Outcomes of pancreaticoduodenectomy in patients with chronic hepatic dysfunction including liver cirrhosis: results of a retrospective multicenter study by the Japanese Society of Hepato-Biliary-Pancreatic Surgery
Abstract
Background
Since there is no reliable evidence on the safety of pancreaticoduodenectomy (PD) in chronic hepatic dysfunction (CHD) including liver cirrhosis (LC), the effects of CHD on patients undergoing PD were investigated.
Methods
This multi-institutional retrospective study analyzed 529 patients with CHD, including 105 patients diagnosed with LC, who underwent PD at 82 high-volume institutions between 2004 and 2013.
Results
The in-hospital mortality rate was 5.9%. The incidence of postoperative hepatic decompensation upon discharge and refractory ascites was 10.2% and 8.9%, respectively. For hepatic decompensation, the serum aspartate aminotransferase (AST) of more than 50 IU/l and portal hypertension (PHT) were independent significant risk factors. For refractory ascites, prothrombin activity of <70%, serum AST of more than 50 IU/l and advanced PHT with collaterals were significant risk factors. Five-year overall survival was 57.8% in Child A and 24.8% in Child B patients (P < 0.0001). The Child B/C patients were divided into two groups according to an AST-platelet ratio index (APRI) of 1.0; the APRI of <1.0 yielded a significantly higher survival rate than their counterpart (43.2% vs. 14.7%, P = 0.04).
Conclusions
In addition to PHT, pre-operative evaluation of AST and APRI may be helpful for patient selection for PD in patients with CHD.
Introduction
Poorly compensated chronic hepatic dysfunction (CHD) or liver cirrhosis (LC) has been recognized as a high-risk complication for surgical procedures, because perioperative morbidity and mortality have been observed to be much higher in patients with CHD or LC than in controls 1-4. The outcomes of surgery in patients with LC are also known to differ according to the degree of liver damage and the invasiveness of the type of surgery performed. Laparoscopic cholecystectomy and inguinal hernia repair are less invasive procedures, while intraperitoneal organ surgery for tumors and cardiovascular surgery are considered to be high-risk; the reported mortality for each of these surgeries, respectively, is 0%, 2.5%, 35%, and 80% 5, 6. When analyzed according to the Child–Pugh classification, mortality in major abdominal surgery was reported to be 10%, 30% and 76–82% for Child class A, B and C, respectively 7.
For patients with malignant diseases of the periampullary region or pancreatic cancer, pancreaticoduodenectomy (PD) is the only curative procedure, which is one of the major and complex abdominal surgical procedures. Due to improvements in surgical techniques and perioperative management, PD can be performed safely at high-volume centers with a postoperative mortality rate of <3% 8, while the postoperative morbidity rate ranges from 20% to 60% 9-11. Traditionally, LC has been considered as a contraindication for pancreatic surgery 12. At present, only a few studies with small sample sizes have been published regarding the outcomes of PD in patients with LC 13-16. These studies demonstrated that the Child–Pugh classification was a prognostic factor of mortality and morbidity, which have been reported as 4% and 79%, respectively, in Child class A, and 55% and 100% respectively, in Child class B 13. Those recent studies suggest that LC should not be considered as an absolute contraindication to PD, particularly in patients with Child–Pugh A cirrhosis 13-18. However, it remains uncertain whether or not the Child–Pugh classification is sufficient to assess the risk of PD, and currently, there are no other prognostic or predictive factors other than the Child–Pugh score. Because no reliable evidence on the safety of PD in patients with compensated CHD or LC is available so far, the current indication for PD differs significantly depending on the experience of the surgeon and the institution.
Portal hypertension (PHT) is associated with intraoperative bleeding and blood transfusion 19, while postoperative pancreatic fistula (POPF) has been reported to be more common in patients with LC 14. In addition to the risk of increasing perioperative complications specific to PD, there may also be risks of severe LC-related complications such as postoperative hepatic decompensation including refractory ascites.
Furthermore, patients’ prognosis, which is potentially worsened by LC-related events, should be considered for the indication of PD in patients with CHD in the middle to long term.
The aim of this multi-institutional retrospective study was to identify the effect of CHD on morbidity and mortality in patients undergoing PD.
Methods
Eighty-two of 210 institutions (39.0%) certified by the Japanese Society of Hepato-Biliary-Pancreatic Surgery participated in this study, which was conducted in 2015. A total of 16,584 patients had undergone PD among the 82 registered institutions for various indications between 2004 and 2013. Among these patients, the clinical data of 532 patients (3.2%) with CHD, including patients diagnosed with LC, were registered. A data sheet was used to collect data and it was designed to examine various pre-, intra-, and postoperative variables. CHD was diagnosed at each institution based on clinical data, imaging studies, intraoperative findings, and/or histopathological diagnosis. All data confirmed the validity of diagnosis of CHD using preoperative data including etiology of CHD, pathological diagnosis, laboratory data, image studies, and endoscopic findings. Of the total cases, three were omitted due to incomplete data. This retrospective study was approved first by the institutional review board of the Jikei University School of Medicine (approval number: 26-351) and at the ethics committee of each institution.
Definition of chronic hepatic dysfunction in this study
Chronic hepatic dysfunction in the current study was defined as one or more of the following three patterns of diagnoses because hepatic function correlates well with the degrees of liver fibrosis 20, 21: (1) pre-operative clinical diagnosis of LC or chronic hepatitis based on clinical course, physical findings, laboratory data and imaging studies; (2) intraoperative clinical diagnosis of LC or chronic hepatitis based on macroscopic findings in the liver; or (3) pathological diagnosis of LC or chronic hepatitis based on liver specimens.
Classification of postoperative complications
Postoperative pancreatic fistula was defined according to the guidelines of the International Study Group on Pancreatic Fistula 22. Delayed gastric emptying (DGE) was graded according to the International Study Group of Pancreatic Surgery consensus definition 23. Postoperative hepatic dysfunction was graded according to grading of post-hepatectomy liver failure by the International Study Group of Surgery 24. Other morbidities were graded according to the Clavien-Dindo classification 25, and adverse events of grade IIIA or more were defined as morbidity.
Assessments
The primary endpoints were the incidence of postoperative hepatic decompensation and in-hospital mortality following PD. The secondary endpoints were the incidence of postoperative deterioration of hepatic function on discharge, morbidity including refractory ascites and long-term patient survival. The associated factors of hepatic decompensation or deterioration of hepatic function, and prognostic factors were also investigated.
Definition of terms and cutoff value of variables
Hepatic decompensation
Hepatic decompensation is a series of symptoms associated with declining liver function. In the current study, the following clinical findings were evaluated at the time of discharge after PD: refractory ascites, hepatic encephalopathy (grade 1 or higher) associated with hyperammonemia or jaundice (total bilirubin [T-Bil] 2.0 mg/dl or higher in the absence of obstructive jaundice) or Child–Pugh class C.
Refractory ascites
Refractory ascites refers to the following postoperative statuses: (1) uncontrolled ascites at time of discharge, and (2) a peritoneovenous shunt made during the hospitalization period in which PD was performed.
Deterioration of hepatic function
Deterioration of hepatic function was considered to be a shift from a Child–Pugh class A to B, class A to C, or class B to C following PD. Patients whose pre-operative serum T-Bil levels were 2.0 mg/dl or higher were excluded from the analyses regarding deterioration of hepatic function, because pre-operative Child–Pugh score could be variable according to elevated serum T-Bil caused by obstructive jaundice.
