Selective modification of bifunctional heterocyclic compounds containing amino and thioamide groups in acetic acid medium
Abstract
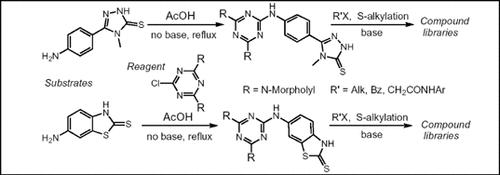
In acetic acid medium, 2-chloro-4,6-dimorpholin-4-yl-[1,3,5]triazine undergoes amination by bifunctional heterocyclic compounds containing both amino and thioamide groups. The reactions are high-yielding and selective; the thioamide functions are unaffected under the requisite conditions. The reactions do not proceed in satisfactory yields in other solvents and, thus, require acetic acid. The resulting thioamides, consisting of multiple biologically relevant residues, are easily alkylated at the sulfur (thiolthione) centers to furnish new thioethers in subsequent steps.