Molecular aspects of cholangiocarcinoma
Abstract
Novel targets for therapeutic or chemopreventive approaches against cholangiocarcinoma (CCA) are urgently needed. In this review article, we discuss the molecular aspects of CCA including the role of erbB receptor tyrosine kinases (RTKs), downstream signaling pathways of these erbB RTKs, inflammatory mediators during gallbladder carcinogenesis and bile acids based on our study using a mouse model for human CCA (BK5.erbB2 mice) as well as additional information in the literature.
Introduction
Cholangiocarcinoma (CCA), which includes gallbladder carcinomas (GBCs) and cancers of the intra- and extrahepatic biliary tree, are relatively infrequent but highly lethal malignancies 1. Although there have been advances in the diagnosis and management of CCA, these cancers still prove challenging to treat due to their insensitivity to conventional therapies and the inability to detect early tumor formation. These factors render GBC nearly incurable with a 5-year survival rate of only 5–21% 2-6. Novel targets for therapeutic or chemopreventive approaches are urgently needed.
To date, very few studies have addressed the molecular and cellular mechanisms underlying the development of CCA. We previously generated transgenic mice that overexpress wild type rat erbB2 in epithelial tissues under the control of the bovine keratin 5 (BK5) promoter (BK5.erbB2 mice) 7. Overexpression of the erbB2 in basal epithelial cells of the gallbladder led to the development of adenocarcinoma of the gallbladder and cystic duct in 90% of these transgenic mice. This was the first direct demonstration that erbB2 overexpression could lead to the development of CCA 7. We have shown that BK5.erbB2 mice are a valid model for investigating mechanisms underlying development of GBCs and other CCAs.
In this article, we discuss the molecular aspect of CCA including the role of erbB receptor tyrosine kinases (RTKs), downstream signaling pathways of these erbB RTKs, inflammatory mediators during gallbladder carcinogenesis and bile acids based on our study using BK5.erbB2 mice as well as additional information in the literature (review in 8, 9). Understanding the signaling pathways altered in CCA will provide critical clues for novel therapeutic and chemopreventive strategies using drugs and/or agents that selectively target these specific pathways.
Role of ErbB RTKs and their downstream signaling pathways in the development of CCA
EGFR and erbB2 in CCA
Upregulation of the RTK, erbB2, contributes to tumorigenesis in a number of tissues, however, little is known about its role in CCA including GBC. ErbB2 overexpression has been reported in a significant percentage of human CCA 6, 10-20. Similarly, overexpression and activation of epidermal growth factor receptor (EGFR) has been reported in 30–60% of CCA samples 11, 12, 21, 22.
We previously reported BK5.erbB2 transgenic mice, in which erbB2 was targeted to overexpress in epithelial tissues, developed spontaneous GBC and intrahepatic CCA with incidences of approximately 90 and 30%, respectively, by 2 months of age (Fig. 1b) 7. Figure 1a shows the cDNA construct of BK5.erbB2 mouse. The majority of the gallbladder tumors completely filled the lumen (Fig. 1c) 7. Most of the tumors were diagnosed as well-differentiated adenocarcinomas. Carcinoma cells frequently invaded into the surrounding connective tissues. Microscopic analysis of H&E-stained serial sections showed that tumor cells of the common bile duct had invaded into the pancreatic duct (Fig. 1d) 7. In addition, the ampulla of Vater was dilated, and hyperplasia of the epithelium was observed in transgenic mice (Fig. 1e) 7. Pronounced congestion of bile, inflammation, necrosis, hyperplasia of biliary duct cells, and/or tumor development (CCA) was also observed frequently in intrahepatic biliary ducts of transgenic mice (Fig. 1f) 7. The localization of transgene expression in the biliary tract was determined by indirect immunofluorescence staining. Persistent expression of the transgene was observed in both the epithelium and tumor lesion from gallbladder (Fig. 1g) and intrahepatic biliary ducts (data not shown) of BK5.erbB2 mice. Endogenous erbB2 expression was only weakly detectable in both the intrahepatic biliary duct (data not shown) and gall bladder from nontransgenic mice (Fig. 1h).
