncertainties and detection limits, are given in Table 2. The combined uncertainties (coverage factor k = 1) involve all important uncertainty sources in the sample preparation and analytical steps.
| Sample | Unit | Si concentration | Detection limit |
|---|---|---|---|
| Brewing water | mg/L | 7.1 ± 0.9 | 2.2 |
| 7.4 ± 1 | 2.3 | ||
| Barley malt | mg/kg | 1671 ± 33 | 65 |
| 1490 ± 31 | 66 | ||
| Sweet wort | mg/L | 51.3 ± 1.2 | 4 |
| 57.1 ± 1.5 | 5.2 | ||
| Spent grain | mg/kg | 984 ± 14.4 | 36 |
| 917 ± 13.7 | 34 | ||
| Hopped wort | mg/L | 70.1 ± 2 | 7.9 |
| 67.3 ± 1.9 | 7.3 | ||
| CO2 hop extract | mg/kg | 12.5 ± 0.5 | 2.3 |
| Hop pellets | mg/kg | 4607 ± 83 | 147 |
| 4920 ± 94 | 153 | ||
| Yeast | mg/kg | 29.9 ± 2.8 | 16 |
| 23.8 ± 2.5 | 14 | ||
| Hot break | mg/kg | 1079 ± 20 | 63 |
| 1070 ± 21 | 66 | ||
| Unfiltered beer | mg/L | 35.4 ± 0.9 | 3.5 |
| 28.7 ± 0.8 | 3.6 | ||
| Filtered beer | mg/L | 30.4 ± 0.8 | 3 |
| 32.3 ± 0.8 | 3.4 |
The highest concentration of silicon was found in the hop pellets, about three times higher than in the barley malt. This was surprising at first, as barley malt is considered to be a major silicon source in beer 9, 12. However, hops are used in much smaller quantities than malt, decreasing the silicon contribution to the beer. The hop processing method is also important, as shown by the very low silicon concentration in the hop extract. Nevertheless, highly hopped beers are expected to contain higher silicon levels 9.
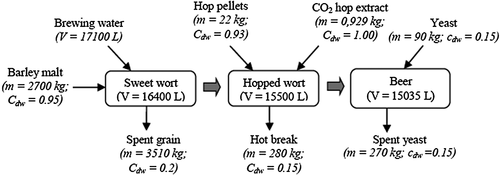
Figure 1 shows the changes in the silicon concentration during the brewing process. The chart results from the data listed in Table 2. The mass balance is based on the data in Fig. 2, showing the masses of the analysed materials along with their dry weight concentrations (cdw) in parts by weight. The concentration of silicon increased significantly after the mashing process, demonstrating the major role of barley malt as a silicon source in beer. The contribution of the brewing water to the concentration of silicon in the sweet wort was much lower, but not insignificant. Furthermore, it is likely that the use of silicon-rich water will lead to an increased beer silicon concentration 12. The mass balance of silicon confirmed that barley malt is the major silicon source in beer – barley malt alone contained 95% of the total silicon in the raw materials. About 20% of the barley malt silicon was released into the sweet wort. The concentration of silicon further increased after wort boiling. However, this was small compared with the mass balance results. The total silicon content of the hot break (mean value 301 g), formed during the boiling and removed before the fermentation, was about three times higher than the total silicon content in the hop pellets (mean value 105 g). Therefore, the total silicon content in the hopped wort had to be lower than in the sweet wort. Similarly, in the work of Casey and Bamforth 9, hops introduced approximately 14% of the silicon at the boiling stage, but more silicon was removed with the hot break than was introduced by the hops. The same conclusion was made also by Cejnar et al. 16, where 11% of the silicon in pale wort and 6% of the silicon in dark wort was lost. However, owing to water evaporation, the wort thickens during the boiling phase, increasing the concentration of silicon.