Portal hypertension
Portal hypertension in the current study was defined as one or more of the following two patterns of diagnoses: (1) pre-operative clinical diagnosis of gastroesophageal varices or portal hypertensive gastropathy based on endoscopic findings; (2) pre-operative clinical diagnosis of splenomegaly with a diameter of 12 cm or more, ascites, and collateral pathways formation based on imaging.
Aspartate aminotransferase-platelet ratio index
The aspartate aminotransferase-platelet ratio index (APRI): aspartate aminotransferase (AST) level (IU/l)/AST (upper limit of normal; set at 30 in the present study) (IU/l)/platelet count (109/L) × 100) has been shown to be a simple and useful indicator of hepatic fibrosis or cirrhosis 26, 27, which was applied in the present study. A cutoff value of APRI in the present study was set at 1.0.
Cutoff value for platelets
Significant hepatic fibrosis can be observed when the platelet count is approximately 150,000/μl or below, and platelet count is a strong indicator of hepatic cirrhosis when it is 100,000/μl or below 28. Also, the cutoff value of platelet for fibrosis related to hepatic cirrhosis differs depending on the type of underlying chronic hepatitis, 127,000/μl or below for hepatitis C, and 160,000/μl or below for non-alcoholic fatty liver disease 29. Therefore, a cutoff value of platelets count in the present study was set at 150,000/μl referring a receiver operating characteristic curve (ROC) for the association of hepatic decompensation.
Determination of cutoff value of variables
Some clinical continuous variables were classified into two groups for the logistic regression models and the Cox proportional hazard models, as follows: T-Bil, <2.0 or ≥2.0 mg/dl, according to a previous study 30 and consideration of Child–Pugh classification; hemoglobin, <10.0 or ≥10.0 g/dl, according to previous studies 6, 30: and albumin, <4.0 or ≥4.0 g/dl, prothrombin time, <70.0 or ≥70.0%, based on the normal limit. Cutoff values of AST, intraoperative blood loss, and platelets were determined referring a ROC analysis for the association of hepatic decompensation, which were 50 IU/l, 1,500 ml, and 150,000/μl, respectively. The cutoff value of operative time was determined by the median.
Statistical analysis
Results are presented as medians with interquartile ranges. Continuous variables were compared between study groups using the Student's t-test for normally distributed data and the Mann–Whitney U-test for non-normally distributed data. Categorical data were compared using the χ2 test and Fisher's exact test, as appropriate. Univariate analysis of risk factors for deterioration of hepatic function or hepatic decompensation was performed in relation to the following pre-operative and perioperative variables using a logistic regression model. Significant prognostic factors, which were revealed by univariate analysis, were entered into a multivariate regression model.
Kaplan–Meier survival curve estimates and log-rank tests were used to compare survival rates. Cox proportional hazards analyses were performed to estimate risk factors for survival. All analyses for survival were limited within the cases, in which curative operation was performed (R0). All P-values were two-sided, and P < 0.05 was considered statistically significant. All analyses were performed using Stata Statistical Software (Version 12.0; StataCorp LP, College Station, TX, USA).
Results
Clinical characteristics of patients with CHD
The characteristics of 529 patients registered in the current study are summarized in Table 1. A total of 424 patients (80%) were male and the median age was 70 years. Pancreatic cancer (40.3%) was the most predominant indication for PD. One hundred and five patients (19.8%) had a clinical diagnosis of LC. Pre-operative biliary drainage was performed in 43.7% of the patients. For reference of patient background in the present study, mortality and morbidity from two studies, in which the frequency of post-PD complications was summarized using all registered cases of the Japanese National Clinical Database (NCD), were cited, and the perioperative parameters that can be compared with the present study are listed in Table S1 30, 31.
| n = 529 | |
|---|---|
| Pre-operative factors | |
| Age (years) | 70 [63–75] |
| Gender (male) | 424 (80.2%) |
| Etiology of chronic hepatitis | |
| HCV | 292 (55.2%) |
| HBV | 89 (16.8%) |
| Alcoholic | 65 (12.3%) |
| NASH | 16 (3.0%) |
| Others | 67 (12.7%) |
| Indication | |
| Pancreatic cancer | 213 (40.3%) |
| Bile duct cancer | 87 (16.4%) |
| IPMA/IPMC | 83 (15.7%) |
| Ampulla of Vater cancer | 72 (13.6%) |
| Duodenal cancer | 17 (3.2%) |
| Neuroendocrine tumor | 15 (2.8%) |
| Others | 42 (7.9%) |
| Comorbidity | |
| HCC (history of treatment) | 48 (9.1%) |
| Old myocardial infarction/Angina pectoris | 29 (5.5%) |
| Respiratory disorders | 53 (10.0%) |
| Diabetes mellitus | 154 (29.1%) |
| Clinical diagnosis of liver cirrhosis | 105 (19.8%) |
| WBC (/μl) | 5,000 [4,100–6,100] |
| Hb (g/dl) | 12.3 [11.2–13.5] |
| PLT (×103/μl) | 178 [138–230] |
| AST (IU/l) | 32 [23–53] |
| ALT (IU/l) | 31 [18–59.5] |
| T-Bil (mg/dl) | 0.8 [0.6–1.4] |
| Serum creatinine (mg/dl) | 0.7 [0.6–0.83] |
| Serum sodium (mEq/l) | 140 [138–142] |
| Albumin (g/dl) | 3.8 [3.4–4.2] |
| Total cholesterol (mg/dl) | 161 [137–189] |
| Triglyceride (mg/dl) | 100 [70–142] |
| Choline esterase (mU/ml) | 217 [169–272] |
| CRP (mg/dl) | 0.15 [0.05–0.54] |
| HbA1c (%) | 6.0 [5.4–6.9] |
| PT (%) | 91 [77–101] |
| PT-INR | 1.04 [0.98–1.13] |
| Blood ammonia (μg/dl) | 40 [26–60] |
| ICG-R15 (%) | 13.5 [9–20.9] |
| AT-III (%) | 91.4 [61–104.3] |
| Encephalopathy | 2 (0.4%) |
| Preoperative biliary drainage | 231 (43.7%) |
| Varices | 31 (5.9%) |
| Splenomegaly | 32 (6.9%) |
| Ascites | |
| Slight | 19 (3.6%) |
| Mild | 1 (0.2%) |
| Collateral channels | 29 (5.5%) |
| NACRT | 29 (5.5%) |
| Surgical factors | |
| Operative time (min) | 500 [408–594] |
| Blood loss (ml) | 911 [150–1,586] |
| Operative procedure | |
| PD | 116 (21.9%) |
| SSPPD | 200 (37.8%) |
| PPPD | 212 (40.1%) |
| Lymph node dissection (D2 or D2 + α) | 419 (79.2%) |
| Concomitant resection of portal vein | 64 (12.1%) |
| Resection of peri-SMA nerves | 217 (41.0%) |
| Transfusion of red blood cells | 178 (33.6%) |
| Transfusion of frozen fresh plasma | 99 (18.7%) |
| Curabilitya | |
| R1 | 44 (11.3%) |
| R2 | 12 (3.1%) |
- Display: n (%) or median [interquartile range (IQR)]
- ALT aspartate aminotransferase, AST aspartate aminotransferase, AT-III antithrombin III, CRP C-reactive protein, Hb hemoglobin, HBV hepatitis B virus, HCC hepatocellular carcinoma, HCV hepatitis C virus, ICG-R15 indocyanine green retention rate at 15 min, IPMA intraductal papillary mucinous adenoma, IPMC intraductal papillary mucinous carcinoma, NACRT neoadjuvant chemoradiotherapy or chemotherapy, NASH non-alcoholic steatohepatitis, PD pancreaticoduodenectomy = Classic Whipple, PLT platelet count, PPPD pylorus-preserving pancreaticoduodenectomy, PT prothrombin time, PT-INR prothrombin time-international normalized ratio, SMA superior mesenteric artery, SSPPD substomach-preserving pancreaticoduodenectomy, T-Bil: total bilirubin, WBC white blood cell
- a Calculated for patients with malignancy
Mortality, incidence of postoperative hepatic decompensation, and incidence of deterioration in hepatic function
Postoperative mortality, hepatic decompression, and deterioration in hepatic function are summarized in Table 2. The in-hospital mortality rate was 5.9% (31/529). Among these 31 patients, 26 had complications of hepatic decompensation. Twenty-two of these deaths were also complicated by other factors as follows; recurrence or metastasis of primary cancer in seven, infection in six, POPF in three, other diseases in three, hemorrhage in two, and heart disease in one patient. Genuine hepatic decompensation accounted for 22.6% of the patients and constituted the majority of in-hospital mortalities. However, there were only seven registered cases of hepatic decompensation.