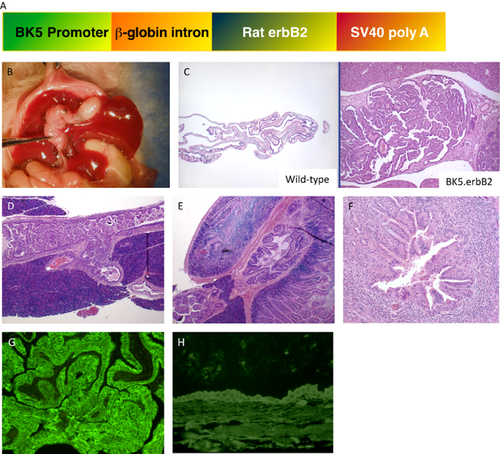
(a) DNA construct used to generate BK5.erbB2 mice. (b) Gross appearance and histological evaluations of cholangiocarcinoma (CCA) in BK5.erbB2 mice at 3 months of age. (c) H&E staining of gallbladder in wild type mouse and (left) BK5.erbB2 mouse (right)). (d) The junction of the pancreatico-biliary duct (JPBD) in a 3-month-old BK5.erbB2 mouse. (e) H&E staining of the ampulla of Vater. (f) Intrahepatic CCA from a 3-month-old BK5.erbB2 mouse. (g and h) Expression of erbB2: Immunostaining for erbB2 in gallbladder from BK5.erbB2 (g) and wild type (h) of 3-month-old mouse. Figures are adopted from Kiguchi et al. 2001 7
Western blot analysis of gallbladder tissue lysates showed that the level of erbB2 protein was significantly elevated in BK5.erbB2 mice compared to that of wild type mice, as expected (Fig. 2) 7, 9. ErbB2 was also hyperphosphorylated (p-erbB2) after adjustment for total erbB2 protein level (Fig. 2). Interestingly, the level of EGFR protein (but not erbB3 or erbB4 protein) was elevated and hyperphosphorylated on tyrosine residues in gallbladder tissue from BK5.erbB2 mice 7. Additional analyses by immunoprecipitation of EGFR and erbB2 followed by Western blot analysis for erbB2 and EGFR, respectively, confirmed elevated heterodimer formation between erbB2 and EGFR in gallbladder tissue of BK5.erbB2 mice 7.
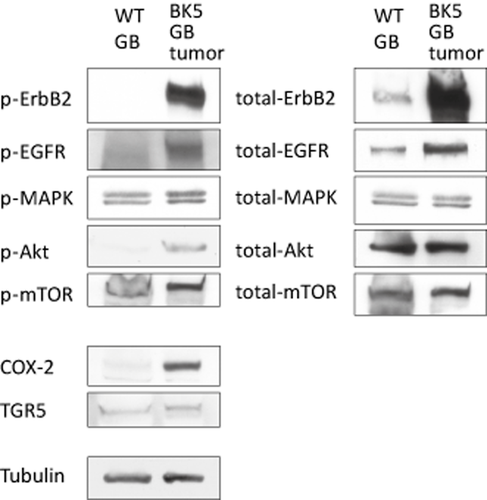
Analysis of protein expression and kinase activation in gallbladder from wild type and BK5.erbB2 mice. Whole tissue lysates from wild type and BK5.erbB2 mice were analyzed via Western blot with antibodies against indicated molecules. Tubulin was used as an internal control. Figures are adopted from Kiguchi and DiGiovanni 2014 9
Administration of the selective erbB2/EGFR inhibitor, GW2974, showed a potent chemopreventive and therapeutic effect on GBC in this model 23. Figure 3 shows the therapeutic effect of GW2974 24. Treatment with GW2974 resulted in a significant decrease in the incidence of GBC to 3% (Fig. 3a) 23. These reductions corresponded to a 95% decrease in tumor incidence compared with BK5.erbB2 mice receiving the control diet, which had a GBC incidence of 72% as determined by histopathological examination. The impact of treatment is evident in the ultrasound images in Figure 3b 23. H&E staining in the right panels clearly show the dramatic regression of the tumor with only hyperplasia remaining (Fig. 3b–d). Treatment with GW2974 resulted in decreased levels of both erbB2 and EGFR. Levels of p-erbB2 and p-EGFR were also markedly reduced 23. Nearly complete inhibition of tumor development by GW2974 suggests a level of erbB2 dependency during gallbladder tumor development in BK5.erbB2 mice.
