Convenient synthesis of some 2-substituted 4(3H)-quinazolinone derivatives
I. Philipova
Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev str., Block 9, 1113 Sofia, Bulgaria
Search for more papers by this authorG. Dobrikov
Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev str., Block 9, 1113 Sofia, Bulgaria
Search for more papers by this authorK. Krumova
Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev str., Block 9, 1113 Sofia, Bulgaria
Search for more papers by this authorJ. Kaneti
Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev str., Block 9, 1113 Sofia, Bulgaria
Search for more papers by this authorI. Philipova
Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev str., Block 9, 1113 Sofia, Bulgaria
Search for more papers by this authorG. Dobrikov
Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev str., Block 9, 1113 Sofia, Bulgaria
Search for more papers by this authorK. Krumova
Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev str., Block 9, 1113 Sofia, Bulgaria
Search for more papers by this authorJ. Kaneti
Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev str., Block 9, 1113 Sofia, Bulgaria
Search for more papers by this authorAbstract
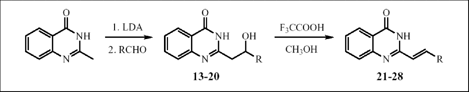
A series of 2-substituted-4(3H)-quinazolinones 13-20 has been synthesized in good yields using the reaction of double lithiated 2-methylquinazolinone-4 with a variety of aromatic aldehydes. They have been easily transformed in high yields into the corresponding 2-substituted conjugated derivatives 21-28 bearing terminal aryl groups by F3CCOOH mediated dehydration.
References
- 1
Z. Widdige,
Prakt. Chem.,
36,
141
(1887).
10.1002/prac.18870360115 Google Scholar
- 2 W. L. F. Armarego, Adv. Heterocycl. Chem., 24, 1 (1979).
- 3 J. P. Mayer, G. S. Lewis, M. J. Curtis, J. Zhang, Tetrahedron lett., 38, 8445 (1997).
- 4 J. B. Jiang, D. P. Hessan, B. A. Dusak, D. L. Dexter, G. J. Kang, E. Hamel, J. Med. Chem., 33, 1721 (1990).
- 5 S. Eguchi, T. Suzuki, J. Okawa, Y. Matsu, E. Yashima, Y. Okamoto, J. Org. Chem., 61, 7316 (1996).
- 6 J. Bergman, A. Brynolft, Tetrahedron, 46, 1295 (1990).
- 7 I. K. Kacker, S. H. Zaheer, J. Ind. Chem. Soc., 28, 344 (1951).
- 8 J. F. Wolfe, T. L. Rathman, M. C. Sleevi, J. A. Campbell, T. D. Greenwood, J. Med. Chem., 33, 161 (1990).
- 9 W. M. Welch, F. E. Ewing, J. Huang, F. S. Menniti, M. J. Pagnozzy, K. Kelly, P. A. Seymour, V. Guanowsky, S. Guhan, M. R. Guimm, D. Critchett, J. Lazzaro, A. H. Ganong, K. M. DeVries, T. L. Staigers B. L. Chenard, Bioorg. Med. Chem. Lett., 11, 177 (2001).
- 10 A. G. A. Helby, M. H. A. Wahab, Acta Pharm., 53, 127 (2003).
- 11 B. R. Baker, R. E. Schaub, J. P. Joseph, F. J. McEvoy, J. E. Williams, J. Org. Chem., 17, 35, 52, 141, 149, 157, 164 (1952).
- 12 S. Kobayashi, M. Ueno, R. Suzuki, H. Ishitani, H.-S. Kim, Y. Wataya, J. Org. Chem., 64, 6833 (1999).
- 13 Y. Takaya, H. Tasaka, T. Chilba, K. Uwai, M.-A. Tanitsu, H.-S. Kim, Y. Wataya, M. Miura, M. Takeshita, Y. Oshima, J. Med. Chem., 42, 3163 (1999).
- 14 S. Uesato, Y. Kuroda, M. Kato, Y. Fujiwara, Y. Hase, T. Fujita, Chem. Pharm. Bull., 46, 1 (1998).
