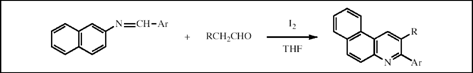
I2-catalyzed reactions of schiff base and alkyl aldehyde towards benzo[f]quinoline derivatives
Qing Li
School of Chemistry and Chemical Engineering, Xuzhou Normal University, Xuzhou, Jiangsu 221116 China
Search for more papers by this authorChang-Sheng Yao
School of Chemistry and Chemical Engineering, Xuzhou Normal University, Xuzhou, Jiangsu 221116 China
Search for more papers by this authorMei-Mei Zhang
The Key Laboratory of Biotechnology for Medical Plant of Jiangsu Province, Xuzhou, Jiangsu 221116 China
Search for more papers by this authorShu-Jiang Tu
School of Chemistry and Chemical Engineering, Xuzhou Normal University, Xuzhou, Jiangsu 221116 China
The Key Laboratory of Biotechnology for Medical Plant of Jiangsu Province, Xuzhou, Jiangsu 221116 China
Search for more papers by this authorXiang-Shan Wang
School of Chemistry and Chemical Engineering, Xuzhou Normal University, Xuzhou, Jiangsu 221116 China
The Key Laboratory of Biotechnology for Medical Plant of Jiangsu Province, Xuzhou, Jiangsu 221116 China
Search for more papers by this authorQing Li
School of Chemistry and Chemical Engineering, Xuzhou Normal University, Xuzhou, Jiangsu 221116 China
Search for more papers by this authorChang-Sheng Yao
School of Chemistry and Chemical Engineering, Xuzhou Normal University, Xuzhou, Jiangsu 221116 China
Search for more papers by this authorMei-Mei Zhang
The Key Laboratory of Biotechnology for Medical Plant of Jiangsu Province, Xuzhou, Jiangsu 221116 China
Search for more papers by this authorShu-Jiang Tu
School of Chemistry and Chemical Engineering, Xuzhou Normal University, Xuzhou, Jiangsu 221116 China
The Key Laboratory of Biotechnology for Medical Plant of Jiangsu Province, Xuzhou, Jiangsu 221116 China
Search for more papers by this authorXiang-Shan Wang
School of Chemistry and Chemical Engineering, Xuzhou Normal University, Xuzhou, Jiangsu 221116 China
The Key Laboratory of Biotechnology for Medical Plant of Jiangsu Province, Xuzhou, Jiangsu 221116 China
Search for more papers by this authorAbstract
References and Notes
- 1 Brock, E. D.; Lewis, D. M.; Yousaf, T. I.; Harper, H. H., (The Procter and Gamble Company USA) WO 9951688, 1999.
- 2a Beagley, P.; Blackie, M. A. L.; Chibale, K.; Clarkson, C.; Meijboom, R.; Moss, J. R.; Smith, P.; Su, H. Dalton Trans. 2003, 3046–3051. 2b Sawada, Y.; Kayakiri, H.; Abe, Y.; Mizutani, T.; Inamura, N.; Asano, M.; Hatori, C.; Aramori, I.; Oku, T.; Tanaka, H. J. Med. Chem. 2004, 47, 2853–2863. 2c Ma, Z.; Hano, Y.; Nomura, T.; Chen, Y. Bioorg. Med. Chem. Lett. 2004, 14, 1193–1196. 2d Denton, T. T.; Zhang, X.; Cashman, J. R. J. Med. Chem. 2005, 48, 224–239. 2e Fokialakis, N.; Magiatis, P.; Chinou, L.; Mitaku, S.; Tillequin, F. Chem. Pharm. Bull. 2002, 50, 413–414. 2f Fossa, P.; Mosti, L.; Menozzi, G.; Marzano, C.; Baccichetti, F.; Bordin, F. Bioorg. Med. Chem. 2002, 10, 743–751. 2g Ryckebusch, A.; Derprez-Poulain, R.; Maes, L.; Debreu-Fontaine, M. A.; Mouray, E.; Grellier, P.; Sergheraert, C. J. Med. Chem. 2003, 46, 542–557. 2h Morgan, L. R.; Jursic, B. S.; Hooper, C. L.; Neumann, D. M.; Thangaraj, K.; Leblanc, B. Bioorg. Med. Chem. Lett. 2002, 12, 3407–3411.