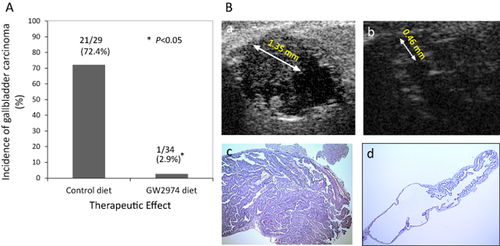
Therapeutic effect of tyrosine kinase inhibitor, GW2974, on the incidence of gallbladder carcinoma (GBC) in BK5.erbB2 mice
(a) Incidence of GBC in BK5. erbB2 mice. Two month-old BK5 erbB2 mice were treated with AIN76 control diet or AIN76 diet containing 200 ppm GW2974 for one month. *, P < 0.01 B. Effects of GW2974 detected by ultrasound and (b) histologic analyses. Regression of GBC by GW2974 treatment as detected by ultrasound biomicroscopy. All images are from a single animal depicting the response representative of the treatment group. (a) Ultrasound image of GBC (maximum size: 1.35 mm) before GW2974 treatment. (b) Ultrasound image of gallbladder on the 23rd day of treatment; this image indicates regression of the carcinoma (observed size: 0.46 mm). (c) Gallbladder of BK5.erbB2 mouse receiving AIN76 control diet, (d) Typical histologic features of the gallbladder from BK5.erbB2 mice treated with AIN76 diet containing 200 ppm GW2974. Figures are adopted from Kiguchi et al. 2005 23
Furthermore, we recently reported that a histone deacetylase inhibitor (HDAC), PCI-24781 showed a potent therapeutic effect against GBC in these mice by reducing erbB2 expression 25. In this study, PCI-24781 (50 mg/kg per day, i.p. injection twice a day) was delivered to BK5.erbB2 mice for 4 weeks. Each group of mice was screened via the ultrasound image to ensure they had GBCs when the treatment started. Treatment of BK5.erbB2 mice with PCI-24781 for one month prevented 79% of GBC cases from progression and showed a clinical effect in 47% of cases (Fig. 4a) 25. We also confirmed the evidence for a potent inhibitory effect on tumor cell growth in human CCA cell lines treated with PCI-24781. In both in vitro and in vivo models, this effect was associated with downregulation of erbB2 mRNA and erbB2 protein/activity and upregulation of acetylated histone and acetylated tubulin (Fig. 4b,c) 25. These results indicate that the significant therapeutic/inhibitory effect that this HDAC has on the development of gallbladder tumors is due to its ability to block the activation of erbB2.