| n = 529 | |
|---|---|
| In-hospital mortality | 31 (5.9%) |
| Hepatic decompensation | 7 (22.6%) |
| Primary cancer recurrence | 7 (22.6%) |
| Infection | 6 (19.4%) |
| POPF | 3 (9.7%) |
| Hemorrhage | 2 (6.5%) |
| Heart | 1 (3.2%) |
| Others/unknown | 5 (16.1%) |
| Death accompanying hepatic decompensation among the 31 patients | 26/31 (83.9%) |
| Mortality according to the clinical diagnosis | |
| Liver cirrhosis | 8/105 (7.6%) |
| Chronic hepatitis | 23/424 (5.4%) |
| Mortality of emergent cases (P = 0.04) | |
| Elective | 30/526 (5.7%) |
| Emergency | 1/3 (33.3%) |
| Mortality according to Child–Pugh class | |
| A | 23/427 (5.4%) |
| B | 7/98 (7.1%) |
| C | 1/4 (25%) |
| Mortality according to MELD score | |
| ~9 | 20/370 (5.4%) |
| 10–14 | 8/91 (8.8%) |
| 15~ | 2/13 (15.4%) |
| Mortality according to existence of portal hypertension | |
| Yes | 7/63 (11.1%) |
| No | 230/446 (5.2%) |
| Frequency of hepatic decompensation upon discharge | 54 (10.2%) |
| In-hospital mortality among the 54 patients | 26/54 (48.1%) |
| Frequency of deterioration in hepatic impairment upon discharge | 119/405 (29.4%) |
| Child–Pugh class A to B | 107/372 (28.8%) |
| Child–Pugh class A to C | 9/372 (2.4%) |
| Child–Pugh class B to C | 3/32 (9.4%) |
| Frequency of postoperative refractory ascites | 47/529 (8.9%) |
- MELD Model for End-Stage Liver Disease, POPF postoperative pancreatic fistula
Regarding surgical indications among the patients who died in the hospital during the hospitalization period in which PD was performed, 22 of the 31 patients (71%) had pancreatic cancer. In addition, there were two cases each (6.5%) of cholangiocarcinoma, papillary carcinoma, duodenal cancer, as well as one case (3%) of pancreatic neuroendocrine tumor, intraductal papillary mucinous adenoma, and benign disease. The most common cause of death among the 22 patients with pancreatic cancer was recurrence of primary disease in six, followed by hepatic failure in four, POPF in four, death by other illnesses in three, infection in two, hemorrhage in one, and unknown causes in two patients. Additionally, five of six patients with recurrence of pancreatic cancer had experienced complications of Clavien-Dindo level III or higher, and the median hospitalization time of the six patients was 68 days (34–95 days). Meanwhile, the causes of death in the nine patients without pancreatic cancer were infection in four, hepatic failure in three, hemorrhage in one, and recurrence of underlying illness in one patient.
Among the 529 patients, 54 (10.2%) had hepatic decompensation at the time of discharge. Twenty-six (48.1%) of these 54 patients died during the hospitalization. Deterioration in hepatic function represented by the Child–Pugh classification at time of discharge was encountered in 29.4%.
Operative data, hospitalization and postoperative complications
Table 3 displays perioperative data, hospitalization and morbidity and compared the patients in Child class A and B plus C. The patients with Child class B plus C had significantly more intraoperative blood loss, higher incidence of postoperative ascites, hyperammonemia, surgical site infection, bile leakage and portal vein thrombosis. The incidences of POPF and DGE were not significantly different between the two groups.
| Total (n = 529) | Child A (n = 427) | Child B, C (n = 98, 4) | P-value | |
|---|---|---|---|---|
| Operative time (min) | 500 [408–594] | 494 [404–594] | 528 [425–595] | 0.47 |
| Intraoperative blood loss (ml) | 911 [559–1,586] | 860 [551–1,524] | 1,154 [750–1,980] | 0.03 |
| Postoperative intensive care unit stay (days) | 1 [0–2] | 1 [0–2] | 1 [0–2] | 0.10 |
| Postoperative hospital stay (days) | 34 [24–52] | 34 [24–51] | 36 [23–61] | 0.84 |
| Morbidity (total) | 233 (44.0%) | 182 (42.7%) | 51 (50.0%) | 0.18 |
| Morbidity | ||||
| Postoperative pancreatic fistula | 117 (22.1%) | 97 (22.7%) | 20 (19.6%) | 0.50 |
| Intra-abdominal abscess or infection | 92 (17.4%) | 73 (17.1%) | 19 (18.6%) | 0.71 |
| Ascites | 40 (7.6%) | 24 (5.6%) | 16 (15.7%) | 0.001 |
| Delayed gastric emptying | 36 (6.8%) | 31 (7.3%) | 5 (4.9%) | 0.40 |
| Pulmonary edema | 30 (5.7%) | 22 (5.2%) | 8 (7.8%) | 0.29 |
| Reoperation | 28 (5.3%) | 20 (4.7%) | 8 (7.8%) | 0.20 |
| Hyperammonemia | 26 (4.9%) | 17 (4.0%) | 9 (8.8%) | 0.04 |
| Sepsis | 26 (4.9%) | 21 (4.9%) | 5 (4.9%) | 1.00 |
| Intra-abdominal hemorrhage | 25 (4.7%) | 18 (4.2%) | 7 (6.9%) | 0.26 |
| Surgical site infection | 22 (4.2%) | 14 (3.3%) | 8 (7.8%) | 0.04 |
| Wound dehiscence | 17 (3.2%) | 13 (3.0%) | 4 (3.9%) | 0.65 |
| Pleural effusion | 17 (3.2%) | 14 (3.3%) | 3 (2.9%) | 0.86 |
| Pneumonia | 16 (3.0%) | 12 (2.8%) | 4 (3.9%) | 0.56 |
| Encephalopathy | 13 (2.5%) | 8 (1.9%) | 5 (4.9%) | 0.08 |
| Renal dysfunction | 13 (2.5%) | 11 (2.6%) | 2 (2.0%) | 0.72 |
| Bile leakage | 11 (2.1%) | 6 (1.4%) | 5 (4.9%) | 0.03 |
| Idiopathic bacterial peritonitis | 10 (1.9%) | 6 (1.4%) | 4 (3.9%) | 0.09 |
| Gastrointestinal hemorrhage unrelated to PHT | 9 (1.7%) | 6 (1.4%) | 3 (2.9%) | 0.28 |
| Liver abscess | 6 (1.1%) | 5 (1.2%) | 1 (1.0%) | 0.87 |
| Leakage of GJ/DJ anastomosis | 5 (1.0%) | 4 (0.9%) | 1 (1.0%) | 0.97 |
| Deep vein thrombosis | 4 (0.8%) | 3 (0.7%) | 1 (1.0%) | 0.77 |