- 15 S. Kobayashi, M. Ueno, R. Suzuki, H. Ishitani, Tetrahedron Lett., 40, 2175 (1999).
- 16
St. von Niementowski,
J. Prakt. Chem.,
51,
564
(1895).
10.1002/prac.18950510150 Google Scholar
- 17 M. T. Bogert, A. H. Gotthelf, J. Am. Chem. Soc., 22, 522 (1900).
- 18
J. F. Meyer,
E. C. Wagner,
J. Org. Chem.,
8,
238
(1943).
10.1021/jo01191a005 Google Scholar
- 19 B. P. Bandgdar, Synth. Commun., 27, 2065 (1997).
- 20 E. M. Filachione, J. H. Lengel, C. H. Fisher, J. Am. Chem. Soc., 66, 494 (1944).
- 21 P. Reddy, P. P. Reddy, T. Vasantha, Heterocycles, 60, 183 (2003).
- 22 A. Witt, J. Bergman, Tetrahedron, 56, 7245 (2000).
- 23 Yu. M. Volovenko, O. V. Khilya, T. A. Volovenko, T. V. Shokol, Chem. Heterocyclic Compounds, 38, 314 (2002).
- 24 S. Kovalenko, I. Belenichev, V. Nikitin, A. Karpenko, Acta Pol. Pharm., 60, 4, 275 (2003).
- 25 M. T. Bogert, G. D. Beal, G. D. C. G. Amend, J. Am. Chem. Soc., 32, 1654 (1910).
- 26 Karamchand Premchand Privaet Ltd, Ger. Offent., 2114607; Chem. Abstr., 78, 4275 (1973).
- 27 B. L. Chenard, W. M. Welch Jr., M. K. Devries, PCT Int. Appl. WO. 9838, 187; Chem. Abstr., 129, 230734 (1998).
- 28 B. L. Chenard, W. M. Welch Jr., Eur. Pat. Appl. EP., 884, 316; Chem. Abstr., 130, 66508 (1999).
- 29 W. M. Welch Jr., M. K. Devries, PCT Int. Appl. WO., 98 38, 174; Chem. Abstr., 129, 230733 (1998).
- 30 B. L. Chenard, A. R. Reinhold, W. M. Welch Jr., Eur. Pat. Appl. EP., 884, 310; Chem. Abstr., 130, 66507 (1999).
- 31 B. L. Chenard, K. D. Shenk, Eur. Pat. Appl. EP., 934, 934; Chem. Abstr., 131, 144610 (1999).
- 32 E. C. Lawson, W. A. Kinney, M. J. Constanzo, W. J. Hoekstra, J. A. Kaufmann, D. K. Luci, R. Santulli, B. A. Tounge, S. C. Yabut, P. Andrade-Gordon, and B. E. Maryanoff, Lett. Drug Design & Discovery 1, 14 (2004).
- 33 G. Constantino, A. Macchiarulo, E. Camadoni,. R. Pellicciari, J. Med. Chem., 44, 3786 (2001).
- 34 G. Kulcsar, T. Kalai, E. Osz, C. P. Sar, J. Jeko, B. Sumegi, K. Hideg, ARKIVOC, 121 (2003).
- 35 A. Ogiso, H. Tsukahara, T. Nishimoto, T. Misawa, K. Takuma, Japan. Pat. 280620 (2000) Chem. Abstr. 133, P288936v (2000).
- 36 S. M. Bakalova, A. G. Santos, I. Timcheva, J. Kaneti, I. L. Filipova, G. M. Dobrikov, V. D. Dimitrov, J. Mol. Structure (Theochem), 710, 229 (2004).
- 37 T. P. Murray, J. V. Hay, D. E. Protlock, J. F. Wolfe, J. Org. Chem., 39, 595 (1974).
- 38 K. Smith, G. A. El-Hiti, M. F. Abdel-Megeed, M. A. Abdo, Collect. Czech. Chem. Commun., 64, 515 (1999).