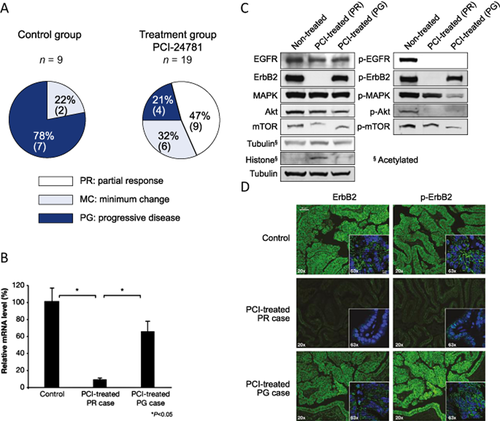
Effect of PCI-24781
(a) Therapeutic evaluation of PCI-24781 on GBC in BK5.erbB2 mice. MC Minimum Change, PG Progressive Disease, PR Partial Response
(b) Relative ErbB2 mRNA levels in gallbladders treated with PCI-24781
(c) Western blot analysis of the levels of erbB2/EGFR and their downstream molecules in gallbladders from non-treated BK5.erbB2 mice, treated gallbladders diagnosed as PR, and treated gallbladders diagnosed as PG
(d) ErbB2 and p-erbB2 expression in gallbladders from non-treated BK5.erbB2 mice, treated gallbladders diagnosed as PR, and treated gallbladders diagnosed as PG (scale bar, 40 lm). Figures are adopted from Kitamura et al. 2012 25
In addition, erbB2 has been shown to be overexpressed in the neoplastic glandular epithelium of furan- and thioacetamide-induced intestinal-type CCAs in rat liver 26, 27. It has also been reported that erbB2 transfection transformed cultured rat cholangiocytes. These transformed cholangiocytes were tumorigenic when transplanted into isogenic rats, yielding a 100% incidence of CCAs 28. Collectively, these data suggest that altered expression and activity of erbB2 and EGFR are major mechanisms underlying human CCA carcinogenesis (reviewed in 8).
Downstream signaling of EGFR/erbB2 in the GBC from BK5.erbB2 mice
The activation status of signaling molecules downstream of erbB2/EGFR in the GBC from BK5.erbB2 mice was also examined. Although total protein levels of MAPK were not changed (Fig. 2), the level of p-MAPK was increased in the gallbladder of transgenic mice. Furthermore, p-Akt, but not total Akt level, was elevated in the gallbladder of BK5.erbB2 mice (Fig. 2). We have reported that mTOR and other signaling molecules both immediately upstream (Akt, MAPK) and downstream (p70S6K) of mTOR are hyperphosphorylated in gallbladder tissues from BK5.erbB2 mice compared to corresponding tissue from wild type mice (Fig. 3 and 8, 29). Significant reduction of the incidence and severity of GBCs was observed in BK5.erbB2 mice treated with rapamycin by i.p. injection (5 mg/kg BW, once daily for 14 days) in a dose-dependent manner 29. Tumors responsive to treatment exhibited a higher number of apoptotic cells. Although rapamycin treatment didn't change the level of p-mTOR (Ser2448) but led to decreased p70 S6 kinase (Thr389) in gallbladder tissue, but as assessed by both Western blot (Fig. 5a,b) 29 and immunofluorescence analyses 29. Immunofluorescence staining of p-Akt and p-mTOR were done in 27 human gallbladder adenocarcinoma (stage IIB diagnosed by the criteria of the American Joint Committee on Cancer) and seven normal human gallbladders. The results showed that elevated levels of p-Akt were observed in 74.1% (20 of 27) of human GBC specimens and in only 28.6% (2 of 7) of normal human gallbladder specimens. Elevated levels of p-mTOR also were observed in 92.6% (25 of 27) of human GBC specimens and in only 28.6% (2 of 7) of normal human gallbladder specimens 29. These data reveal that human gallbladder adenocarcinoma has elevated p-Akt and p-mTOR levels compared with noncancerous tissue. Representative images of immunofluorescence staining are shown in Figure 5c 29. Rapamycin and related drugs may be effective therapeutic agents for the treatment of human GBC with activated Akt/mTOR pathway 29.