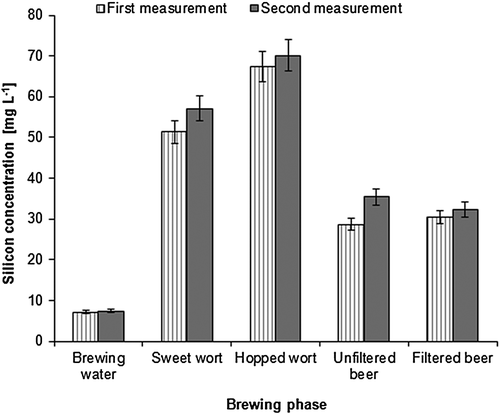
The wort was then cooled to the fermentation temperature, charged with oxygen and fermentation followed. During the fermentation, the concentration of silicon was reduced by half. The loss of silicon was probably caused by its sorption onto yeast and other solid particles that were subsequently removed from the beer. The effect of fermentation was assessed previously. The concentration of silicon was either not influenced by fermentation 12 or decreased only slightly 9. However, the brewing styles used by these authors, especially the courses of fermentation, were significantly dissimilar from the lager beer production used in the current work. In the first case, sweet wort at 18 °C for 6 days was fermented and in the second one, wort with 15 bitterness units was prepared and fermented at 20 °C for 12 days. Thus, fermentation conditions could play a considerable role in the binding of silicon to the solid particles of fermented wort.
The impact of the kieselguhr filtration on the concentration of silicon in beer was insignificant. The impact of filtration was investigated previously by Walker et al. 12, where the concentration of silicon was decreased by one-third, and by Casey and Bamforth 9, where two attempts were carried out. In the first attempt, the concentration of silicon increased significantly. In the second attempt, the concentration did not change significantly. The type of filtration material used appears to be important. The loss of silicon is possible by the adsorption of silicon onto filtration material or onto captured cells 12, but there is no material reported that releases silicon into the beer. In this context, kieselguhr, which contains a reasonable level of silicon, was examined. No leaching of silicon into the beer was observed 9, 12.
The finished beer contained slightly above 10% of the silicon present in the raw materials. Around 74% remained in the spent grain and around 7% was lost with the hot break. This is in agreement with the available literature. Walker et al. 12 stated that beer contained 19% of silicon from the barley malt, and in the work of Casey and Bamforth 9, only 4% of the silicon present in the raw materials was found in the beer.
Silicon content in beer samples
The concentration of silicon in the analysed beers, their original gravities and the concentrations of silicon in the corresponding brewing waters, together with combined uncertainties and detection limits, are shown in Table 3. The concentration of silicon in the beer samples ranged from 13.7 to 44.2 mg/L and depended strongly on the type and quantity of raw materials used and also on the brewing technology. Generally, the silicon content increased as the original gravity of beer increased. This suggests that the most important factor influencing the concentration of silicon in beer was the amount of barley malt used in the mashing process. However, the content of silicon in barley depends on the variety and appears to be at least partially genetically determined 17. Thus, selection of a particular variety can strongly influence the concentration of silicon in beer. Further, adjuncts – used by many breweries – contribute to the original gravity as well. As adjuncts can contain less silicon than barley malt; this usually leads to a decreased concentration of silicon in the beer 9. Brand 1 was a beer with a high proportion of sugar adjuncts, which explained its low silicon concentration. Brand 3 was brewed from barley malt without adjuncts and its silicon concentration was relatively high. The concentration of silicon in Brand 2 was surprising, as it is also brewed solely from barley malt. However, Brewery B used microfiltration for the filtration of beer, where silicon loss owing to the sorption of orthosilicic acid on the filtration membrane is possible 12. Brands 4 and 5 were traditionally brewed beers and their silicon concentrations were the highest.
