Facile routes to 1,2,4-triazolo[3,4-b][1,3,4]thiadiazines and 1,2,4-triazino[3,4-b][1,3,4]thiadiazine by heteropolyacides
Radineh Motamedi
Department of Chemistry, School of sciences, Payame nourUniversity, Delijan, Iran
Search for more papers by this authorMajid M. Heravi
Department of Chemistry, School of sciences Azzahra University, Vanak, Tehran, Iran
Search for more papers by this authorFatemeh F. Bamoharram
Department of Chemistry, Islamic Azad University-Mashhad Branch, Mashhad, Iran
Search for more papers by this authorAnahita Haeri
Department of Chemistry, School of sciences Azzahra University, Vanak, Tehran, Iran
Search for more papers by this authorRadineh Motamedi
Department of Chemistry, School of sciences, Payame nourUniversity, Delijan, Iran
Search for more papers by this authorMajid M. Heravi
Department of Chemistry, School of sciences Azzahra University, Vanak, Tehran, Iran
Search for more papers by this authorFatemeh F. Bamoharram
Department of Chemistry, Islamic Azad University-Mashhad Branch, Mashhad, Iran
Search for more papers by this authorAnahita Haeri
Department of Chemistry, School of sciences Azzahra University, Vanak, Tehran, Iran
Search for more papers by this authorAbstract
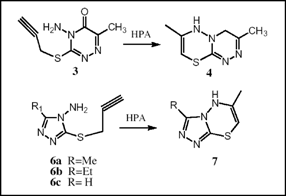
Cyclization of 4-amino-6-methyl-3-propargylmercapto-1,2,4-triazine-5-one 3 and 4-amino-3-propargyl mercapto-1,2,4-triazole derivatives 6 were afforded 1,2,4-triazino[3,4-b][1,3,4]thiadiazines 4 and 1,2,4-triazolo[3,4-b][1,3,4]thiadiazines 7 in presence of heteropolyacids, H14[NaP5W29MoO110] and H6P2W18O60 in high yields. Among used heteropoly acids, the yields were higher with H14-P5Mo, caused to their high acid strengths.
References and Notes
- 1 Invidiata, F. P.; Furno, G.; Lampronti, L.; Semoni, D. J. J. Heterocyclic Chem. 1997, 34, 1255.
- 2 Heindel, N. D.; Reid, J. R. Org. Prep. Proced. Int. 1981, 13, 123.
- 3 Chadha, V. K. J. Indian Chem. Soc. 1978, 55, 817.
- 4 Chadha, V. K.; Sharma, G. R. J. Indian Chem. Soc. 1980, 57, 1112.
- 5 Molina, P.; Alajarin, M. J. Chem. Soc. Perkin Trans. I 1987, 1853.
- 6 Mahan, J.; Alajarin, G. S. R. J. Chem. Soc, Perkin Trans. I 1987, 1853.
- 7 Omar, A. M. M. E.; Aboulmafe, O. M. J. Heterocyclic Chem. 1986, 23, 1339.
- 8 Ghannoum, M. A.; Eweiss, N. F.; Bahajaj, A. A.; Quereshi, M. A. Microbios 1983, 37, 151.
- 9 Rasad, A. R.; Ramalengamat, T.; Ras A. B.; Drawn, P. W.; Sattur, P. B. Indian J. Chem. 1986, 26B, 556.
- 10 Falke, D.; Rada, B. Acta Virol, 1970, 14, 115.
- 11 Sdwell, R. W.; Dixon, G. J.; Schabel, F. M. Jr. Appl. Microbiol. 1968, 16, 370.
- 12 Creasey, W. A.; Fink, M. E.; Handschurnacker, R. E.; Calabresi, P. Cancer Res. 1963, 23, 444.
- 13
Walters, T. R.;
Aur, R. J.;
Hernandez, A. K.;
Veetli, T.;
Penkel, D.
Cancer,
1963,
29,
1057.
10.1002/1097-0142(197204)29:4<1057::AID-CNCR2820290455>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 14 Malolcsy, G. Acta. Phytopathol. 1966, 1, 245.
- 15 Deshmukh, A. A.; Mody, M. K.; Ramalengant, T.; Sattur, P. B. Indian J. Chem. 1985, 25B, 793.