| Portal vein thrombosis | 3 (0.6%) | 1 (0.2%) | 2 (2.0%) | 0.04 |
| Gastrointestinal hemorrhage related to PHT | 3 (0.6%) | 2 (0.5%) | 1 (1.0%) | 0.54 |
- Display: median [interquartile range [IQR)] or n (%). Bold denotes a value of P < 0.05
- DJ duodenojejunostomy, GJ, gastrojejunostomy, PHT portal hypertension
Analyses of risk factors for hepatic decompensation upon discharge
Results of univariate and multivariate analyses for elucidating predictors of hepatic decompensation are summarized based on the components of Child–Pugh scoring system, for the 529 patients registered in the current study (Table 4 and Table S2). According to the multivariate analysis, the factors significantly that correlated with the worst state for each component of the Child–Pugh classification were as follows: hepatic encephalopathy was associated with serum AST more than 50 IU/l and PHT; serum T-Bil more than 2.0 mg/dl was associated with AST more than 50 IU/l, PHT and intraoperative blood loss more than 1,500 ml; serum albumin <2.8 g/dl was associated with platelet count <150,000/μl, T-Bil more than 2.0 mg/dl, AST more than 50 IU/l and PHT; and prothrombin activity <70% was associated with platelet count <150,000/μl, prothrombin activity <70%, PHT and neoadjuvant chemoradiation therapy (NACRT). For refractory ascites, significant independent risk factors consisted of serum AST more than 50 IU/l, prothrombin activity <70% and advanced PHT, in which varices or collateral channels developed. Multivariate analysis determined that the serum AST of more than 50 IU/l and PHT were independent factors predictive of hepatic decompensation in the total population registered in the current study.
| Variables | Hepatic encephalopathy (n = 10) | T-Bil ≥2.0 (n = 23) | Alb <2.8 g/dl (n = 128) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | ||||||||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| PLT (×103/μl) | ~149.99/150~ | 0.45 | 0.13–1.59 | 0.22 | 0.41 | 0.18–0.95 | 0.04 | 0.53 | 0.35–0.80 | 0.003 | 0.53 | 0.32–0.87 | 0.01 | ||||||
| T-Bil (mg/dl) | ~1.999/2.0~ | 0.62 | 0.08–4.99 | 0.66 | 2.67 | 1.06–6.73 | 0.04 | 1.87 | 0.64–5.48 | 0.25 | 1.95 | 1.16–3.30 | 0.01 | 1.89 | 1.004–3.54 | 0.048 | |||
| AST (IU/l) | ~50/51~ | 4.03 | 1.12–14.48 | 0.03 | 4.04 | 1.10–14.88 | 0.04 | 3.55 | 1.52–8.29 | 0.003 | 3.15 | 1.21–8.21 | 0.02 | 1.95 | 1.27–3.00 | 0.002 | 1.78 | 1.08–2.94 | 0.03 |
| PT (%) | ~69.9/70.0~ | 0.78 | 0.10–6.42 | 0.82 | 0.53 | 0.15–1.89 | 0.33 | 0.48 | 0.25–0.95 | 0.04 | 0.62 | 0.30–1.28 | 0.20 | ||||||
| Portal hypertension | Yes/No | 4.94 | 1.35–18.04 | 0.02 | 4.37 | 1.15–16.69 | 0.03 | 4.20 | 1.70–10.37 | 0.002 | 3.06 | 1.14–8.18 | 0.03 | 2.66 | 1.52–4.65 | 0.001 | 2.20 | 1.14–4.24 | 0.02 |
| NACRT | Yes/No | 1.96 | 0.24–16.06 | 0.53 | 2.76 | 0.77–9.91 | 0.12 | 1.20 | 0.51–2.82 | 0.67 | |||||||||
| Blood loss (ml) | ~1,500/1,501~ | 1.86 | 0.52–6.68 | 0.34 | 7.33 | 2.94–18.25 | 0.001> | 6.28 | 2.44–16.18 | 0.001> | 2.17 | 1.41–3.35 | 0.001> | ||||||
| Variables | PT <70% (n = 60) | Refractory ascites (n = 40)a | Hepatic decompensation (n = 54) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | ||||||||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| PLT (×103/μl) | ~149.99/150~ | 0.36 | 0.19–0.67 | 0.001 | 0.44 | 0.20–0.97 | 0.04 | 0.43 | 0.22–0.84 | 0.01 | 0.51 | 0.29–0.91 | 0.02 | 0.63 | 0.32–1.22 | 0.17 | |||
| T-Bil (mg/dl) | ~1.999/2.0~ | 0.51 | 0.21–1.25 | 0.14 | 2.05 | 0.93–4.55 | 0.08 | 2.45 | 1.28–4.71 | 0.01 | 1.72 | 0.80–3.69 | 0.16 | ||||||
| AST (IU/l) | ~50/51~ | 0.78 | 0.40–1.51 | 0.47 | 2.03 | 1.03–4.00 | 0.04 | 2.13 | 1.05–4.33 | 0.04 | 2.27 | 1.25–4.11 | 0.01 | 1.93 | 1.002–3.73 | 0.049 | |||
| PT (%) | ~69.9/70.0~ | 0.36 | 0.15–0.83 | 0.02 | 0.27 | 0.10–0.76 | 0.01 | 0.20 | 0.09–0.46 | 0.001> | 0.27 | 0.11–0.65 | 0.003 | 0.64 | 0.25–1.61 | 0.34 | |||
| Portal hypertension | Yes/No | 4.17 | 1.92–9.05 | 0.001> | 3.20 | 1.24–8.22 | 0.02 | 2.98b | 1.27–6.97 | 0.01 | 2.93 | 1.19–7.25 | 0.02 | 2.70 | 1.35–5.40 | 0.01 | 2.23 | 1.03–4.86 | 0.04 |
| NACRT | Yes/No | 5.22 | 1.26–21.63 | 0.02 | 9.92 | 1.70–57.97 | 0.01 | 0.93 | 0.21–4.09 | 0.92 | 1.90 | 0.69–5.19 | 0.21 | ||||||
| Blood loss (ml) | ~1,500/1,501~ | 2.92 | 1.56–5.47 | 0.001 | 2.06 | 0.91–4.63 | 0.08 | 1.86 | 0.95–3.65 | 0.07 | 4.34 | 2.43–7.76 | 0.001> | ||||||
- Bold denotes a value of P < 0.05. Alb serum albumin, AST aspartate aminotransferase, CI confidence interval, Hb hemoglobin, HR hazard ratio, NACRT neoadjuvant chemoradiotherapy or chemotherapy, PLT platelet count, Portal hypertension patients in whom splenomegaly, esophageal varices, and collateral channels were observed, PT prothrombin time, T-Bil total bilirubin
- a Analyses were performed excluding patients who died with other reasons except liver failure
- b Analyzed in patients with findings of esophageal varices or collateral channels
In the AST >50 IU/l group, a diagnosis of LC was made more frequently (36% vs. 26% when AST <50 IU/l, P = 0.047), and complications of hyperbilirubinemia (32% vs. 8% when AST <50 IU/l, P <0.001) and hypoalbuminemia were more common (75% vs. 58% when AST <50 IU/l, P = 0.001); moreover, cases in which biliary drainage was performed for jaundice were more common (61% vs. 37% when AST <50 IU/l, P < 0.001).