- 3 Sakata, G.; Makino, K.; Karasawa, Y. Heterocycles 1988, 27, 2481–2515,
- 4a Demeunynck, M.; Moucheron, C.; Mesmaeker, A. K.-D. Tetrahedron Lett. 2002, 43, 261–264. 4b Baraznenok, I. L.; Nenajdenko, V. G.; Balenkova, E. S. Eur. J. Org. Chem. 1999, 937–941. 4c Ali, M. M.; Tasneem, K. C.; Rajanna, P. K.; Prakash, S. Synlett 2001, 251–253. 4d Palacios, F.; de Retana, Ochoa A. M.; Oyarzabal, J. Tetrahedron 1999, 55, 5947–5964. 4e Charpentier, P.; Lobregat, V.; Levacher, V.; Dupas, G.; Queguiner, G.; Bourguignon, J. Tetrahedron Lett. 1998, 39, 4013–4016. 4f Cho, C. S.; Kim, B. T.; Kim, T. J.; Shim, S. C. Chem. Commun. 2001, 2576–2577. 4g Uchiyama, K.; Hayashi, Y.; Narasaka, K. Synlett 1997, 445–447. 4h Crousse, B.; Begue, J.-P.; Bonnet-Delpon, D. J. Org. Chem. 2000, 65, 5009–5013. 4i Lin, X. F.; Cui, S. L.; Wang, Y. G. Tetrahedron Lett. 2006, 47, 4509–4512. 4j Lin, X. F.; Cui, S. L.; Wang, Y. G. Tetrahedron Lett. 2006, 47, 3127–3130.
- 5a
Skraup, H.
Chem. Ber.
1880,
13,
2086–2087.
5b
Mansake, R. H.;
Kulka, M.
Org. React.
1953,
7,
59–61.
5c
Doebner, O.;
Miller, V. W.
Chem. Ber.
1881,
14,
2812–2813.
10.1002/cber.188101402258 Google Scholar5d Conrad, M.; Limbach, L. Chem. Ber. 1887, 20, 944–945.10.1002/cber.188702001215 Google Scholar5e Combes, A. Compt. Rend. 1888, 106, 142–143. 5f Pfitzinger, W. J. Prakt. Chem. 1886, 33, 100.10.1002/prac.18850330110 Google Scholar
- 6 Whitmore, F. C.; Rothrock, H. S. J. Am. Chem. Soc. 1933, 55, 1106–1109.
- 7 Rutherford, K. G.; Mamer, O. A.; Prokipcak, J. M.; Jobin, R. A. Can. J. Chem. 1966, 44, 2337–2339.
- 8 Jenner, G. Tetrahedron Lett. 1988, 29, 2445–2448. 15. Hessian, K. O.; Flynn, B. L. Org. Lett. 2003, 5, 4377–4380.
- 9 Bandgar, B. P.; Shaikh, K. A. Tetrahedron Lett. 2003, 44, 1959–1961.
- 10 Sun, J.; Dong, Y.; Cao, L.; Wang, X.; Wang, S.; Hu, Y. J. Org. Chem. 2004, 69, 8932–8934.
- 11 Ramalinga, K.; Vijayalakshimi, P.; Kaimal, T. N. B. Tetrahedron Lett. 2002, 43, 879–882.
- 12 Chavan, S. P.; Kale, R. R.; Shivasankar, K.; Chandake, S. I.; Benjamin, S. B. Synthesis 2003, 2695–2698.
- 13a Yadav, J. S.; Reddy, B. V. S.; Sadasiv, K.; Satheesh, G. Tetrahedron Lett. 2002, 43, 9695–9697. 13b Wang, S. Y.; Ji, S. J.; Loh, T. P. Synlett 2003, 2377–2379.
- 14a Ji, S. J.; Wang, S. Y.; Zhang, Y.; Loh, T. P. Tetrahedron 2004, 60, 2051–2055. 14b Ke, B. W.; Qin, Y.; He, Q. F.; Huang, Z. Y.; Wang, F. P. Tetrahedron Lett. 2005, 46, 1751–1753.
- 15 Crystal data for 3o: C25H16BrN M = 410.30, Pale yellow block crystals, 0.78 × 0.55 × 0.17 mm, Monoclinic, space group P 21/c, a = 12.704 (2), b = 9.4917 (14), c = 15.142 (3) Å, β = 94.612 (4) °, V = 1819.9 (5)3, Z = 4, Dc = 1.497 g.cm−3. F(000) = 832, μ(MoKα) = 2.268 mm−1. Intensity data were collected on Rigaku Mercury diffractometer with graphite monochromated MoKα radiation (λ = 0.71070 Å) using ω scan mode with 3.03 ° < θ < 25.35 °. 3336 unique reflections were measured and 3034 reflections with I>2σ(I) were used in the refinement. Structure solved by direct methods and expanded using Fourier techniques. The final cycle of full-matrix least squares technique to R = 0.0423 and wR = 0.0814.