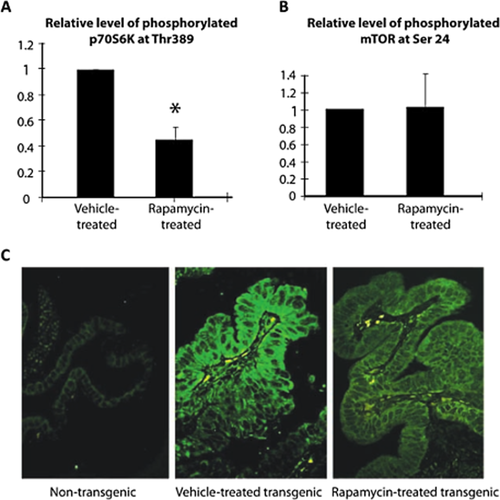
Levels of p-p70S6K and p-mTOR in the gallbladders of BK5.erbB2 mice treated with vehicle or 5 mg/kg rapamycin
(a) Western blot analysis for p-Thr389 p70S6K, total p70S6K, and h-tubulin and relative level of p-p70S6K to total p70S6K in vehicle-treated or rapamycin-treated gallbladders. (b) Western blot analysis for p-mTOR, total mTOR, and h-tubulin and quantitative level of p-mTOR to total mTOR in vehicle-treated or rapamycin-treated gallbladders. (c) Immunofluorescence staining of p-p70S6K in gallbladder tissue of untreated non-transgenic mice, vehicle-treated, and rapamycin-treated (5 mg/kg) BK5.erbB2 mice. Figures are adopted from Wu et al. 2007 29
Interestingly, the therapeutic effect of HDAC inhibitor, PCI-24781 on the GBC developed in BK5.erbB2 mice was associated with a significant reduction in the levels of total erbB2, p-erbB2, p-EGFR (not total EGFR), and the phosphorylated forms of their downstream signaling molecules p-MAPK, p-Akt, and p-mTOR compared to those of gallbladders from non-treated BK5.erbB2 mice (Fig. 4) 25.
These results indicate that cellular response to the upregulation of EGFR/erbB2 is mediated by both Akt signaling and other downstream signaling pathways including Raf/MAPK signaling.
Role of COX-2 in CCA
Accumulating evidence suggests that COX-2, an inducible enzyme responsible for conversion of arachidonic acid to prostaglandins, may play a variety of roles in the gastrointestinal tract including pathogenic processes such as neoplasia 30. Sirica's group reported a strong positive correlation between the immunostaining intensities of erbB2 and COX-2 in CCA. COX-2 was observed not only in the furan-rat CCA model, but also in human CCA 31, supporting the possibility that erbB2 plays a key role in regulating COX-2 expression in neoplastic and precancerous biliary tract epithelial cells. Grossman et al. reported a specific COX-2 inhibitor, but not COX-1 inhibitor, decreased mitogenesis and increased human gallbladder cell apoptosis associated with decreased prostaglandin E2 (PGE2) 32. This suggests that the COX enzymes and the prostanoids may play a role in the development of CCA and that COX-2 inhibitors may have a therapeutic role in CCA 32.
The protein level (determined by immunohistochemistry and Western blot) and mRNA expression (determined by RT-PCR) of COX-2 were significantly elevated in the gallbladder tissue of BK5.erbB2 mice compared to wild type mice (Fig. 3) 7, 33. The level of PGE2 was also found to be elevated in the tissue 33. We also examined the effects of a COX-2 inhibitor, CS-706 on the development of GBCs using the BK5.erbB-2 mouse model. Ultrasound image analysis as well as histologic evaluation revealed a significant therapeutic effect of CS-706 on the GBCs, either as reversion to a milder phenotype or inhibition of tumor progression (Fig. 6) 33. The antitumor effect was associated with inhibition of PGE2 synthesis 33. CS-706 treatment also downregulated the activation of ErbB-2 and EGFR, resulting in decreased levels of phosphorylated Akt and COX-2 in GBCs of BK5.erbB2 mice (Fig. 6) 33.