Analyses of risk factors for deterioration in hepatic function upon discharge
Analyses of risk factors for deterioration associated with Child–Pugh scoring system upon discharge were performed individually for each component of Child–Pugh scores and Child–Pugh score grading (Table 5 and Table S3). In multivariate analyses, significant factors that correlated with deterioration of each component of the Child–Pugh classification were as follows: hepatic encephalopathy was associated with medical history of hepatocellular carcinoma (HCC) and preoperative serum AST more than 50 IU/l; serum T-Bil was associated with platelet count <150,000/μl, intraoperative blood loss more than 1,500 ml and pancreatic cancer as an operative indication; serum albumin was associated with hemoglobin <10.0 g/dl and PHT; prothrombin activity was associated with platelet count <150,000/μl, PHT, NACRT, and intraoperative blood loss of more than 1,500 ml; ascites was associated with platelet count <150,000/μl, prothrombin activity <70%, NACRT and intraoperative blood loss of more than 1,500 ml. In addition to the individual analyses of five components of Child–Pugh score, analyses of risk factors for deterioration associated with Child–Pugh score class were also performed. In a multivariate analysis, significant independent risk factors were hemoglobin level of <10.0 g/dl, platelet count <150,000/μl, serum albumin <4.0 g/dl, PHT and intraoperative blood loss more than 1,500 ml.
| Variables | Hepatic encephalopathy (n = 8) | T-Bil (n = 16) | Alb (n = 276) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | ||||||||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| HCC | Yes vs. No | 7.07 | 1.62–30.91 | 0.01 | 5.84 | 1.29–26.43 | 0.02 | 3.94 | 1.20–12.91 | 0.02 | 1.56 | 0.69–3.55 | 0.29 | ||||||
| Hb (g/dl) | ~9.9/10~ | 1.01 | 0.92–1.11 | 0.82 | 0.99 | 0.86–1.14 | 0.90 | 0.33 | 0.16–0.66 | 0.002 | 0.31 | 0.15–0.63 | 0.001 | ||||||
| PLT (×103/μl) | ~149.99/150~ | 0.82 | 0.19–3.49 | 0.79 | 0.29 | 0.10–0.80 | 0.02 | 0.3 | 0.10–0.87 | 0.03 | 0.69 | 0.43–1.08 | 0.11 | ||||||
| AST (IU/l) | ~50/51~ | 6.08 | 1.43–25.92 | 0.02 | 5.30 | 1.22–23.05 | 0.03 | 2.82 | 1.02–7.79 | 0.045 | 1.004 | 0.61–1.66 | 0.99 | ||||||
| Alb (g/dl) | ~3.9/4.0~ | 1.46 | 0.36–5.91 | 0.60 | 0.85 | 0.30–2.38 | 0.76 | 1.85 | 1.18–2.89 | 0.01 | |||||||||
| PT (%) | ~69.99/70~ | 0.51 | 0.06–4.41 | 0.54 | 1.19 | 0.15–9.41 | 0.87 | 0.74 | 0.32–1.71 | 0.48 | |||||||||
| Portal hypertension | Yes/No | 2.28 | 0.45–11.57 | 0.32 | 3.22 | 1.07–9.66 | 0.04 | 2.33 | 1.09–4.96 | 0.03 | 2.52 | 1.16–5.48 | 0.02 | ||||||
| NACRT | Yes/No | 2.26 | 0.27–19.12 | 0.45 | 2.29 | 0.49–10.67 | 0.29 | 1.82 | 0.66–4.98 | 0.25 | |||||||||
| Blood loss (ml) | ~1,500/1,501~ | 2.85 | 0.70–11.61 | 0.14 | 6.86 | 2.33–20.23 | 0.001> | 5.97 | 2.00–17.84 | 0.001 | 0.95 | 0.59–1.53 | 0.82 | ||||||
| Variables | PT (n = 46) | Ascites (n = 73) | Child–Pugh grade (n = 119) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | ||||||||||||||
| HR | 95% CI | P-value | HR | 95%CI | P-value | HR | 95%CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| HCC | Yes vs. No | 1.08 | 0.41–2.80 | 0.88 | 1.76 | 0.79–3.94 | 0.17 | 1.92 | 0.95–3.90 | 0.07 | |||||||||
| Hb (g/dl) | ~9.9/10~ | 0.37 | 0.08–1.73 | 0.21 | 1.33 | 0.58–3.03 | 0.50 | 0.37 | 0.14–0.98 | 0.046 | 0.26 | 0.08–0.82 | 0.02 | ||||||
| PLT (×103/μl) | ~149.99/150~ | 0.33 | 0.17–0.67 | 0.002 | 0.39 | 0.18–0.88 | 0.02 | 0.54 | 0.32–0.90 | 0.02 | 0.55 | 0.31–0.98 | 0.04 | 0.35 | 0.23–0.55 | 0.001> | 0.44 | 0.26–0.75 | 0.002 |
| AST (IU/l) | ~50/51~ | 0.61 | 0.27–1.39 | 0.24 | 1.27 | 0.71–2.27 | 0.42 | 1.37 | 0.83–2.26 | 0.22 | |||||||||
| Alb (g/dl) | ~3.9/4.0~ | 0.92 | 0.46–1.88 | 0.83 | 0.71 | 0.41–1.21 | 0.21 | 0.47 | 0.30–0.76 | 0.002 | 0.53 | 0.31–0.90 | 0.02 | ||||||
| PT (%) | ~69.99/70~ | 2.41 | 0.67–8.69 | 0.18 | 0.38 | 0.17–0.85 | 0.02 | 0.38 | 0.17–0.88 | 0.02 | 0.69 | 0.31–1.56 | 0.38 | ||||||
| Portal hypertension | Yes/No | 4.31 | 1.90–9.78 | 0.001> | 2.70 | 1.07–6.79 | 0.04 | 1.69 | 0.85–3.37 | 0.13 | 3.72 | 2.05–6.75 | 0.001> | 2.17 | 1.08–4.34 | 0.03 | |||
| NACRT | Yes/No | 5.8 | 1.39–24.28 | 0.02 | 7.40 | 1.58–34.64 | 0.01 | 2.82 | 1.20–6.60 | 0.02 | 3.01 | 1.12–8.13 | 0.03 | 2.31 | 0.99–5.40 | 0.05 | |||
| Blood loss (ml) | ~1,500/1,501~ | 3.14 | 1.55–6.35 | 0.001 | 2.29 | 1.05–4.97 | 0.04 | 2.15 | 1.27–3.65 | 0.01 | 1.83 | 1.02–3.27 | 0.04 | 2.32 | 1.47–3.68 | 0.001> | 2.04 | 1.18–3.50 | 0.01 |
- All analyses were performed for cases with T-Bil <2.0. Bold denotes a value of P < 0.05
- Alb serum albumin, AST aspartate aminotransferase, CI confidence interval, Hb hemoglobin, HCC medical history of hepatocellular cell carcinoma, HR hazard ratio, NACRT neoadjuvant chemoradiotherapy or chemotherapy, PLT platelet count, Portal hypertension patients in whom splenomegaly, esophageal varices, and collateral channels were observed, PT prothrombin time, T-Bil total bilirubin
Long-term survivals and prognostic factors
Figure 1 shows the Kaplan–Meier curves for 5-year overall survival according to the pre-operative Child–Pugh classification. Five-year overall survival was 57.8% for the Child class A and 24.8% for the Child class B (P < 0.0001).