| Sample (original extract; real extract, w/w) | Si in beer | Detection limit | Si in brewing water | Detection limit |
|---|---|---|---|---|
| Brand 1, Brewery A (11.65%; 3.89%) | 13.7 ± 0.8 | 4.6 | 2.56 ± 0.40 | 1.1 |
| 15.0 ± 0.8 | 4.1 | |||
| Brand 2, Brewery B (11.70%; 4.87%) | 16.3 ± 0.7 | 3.5 | 2.78 ± 0.46 | 1.2 |
| 16.8 ± 0.6 | 3.4 | |||
| Brand 3, Brewery C (11.80%; 4.04%) | 25.7 ± 0.6 | 2.1 | 4.49 ± 0.41 | 1.0 |
| 27.5 ± 0.7 | 2.4 | |||
| Brand 4, Brewery D (11.65%; 4.81%) | 30.4 ± 0.6 | 3.0 | 7.13 ± 0.86 | 2.3 |
| 32.3 ± 0.7 | 3.4 | 7.37 ± 0.98 | 2.2 | |
| Brand 5, Brewery E (11.90%; 3.68%) | 43.3 ± 0.9 | 3.5 | Not determined | 3.8 |
| 44.2 ± 0.9 | 3.6 | |||
| Brand 6, Brewery F (9.70%; 3.42%) | 18.5 ± 0.8 | 3.9 | 2.78 ± 0.46 | 1.2 |
| 18.8 ± 0.8 | 3.9 | |||
| Brand 6, Brewery G (9.70%; 3.41%) | 15.5 ± 0.5 | 2.8 | 10.54 ± 0.75 | 1.9 |
| 19.3 ± 0.6 | 3.2 | |||
| Brand 6, Brewery H (9.70%; 3.44%) | 17.9 ± 0.7 | 3.3 | 5.60 ± 0.44 | 1.1 |
| 18.2 ± 0.7 | 3.3 | |||
| Brand 7, Brewery F (11.70%; 3.93%) | 22.6 ± 0.9 | 4.8 | 2.78 ± 0.46 | 1.2 |
| 22.2 ± 0.9 | 4.8 | |||
| Brand 7, Brewery H (11.70%; 3.91%) | 30.5 ± 0.7 | 3.2 | 5.60 ± 0.44 | 1.1 |
| 26.5 ± 0.6 | 2.9 |
Brand 6 was brewed by three distinct breweries, using identical recipes, with one exception – each brewery used its own brewing water. The concentrations of silicon in these three beers were not significantly different from each other, even though the corresponding brewing waters were quite variable as sources of silicon. The results therefore suggest that there is no correlation between the silicon content of brewing water and the final concentration of silicon in the beer. Brewery H uses a mash filter for mash separation, where malt is ground into much smaller particles than in the case of a lauter tun separation. Further, in contrast to the lauter tun separation, the barley husks were ground as well. This is especially important because >80% of the silicon in barley accumulates in the husks 17. This finer grinding can lead to a higher silicon concentration in the beer owing to the greater surface area of the barley grain 12, leading to an increased mass transfer between phases. Brand 7 from Brewery H had a higher silicon concentration than the same brand from Brewery H. For Brand 6, however, no such differences were observed.
Conclusions
The most important factors influencing the silicon concentration in beer were determined. It was confirmed that barley malt is the main source of silicon, as the concentration of silicon significantly increased after the mashing process. This was also apparent from the fact that the concentration of silicon in the high-adjunct beer (Brand 1) was much lower than in the other beers with comparable original gravities. The concentration of silicon in the brewing water was not correlated with the final silicon concentration in the beer. During the wort boil, the total silicon content in solution decreased, most likely because of the sorption of silicon onto the hot break, but the silicon concentration itself increased owing to water evaporation. After fermentation, the concentration of silicon decreased significantly, probably through the sorption of silicon onto yeast. The effect of filtration depended on the filtration material used. The kieselguhr filtration used in the industrial-scale brewing process had virtually no impact on the concentration of silicon, whereas the membrane filtration used in the production of Brand 2, where the sorption of silicon onto the membrane was possible, had an adverse effect on the concentration of silicon.
Acknowledgements
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic, Project no. MSM 6046137305; Research Centre, Project no. 1M0570; and the Czech Science Foundation, Project no. P108/12/G108.