- 16 Heravi, M. M.; Rahimizadeh, M.; Seyf, M.; Davoodnia, A.; Ghassemzadeh, M. Phosphorus, Sulfur and Slicon, 2000, 167, 211.
- 17 Heravi, M. M.; Bakavoli, M. J. Chem. Res. 1995, 11, 480.
- 18 Rani, R. V.; Srinivas, M. R.; Kulkamrani, S. J.; Raghavan K. V. Green Chem. 2001, 3, 305.
- 19 Clark, J. H. Green Chem. 1999, 1, 1.
- 20 Okuhara, T.; Mizuno, N.; Misono, M. Adv. Catal., 1996, 41, 113.
- 21 Kozhevnikov, V. Catal. Rev. Sci. Eng. 1995, 37, 311.
- 22 Mizuno, N.; Misono, M. Chem. Rev. 1999, 98, 199.
- 23 Hu, C.; Hashimoto, M.; Okuhara, T.; Misono, M. J. Catal. 1993, 143, 437.
- 24 Okuhara, T.; Kasai, A.; Misono, M. Catalyst. 1980, 22, 226.
- 25 Yamada, T. Peterotech. (Tokyo), 1990, 13, 627.
- 26 Okuhara, T.; Mishimura, T.; Ohashi, K.; Misono, M. Chem. Lett. 1995, 155.
- 27 Okuhara, T.; Mishimura, T.; Ohashi, K.; Misono, M. Chem. Lett. 1990, 1201.
- 28
Aoshima, A.;
Tonomura, E.;
Yamamatsu, S.
Adv. Technol.
1990,
2,
127.
10.1002/pat.1990.220010202 Google Scholar
- 29a Heravi, M. M.; Derikvand, F.; Bamoharram, F. F. J. Mol. Catal. A Chem. 2005, 242, 173. 29b Heravi, M. M.; Bakhtiari, Kh.; Bamoharram, F. F. Catal. Commun. 2006, 7, 373. 29c Heravi, M. M.; Motamedi, R.; Sefi, N.; Bamoharram, F. F. J. Mol. Catal. A Chem. 2006, 249, 1. 29d Bamoharram, F. F.; Heravi, M. M.; Roshani, M.; Gharib, A.; Jahangir, M. Appl. Catal. A: Gen. 2006, 302, 42. 29e Bamoharram, F. F.; Heravi, M. M.; Roshani, M.; Tavakoli, N. J. Mol. Catal., A: Chem. 2006, 252, 219. 29f Heravi, M. M.; Derikvand, R.; Bamoharram, F. F. J. Mol. Catal. A Chem. 2006, 253, 16. 29g Heravi, M. M.; Bakhtiari, Kh.; Bamoharram, F. F. Catal. Commun 2006, 7, 499. 29h Heravi, M. M.; Motamedi, R.; Sefi, N.; Bamoharram, F. F. Catal. Commun., 2007, 7, 1467.
- 30 Dornow, A.; Menzel, H.; Maix, P. Chem. Ber. 1964, 97, 2173.
- 31a Heravi, M. M.; Khosrofar, P. J. Sci. I. R. Iran, 1996, 7 (2), 86–88. 31b Nuss, J. M.; Levine, B. H. R.; Rennels, A.; Heravi, M. M. Tetrahedron Lett. 1991, 5243. 31c Heravi, M. M.; Bakavoli, M. J. Chem. Soc. Pak.I, 1993, 15 (4), 257. 31d Heravi, M. M.; Nuss, J. M. J. Sci. I. R. Iran. 1993, 4 (3), 194.
- 32 Kharasch, M. S.; Seyler, R. C.; Mayo, F. R. J. Am. Chem. Soc. 1938, 60, 882.
- 33a Heravi, M. M.; Zadmard, R.; Bolourtchian S. M.; Aghapoor, K. I. J. Sci. Tech. 1999, 23 (2), 151–154. 33b Heravi, M. M.; Aghapoor, K.; Nooshabadi, M. A.; Mojtahedi, M. M. Monatshefte für chemie, 1997, 128, 1143–1147.
- 34 Zadmard, R.; Heravi, M. M.; Bolurchian, S. M. Indian J. Heter. Chem. 1998, 7, 239–240.
- 35
Kozhevnikov, I. V.
Russ. Chem. Rev.
1987,
56,
811.
10.1070/RC1987v056n09ABEH003304 Google Scholar