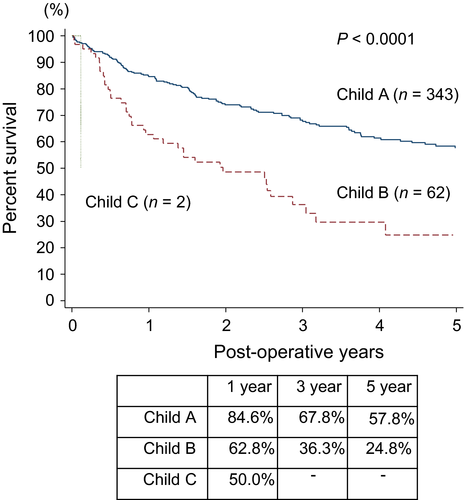
Figure 2 shows the Kaplan–Meier curves for 5-year overall survival according to the pre-operative APRI in patients with Child A vs. B plus C. The difference in 5-year overall survival in relation to APRI was not found in the patients with Child A. The APRI of <1.0 yielded a significantly higher survival rate than their counterpart with Child B and C. Figure 3 shows the Kaplan–Meier curves for 5-year overall survival according to the pre-operative APRI in patients of pancreatic cancer with Child B. The APRI of <1.0 yielded a significantly higher survival rate than their counterpart.
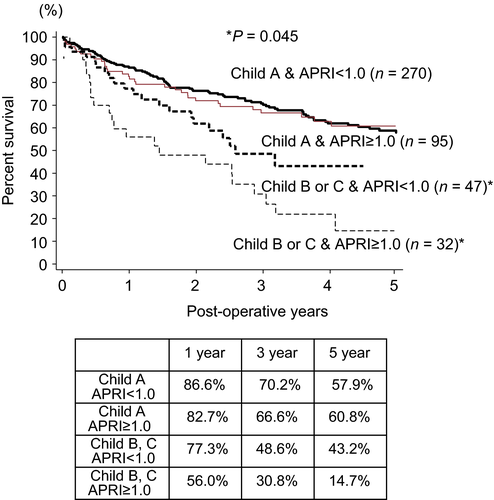

Table 6 provides the results of univariate and multivariate Cox proportional hazards analysis. Risk factors for long-term survival consisted of pancreatic cancer as operative indication, the UICC stage 32, history of HCC treatment, platelet count <150,000/μl, serum T-Bil more than 2.0 mg/dl and presence of preoperative ascites.
| Median survival (years) | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Age (years) | |||||||
| 75– | 6.8 | 1.04 | 0.75–1.45 | 0.81 | |||
| –74 | 5.8 | ||||||
| Sex | |||||||
| Female | 7.1 | 0.86 | 0.63–1.17 | 0.33 | |||
| Male | 5.2 | ||||||
| Diabetes mellitus | |||||||
| Yes | 5.8 | 0.94 | 0.68–1.30 | 0.71 | |||
| No | 5.8 | ||||||
| HCC | |||||||
| Yes | 1.8 | 2.48 | 1.61–3.82 | <0.001 | 1.65 | 1.002–2.74 | 0.049 |
| No | 6.8 | ||||||
| Etiology of liver disease | |||||||
| Non-HCV | 6.7 | 0.95 | 0.70–1.29 | 0.74 | |||
| HCV | 5.7 | ||||||
| PLT (×103/μl) | |||||||
| 150≤ | 7.9 | 0.67 | 0.49–0.91 | 0.01 | 0.65 | 0.45–0.94 | 0.02 |
| 150> | 4.1 | ||||||
| Serum AST (IU/L) | |||||||
| 51≤ | 1.1a | 1.55 | 1.13–2.12 | 0.01 | 1.11 | 0.75–1.64 | 0.60 |
| 51> | 1.9a | ||||||
| Serum T-Bil (mg/dl) | |||||||
| 2.0≤ | 2.2 | 2.39 | 1.65–3.48 | <0.001 | 1.77 | 1.11–2.79 | 0.02 |
| 2.0> | 7.1 | ||||||
| Serum albumin (g/dl) | |||||||
| 4.0≤ | 2.7a | 0.58 | 0.42–0.80 | 0.001 | 0.76 | 0.52–1.13 | 0.18 |
| 4.0> | 1.2a | ||||||
| Serum creatinine (mg/dl) | |||||||
| 0.84≤ | 1.5a | 0.89 | 0.62–1.26 | 0.50 | |||
| 0.84> | 1.7a | ||||||
| Serum sodium (mEq/l) | |||||||
| 138≤ | 6.7 | 0.75 | 0.52–1.08 | 0.12 | |||
| 138> | 4.1 | ||||||
| PT (%) | |||||||
| 70≤ | 6.0 | 0.76 | 0.43–1.35 | 0.36 | |||
| 70> | 5.6 | ||||||
| Splenomegaly | |||||||
| Yes | 1.9 | 1.97 | 1.21–3.21 | 0.01 | 1.05 | 0.58–1.88 | 0.88 |
| No | 6.7 | ||||||
| Ascites | |||||||
| Yes | 1.4 | 2.11 | 1.26–3.52 | 0.01 | 2.08 | 1.15–3.74 | 0.02 |
| No | 6.0 | ||||||
| Indication | |||||||
| Non-pancreatic cancer | 3.6a | 0.25 | 0.19–0.34 | <0.001 | 0.38 | 0.25–0.57 | <0.0001 |
| Pancreatic cancer | 0.7a | ||||||
| Stagea | |||||||
| II, III | 2.0 | 4.07 | 2.73–6.08 | <0.001 | 1.90 | 1.23–2.92 | 0.004 |
| I | 4.7a | ||||||
| Operative time (min) | |||||||
| 501≤ | 4.0 | 1.39 | 1.03–1.87 | 0.03 | 1.12 | 0.78–1.60 | 0.54 |
| 501> | 7.1 | ||||||
| Blood loss (ml) | |||||||
| 1,501≤ | 3.0 | 1.85 | 1.36–2.52 | <0.001 | 1.32 | 0.88–2.00 | 0.18 |
| 1,501> | 7.1 | ||||||
| Transfusion of red blood cells | |||||||
| Yes | 3.2 | 1.91 | 1.41–2.60 | <0.001 | 1.34 | 0.90–2.00 | 0.15 |
| No | 7.9 | ||||||
| APRI | |||||||
| ~1.0 | 7.9 | 1.64 | 1.08–2.49 | 0.02 | |||
| 1.0~ | 3.8 | ||||||
- Bold denotes a value of P < 0.05. APRI aspartate aminotransferase-platelet ratio index, AST aspartate aminotransferase, CI confidence interval, HCC hepatocellular cell carcinoma, HR hazard ratio, PLT platelet count, PT prothrombin time, Stage UICC stage, T-Bil total bilirubin
- a 25% survival time
Discussion
This multi-institutional retrospective study showed the influence of CHD on morbidity, mortality, and mid–long-term survivals, using a larger number of patients with CHD than ever before. The in-hospital mortality rate was 5.4%, 7.1% and 25% for Child class A, B and C, respectively. The incidence of morbidity, postoperative hepatic decompensation upon discharge and refractory ascites was 44.0%, 10.2% and 8.9%, respectively. For hepatic decompensation, PHT and the serum AST of more than 50 IU/l were independent significant risk factors. For refractory ascites, advanced PHT with collaterals, serum AST of more than 50 IU/l and prothrombin activity of <70% were independent significant risk factors. Regarding middle to long-term overall survivals of patients with Child B and C, the APRI of <1.0 yielded a significantly higher survival rate than their counterpart.