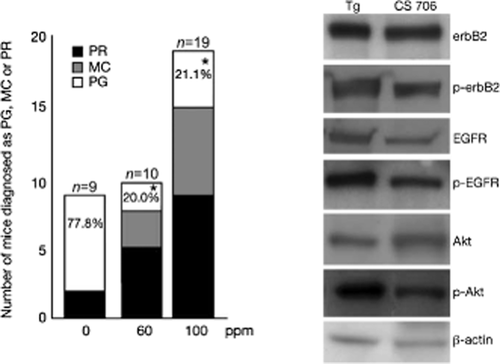
Left panel. Therapeutic effect of CS-706 on gallbladder carcinoma in BK5.erbB-2 mice. Mice were treated with AIN76A control diet (left column), AIN76A diet containing 60 ppm CS-706 (middle column), and AIN76A diet containing 100 ppm CS-706 (right column) for 1 mo. *, P < 0.01. n, total number of mice determined. %, percentage of mice diagnosed as PG. Figures are adopted from Kiguchi et al. 2007 33
Right panel. Western blot analysis of ErbB-2, p-ErbB-2, EGFR, p-EGFR, Akt, and phosphorylated Akt (p-Akt) levels in the gallbladders of BK5.erbB-2 mice on control diet and BK5.erbB-2 mice treated with 100 ppm CS-706
Epithelial cell lysates were prepared from gallbladder tumors from five BK5.erbB-2 mice, and gallbladders from BK5.erbB-2 mice treated with CS-706 were pooled. Figures are adopted from Kiguchi et al. 2007 33
Based on our results, targeting COX-2 could provide a potentially new and effective therapy alone or in combination with other therapeutic agents for patients with CCA. These results suggest that elevated COX-2 may play an important role in the development of CCA in BK5.erbB2 mice.
Role of bile acids in CCA
Exposure to high levels or abnormal composition of bile acid is associated with an increased incidence of cancer of the laryngopharyngeal tract, esophagus, stomach, pancreas, the small intestine, and colon 34. It has been reported that bile acids activate EGFR, MAPK, and PI3-K/Akt signaling pathways in hepatocytes 35, 36. More recent evidence suggests that bile acids may activate RTKs and downstream signaling molecules, indirectly, in a G protein-coupled receptor (GPCR) dependent manner 37 mediated by ADAM family peptidases 38, 39. The role of cell signaling by these organic acids in the development of human CCA remains unknown. A recent study from our laboratory (Kitamura et al. 2013, accepted for publication) demonstrated that the secondary conjugated bile acid, taurochenodeoxycholic acid (TCDC), increased proliferation of primary cultured gallbladder epithelial cells from BK5.erbB2 mice and human CCA cells. TCDC treatment activated erbB2/EGFR and downstream signaling molecules in both primary cultured cells and human CCA cells. TCDC also increased the expression of EGFR ligands and tumor necrosis factor-alpha converting enzyme (TACE) activity in human CCA cells. These results suggest that during the development of CCA, bile acid may act as a promoter when erbB2 is activated in gallbladder epithelial cells.
Role of siRNA in CCA
A miRNA profile revealed that the expression of several miRNAs was significantly altered in GBC in the BK5.erbB2 mice compared with wild type mice, including upregulated miR21, which was downregulated by HDAC inhibitor, PCI-24781 25. Nine miRNAs were significantly upregulated and 13 miRNAs were downregulated in GBC in BK5.erbB2 mice (>2.2-fold, P < 0.05) compared to gallbladders in control mice (Table 1). Treatment with PCI-24781 significantly decreased the expression of some of these miRNAs, including miR-21, miR-142-3p, miR-142-5p, and miR-223, which were upregulated in GBC (Tables 1, 2). PCI-24781 also induced a significant upregulation in the expression of miR-122, which was downregulated in GBC (Tables 1, 2). Since miR-21 has been characterized as an antiapoptotic miRNA and is upregulated in many cancer types including CCA cells 40, 41, miR-21 may also play an important role in the development of GBC in BK5.erbB2 mice. Our results indicate that downregulation of miR-122 may also be a marker of GBC. Interestingly, we found miR-101 was significantly upregulated in GBC treated with PCI-24781. It has been reported that both the expression and function of the enhancer of Zeste homolog 2 (EZH2) in cancer cell lines is inhibited by miR-101 42. These studies suggest PCI-24781-induced miR-101 may regulate the expression of both EZH2 in GBC. We postulate that the posttranscriptional effect of HDAC inhibition on these miRNAs may play an important role in the anticancer activity of PCI-24781.