At present, only a few studies with small sample sizes have been published regarding the outcomes of PD in patients with LC 13-16. Therefore, reliable data and detailed analyses are expected for decision making of indication of PD in patients with CHD. Findings of the current study could help in better selection and management of patients to reduce postoperative mortality and morbidity following PD.
Total mortality in the present study was 5.9%, which is twice that reported in the NCD from Japan (2.9%) 30, 31, although it is much lower than it is supposed to be according to the invasiveness of PD and previous reports 5-7, 13-16. Compared with the overall PD cases according to the NCD, the patients enrolled in the present study had (1) a higher frequency of ischemic heart disease, (2) a higher percentage of patients with a preoperative platelet count of <80,000/μl or <120,000/μl, (3) a higher percentage of patients with prothrombin time-international normalized ratio (PT-INR) of >1.1 or PT-INR >1.25, (4) 5% more patients with an intraoperative bleeding amount of 2,000 ml, (5) 6.5% more patients with an operating time of 6 h or less, (6) a prolonged postoperative hospitalization, (7) a higher incidence of POPF, and (8) a higher incidence of renal failure 30, 31. In spite of poorer perioperative conditions, the cause for lower mortality than that previously reported was inferred as stricter indication for PD as well as more advanced perioperative management.
The Child–Pugh score consists of five factors, so each factor was sub-analyzed in the present study for hepatic decompensation and deterioration of hepatic function. PHT, intraoperative blood loss, the pre-operative T-Bil level of 2.0 mg/dl or greater, low platelet count, low serum albumin, anemia and PT lower than 70% were detected as the independent risk factors, which were identical to the previously reported risk factors in patients with LC for various surgical procedures other than hepatectomy 4, 7, 29, 33, 34. The other risk factors for mortality and morbidity cited in the several previous studies on various surgical procedures, which were not detected or not selected as variables in the current study, were operating time, emergencies, transfusion of red blood cell, platelet or fresh frozen plasma, American Society of Anesthesiologists physical status (ASA), hyponatremia, systolic blood pressure lower than 100 mmHg, encephalopathy, ascites, postoperative infection, chronic heart failure, renal failure and peripheral vascular disorder 7.
As a result, PHT was identified to be strongly correlated with all components of the Child–Pugh scoring system in postoperative hepatic decompensation. Serum AST is an important predictor of hepatic fibrosis or LC in patients with chronic hepatitis C 26. The mechanism is proposed as follows: hepatic fibrosis causes decreased clearance of AST resulting in increased blood AST and impairment of the mitochondria caused by the progression of liver damage 35, 36. In the current analysis, when AST was interpreted as an index of hepatic fibrosis, similar to platelet count, it was able to serve as an index of postoperative hepatic functional impairment, which was shown to be possibly effective as a strong predictive factor of hepatic decompensation. In other words, the degree of hepatic fibrosis indicated by platelet count or AST was another important factor of hepatic decompensation.
To the best of our knowledge, the present study is the first attempt to find risk factors of refractory ascites. In a previous study of 25 patients with gastric cancer complicated by LC (Child class A, 15; Child class B, 10), the incidence of refractory ascites was 6.7% in Child class A and 40.0% in Child class B; however, four of the five patients with refractory ascites were Child class B; and three of these patients did not undergo lymph node dissection 37. The percentage of patients with Child class B that developed refractory ascites was high regardless of lymph node dissection. This suggests that correlation between lymph node dissection and refractory ascites has not been established. The current study also found no correlation between lymph node dissection and refractory ascites.
Regarding refractory ascites, another important finding in the current study is that severity of PHT was critical. That is, PHT with only splenomegaly was not a significant factor for refractory ascites, but severe PHT, in which collateral pathways were formed, was inferred to be a significant risk factor. Refractory ascites was inferred to develop if the collateral pathways were blocked during surgery. Once PHT developed from blocked collateral channels caused by surgical maneuvers, increased permeability of the peripheral branches of the portal vein and variations in circulatory dynamics caused by operative invasion, and decreased synthetic capacity were inferred to be involved in refractory ascites following PD. In addition to decreased PT, increased serum AST was detected. The present study demonstrated that the AST >50 IU/l group had more frequent diagnosis of LC, hyperbilirubinemia and biliary drainage for jaundice. Therefore, the significance of increased serum AST as a risk factor for refractory ascites can be interpreted in two ways. First, similar to platelets, hepatic fibrosis is exhibited; second, the involvement of hepatic impairment caused by obstructive jaundice is exhibited showing the risks of developing refractory ascites for patients with advanced hepatic fibrosis and patients who are undergoing treatment for jaundice but who currently have advanced hepatic damage with serum AST elevation, respectively.
In the present study, the pre-operative Child–Pugh classification, the Model for End-Stage Liver Disease (MELD) score, preoperative clinical diagnosis of LC and APRI were all associated with the occurrence of postoperative hepatic decompensation. Although the Child–Pugh classification and MELD score have been proposed as prognostic factors in previous studies 7, 29, Child–Pugh classification or MELD, individually, is not sufficient enough to predict hepatic decompensation. APRI was proposed as an indicator of fibrosis and can be easily calculated. In a meta-analysis of as many as 40 studies, for patients with APRI of over 1.0, hepatic cirrhosis could be predicted with a sensitivity of 76% and specificity of 72% 26. The risks for Child class B patients could be further classified by using APRI. An APRI greater than or equal to 1.0 in patients with Child class B indicated a very high risk, for whom more careful indication for surgery should be exerted. This result could be attributed to hepatic fibrosis markers, such as platelet count and AST not directly included in the Child–Pugh classification. A recent study reported that hepatic fibrosis evaluated by APRI could predict prognosis after hepatectomy for patients with HCC 38. Similarly, the finding mentioned above can be interrupted as indicating the effect of hepatic fibrosis on mid-term prognosis after PD. Similar to APRI, Fibrosis-4 index was also advocated as a surrogate marker of hepatic fibrosis, which has been found to be highly correlated with histologic evaluation of liver fibrosis in liver biopsy specimens 39, 40. In the present study, APRI was used because of simplicity to be calculated at bedside.
In addition to PHT, the results of the present study showed the importance of preoperative evaluation of hepatic fibrosis or liver damage indicated by AST or APRI. This is the first time, to the best of our knowledge, that the effects of liver fibrosis on the outcomes of PD in patients with CHD have been studied. In clinical settings, preoperative management of patients should consider reducing serum AST of <50 IU/l. For the Child class B patients with APRI ≥1.0, the indication for PD should be decided more carefully. A new risk calculation system including an indicator of hepatic fibrosis may be useful for appropriate indication of PD among patients.
There are limitations to the current study. First, the current indication for PD differs depending on the experience of the surgeon and the institution. This retrospective patient selection is one of the limitations of the current study. The second is that the APRI holds more implications for hepatitis C and hepatitis B 41. Though the main etiologies of CHD were hepatitis C and B infection in the current study, other CHD cases with different etiologies were also included in the analyses, albeit small in number. In detail, the efficacy of APRI and the value of cutoff for each different etiology should be decided with larger cases in the future.
In conclusion, in addition to PHT, evaluation of pre-operative hepatic fibrosis for postoperative hepatic decompensation, including refractory ascites and the mid- to long-term prognosis, was important. These factors should be considered in decision making of PD indication in patients with CHD.
Acknowledgments
We are especially grateful to the 82 leading Japanese institutions that kindly took part in the survey. The order of these institutions in the list below was determined according to the number of patients enrolled. We also would like to express our sincere appreciation to Dr Yoshihiro Shirai (Department of Surgery, The Jikei University, Tokyo, Japan) for his significant contribution to this study. JHBPS funded the use of a professional writing service in the English review of this article.
Department of Surgery, Keio University School of Medicine; Department of Surgery, Kansai Medical University; Department of Surgery and Oncology, Graduate School of Medical Sciences, Kyushu University; Department of Surgery, National Hospital Organization, Kure Medical Center and Chugoku Cancer Center; Department of Surgery, Kurashiki Central Hospital; Department of Hepato-Biliary-Pancreatic Surgery, Tohoku University Graduate School of Medicine; Department of Surgery, Surgical Oncology and Science, Sapporo Medical University; Division of Surgical Oncology, Department of Surgery, Nagoya University Graduate School of Medicine; Department of Hepato-Biliary-Pancreatic Surgery, Tochigi Cancer Center; Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Kurume University School of Medicine; Department of Surgery, Oita Red Cross Hospital; Department of Gastroenterological and Pediatric Surgery, Oita University Faculty of Medicine; First Department of Surgery, Yamagata University Graduate School of Medical Science; Department of Surgery, Okayama Saiseikai General Hospital; Department of Gastroenterological Surgery, Kochi Health Sciences Center; Department of Hepato-Biliary-Pancreatic Surgery, National Hospital Organization, Fukuyama Medical Center; Department of Surgery, Kinki University Faculty of Medicine; Hepatobiliary-Pancreatic and Transplant Surgery, Mie University Graduate School of Medicine; Department of Surgery, Ehime Prefectural Central Hospital; Department of Gastroenterological Surgery II, Hokkaido University Graduate School of Medicine; Division of Hepato-Biliary-Pancreatic Surgery, Department of Surgery, Kobe University Graduate School of Medicine; Division of General Surgery, Japanese Red Cross Kumamoto Hospital; Department of Surgery, Tokushima University Graduate School; Department of Hepato-Biliary-Pancreatic Surgery, Osaka City University Graduate School of Medicine; Department of Gastroenterological Surgery, Graduate School of Life Sciences, Kumamoto University; Hepato-Biliary-Pancreatic Surgery Division, Department of Surgery, Graduate School of Medicine, University of Tokyo; Department of Gastroenterological Surgery, Kanazawa University Hospital; Department of Surgery, National Hospital Organization, Osaka National Hospital; Department of Biliary-Pancreatic Surgery, Fujita Health University; Department of Surgery, Nagasaki University Graduate School of Biomedical Sciences; Department of Gastroenterological Surgery, Fukuyama City Hospital; Department of Gastroenterological Surgery and Oncology, Kitano Hospital; Department of Surgery, Division of Surgical Oncology, Tottori University School of Medicine; Department of Surgery, Hyogo College of Medicine, Nishinomiya; Department of Surgery, Toyama Prefectural Central Hospital; Department of Gastroenterological Surgery, Nagoya City University Graduate School of Medical Sciences; Department of Surgery, National Defense Medical College; Department of Surgery, Nara Medical University; Division of Hepato-Biliary-Pancreatic Surgery, Department of Surgery, University of Miyazaki Faculty of Medicine; Department of Surgery, Jichi Medical University; Division of Digestive and General Surgery, Niigata University Graduate School of Medical and Dental Sciences; Department of Surgery, Saga University Faculty of Medicine; Department of Surgery, Nara Prefecture General Medical Center; Department of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine; Department of Hepato-Biliary-Pancreatic Surgery and Transplantation, Ehime University Graduate School of Medicine; Department of Surgery, Kimitsu Chuo Hospital; Department of Gastrointestinal and Pediatric Surgery, Tokyo Medical University; Department of Surgery, National Hospital Organization, Beppu Medical Center; Department of Hepato-Biliary-Pancreatic Surgery, National Hospital Organization Kyushu Medical Center; Department of Surgery, Saitama Medical Center, Jichi Medical University; Department of Surgery, Teikyo University School of Medicine; Department of Surgery, The Jikei University School of Medicine; Department of Surgery, Kyorin University School of Medicine; Department of Gastroenterological and General Surgery, School of Medicine, Showa University; Second Department of Surgery, Hamamatsu University School of Medicine; Department of Gastroenterological Surgery, Hiroshima City Hiroshima Citizens Hospital; Department of Hepato-Biliary-Pancreatic Surgery, Osaka City General Hospital; Department of Hepatobiliary and Pancreatic Surgery, Graduate School of Medicine, Tokyo Medical and Dental University; Department of Surgery, Toyonaka Municipal Hospital; Department of Surgery, Osaka Medical Center for Cancer and Cardiovascular Diseases; Department of Surgery, Iwate Medical University School of Medicine; Division of gastroenterological surgery, Saitama Cancer Center; Department of Gastroenterological Surgery, Hiroshima Prefectural Hospital; Department of Surgery, Otsu Red Cross Hospital; Department of Surgery, Japanese Red Cross Kyoto Daini Hospital; Department of Surgery, Division of General and Gastroenterological Surgery, Toho University School of Medicine; Department of Surgery, National Hospital Organization, Sendai Medical Center; Department of Surgery, Sendai City Medical Center; Department of Surgery, The Jikei University Daisan Hospital; Department of Gastroenterological Surgery I, Hokkaido University Graduate School of Medicine; Department of Gastroenterological Surgery, Osaka University Graduate School of Medicine; Department of Surgery, Asahikawa Medical University; Department of Gastroenterological Surgery, Akita University Graduate School of Medicine; Department of Gastroenterological Surgery, Kagawa University School of Medicine; Department of Gastroenterological, Breast and Endocrine Surgery, Yamaguchi University Graduate School of Medicine; Department of Digestive Surgery, Breast and Thyroid Surgery, Kagoshima University Graduate School of Medicine and Dental Sciences; Department of Surgery, Yamagata Prefectural Central Hospital; Department of Surgery, The Jikei University Kashiwa Hospital; Division of Hepatobiliary and Pancreatic Surgery, Kanagawa Cancer Center, Kanagawa Prefectural Hospital; Department of Surgery, Nishi Kobe Medical Center; Department of Surgery, Nigata Prefectural Shibata Hospital.
Conflict of interest
None declared.
Author contributions
Study conception and design: Futagawa, Yanaga, Kosuge, Isaji, Hirano, Murakami and Yamaue. Acquisition of data: Futagawa, Yanaga and Suka. Analysis and interpretation of data: Futagawa, Suka and Kosuge. Manuscript drafted by: Futagawa, Yanaga and Kosuge. All authors provided critical revision during the drafting of the manuscript. All authors read and approved the final manuscript.