| Downregulated | Upregulated | ||
|---|---|---|---|
| miR-665 | −8.233 | miR-106a | 2.414 |
| miR-714 | −3.882 | miR-96 | 2.499 |
| miR-763 | −2.880 | miR-223 | 2.612 |
| miR-466f-3p | −2.750 | miR-27a | 2.627 |
| miR-145 | −2.612 | miR-17 | 2.700 |
| miR-193 | −2.588 | miR-15b | 2.793 |
| miR-467e* | −2.511 | miR-142-5p | 2.914 |
| miR-143 | −2.337 | miR-142-3p | 3.582 |
| miR-881* | −2.323 | miR-21 | 5.361 |
| miR-720 | −2.311 | ||
| miR-706 | −2.303 | ||
| miR-122 | −2.255 | ||
| miR-378 | −2.241 | ||
| Downregulated | Upregulated | ||
|---|---|---|---|
| miR-223 | −8.642 ± 2.539 | mmu-miR-193 | 2.273 ± 0.214 |
| mmu-miR-21 | −4.509 ± 0.554 | mmu-miR-101b | 2.348 ± 0.221 |
| mmu-miR-142-3p | −4.360 ± 0.920 | hsa-miR-576-3p | 2.600 ± 0.200 |
| mmu-miR-142-5p | −4.417 ± 0.975 | mmu-miR-451 | 2.652 + 0.251 |
| mmu-miR-205 | −3.702 ± 1.195 | hsa-miR-620 | 3.335 ± 0.315 |
| mmu-miR-141 | −2.189 ± 0.208 | hsa-miR-122* | 11.141 ± 1.031 |
| hsa-miRPlus-B1114 | −2.219 ± 0.121 | mmu-miR-122 | 29.204 ± 14.189 |
Conclusion and future direction
The dismal outcomes that generally result from gallbladder carcinoma and other CCAs explain the pessimism that surrounds treatment of these cancers. Nevertheless, more aggressive surgical techniques and advanced oncologic radiation therapy have led many institutions to report an increase in long-term survival rates. Although these treatments are progressive, the efforts directed toward early detection and novel treatment derived from basic research to determine the mechanisms involved in CCA development may play a key role in improvement of patients' survival. Proposed inhibitory effects of each therapeutic compound on the signaling pathways in GBC of BK5.erbB2 mice are shown in Figure 7.
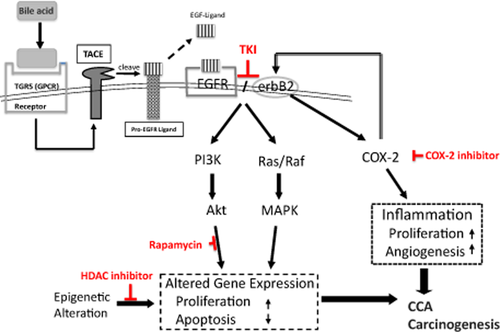
Proposed pathway in which erbB2/EGFR, COX-2, and bile acid may play a role during cholangiocarcinoma (CCA) carcinogenesis. ErbB2 overexpression/activation may accelerate the transactivation cascade of during the development of CCA
New drugs that selectively target specific augmented molecule(s) such as erbB2, COX-2, mTOR, miRNA, and HDAC in CCA may serve as potentially effective adjunct therapeutic strategies for this cancer, for which there is currently no effective medical treatment. In addition, identification of novel candidate gene(s) or protein(s), which regulate these mechanisms, may provide not only potential therapeutic targets, but also novel tumor markers for this lethal disease. The BK5.erbB2 transgenic mouse model provides a unique opportunity to study the mechanisms involved in the development of this cancer.
Acknowledgments
The authors would like to thank Dr Takuya Kitamura (Hoshi General Hospital, Fukushima, Japan), Dr Toru Kawamoto (Tokyo Women's Medical University, Tokyo, Japan), Tetsuo Ajiki (Kobe University School of Medicine, Kobe, Japan), Dr Kevin Connolly (University Medical Center at Brackenridge, Austin, Texas, USA) and Dr John DiGiovanni (College of Pharmacy, University of Texas, Austin, TX, USA) for their contributions and support.
Conflict of interest
None declared.




