Tokyo Guidelines 2018: flowchart for the management of acute cholecystitis
Abstract
We propose a new flowchart for the treatment of acute cholecystitis (AC) in the Tokyo Guidelines 2018 (TG18). Grade III AC was not indicated for straightforward laparoscopic cholecystectomy (Lap-C). Following analysis of subsequent clinical investigations and drawing on Big Data in particular, TG18 proposes that some Grade III AC can be treated by Lap-C when performed at advanced centers with specialized surgeons experienced in this procedure and for patients that satisfy certain strict criteria. For Grade I, TG18 recommends early Lap-C if the patients meet the criteria of Charlson comorbidity index (CCI) ≤5 and American Society of Anesthesiologists physical status classification (ASA-PS) ≤2. For Grade II AC, if patients meet the criteria of CCI ≤5 and ASA-PS ≤2, TG18 recommends early Lap-C performed by experienced surgeons; and if not, after medical treatment and/or gallbladder drainage, Lap-C would be indicated. TG18 proposes that Lap-C is indicated in Grade III patients with strict criteria. These are that the patients have favorable organ system failure, and negative predictive factors, who meet the criteria of CCI ≤3 and ASA-PS ≤2 and who are being treated at an advanced center (where experienced surgeons practice). If the patient is not considered suitable for early surgery, TG18 recommends early/urgent biliary drainage followed by delayed Lap-C once the patient's overall condition has improved. Free full articles and mobile app of TG18 are available at: http://www.jshbps.jp/modules/en/index.php?content_id=47. Related clinical questions and references are also included.
Introduction
Flowcharts for the management of acute cholecystitis (AC) were presented in the Tokyo Guidelines 2007 (TG07) 1 and the Tokyo Guidelines 2013 (TG13) 2. The flowcharts allow practitioners in the clinical setting to understand treatment flow at a glance and have proven useful in the management of AC. There have been significant changes in clinical management since then, including advances in surgical techniques and equipment and progress in multidisciplinary treatment. A number of clinical research papers have been published suggesting various changes in the AC treatment flowchart in TG13. The Tokyo Guidelines flowchart was started as a way to show recommended treatments according to the severity of AC. However, it did not cover issues like physical status such as co-morbidities (especially organ dysfunctions) or other predictive factors/risk factors when choosing a treatment pathway according to severity. In addition, until now Grade III AC was considered not suitable for straightforward laparoscopic cholecystectomy (Lap-C). In the TG18 guidelines, we propose a modified flowchart based on recent recommendations in the clinical setting, particularly evidence reported after the publication of TG13. We also discuss Clinical Questions (CQs) on the evidence underpinning this flowchart.
We stress that this treatment flowchart is aimed at improving the percentage of lives saved by allowing doctors to determine how they can safely treat AC through the use of decision-making criteria even for severe cases.
Criteria for the production of the AC treatment flowchart presented in TG18
- The selection of treatment strategy for patients at each severity grade was based on risk factors. The risk factors used were: predictive factors, Charlson comorbidity index (CCI) score, and the American Society of Anesthesiologists physical status classification (ASA-PS) score.
- Lap-C to treat AC of moderate and severe grades (Grade II and III) should be performed only at advanced centers where experienced surgeons practice, in addition to the conditions described above. An advanced center should have both appropriate personnel and facilities to manage the level of patients being managed. Surgeons should have training and experience in advanced laparoscopic techniques and intensive care unit should be available.
- Lap-C can be performed to treat AC if the conditions described above for each Grade are satisfied.
While considering indications for surgery and emergency drainage, sufficient infusion and electrolyte correction take place, and antimicrobial and analgesic agents are administered while fasting continuing the monitoring of respiratory and hemodynamics. (Level C)
When AC is diagnosed, the severity is determined 3 and initial treatment includes monitoring of respiration and hemodynamics, as well as sufficient intravenous fluid and electrolyte infusion and electrolyte correction and treatment with antimicrobials and analgesics. See the paper by Miura et al. for more details on initial treatment 4. The approaches specified in papers by Gomi et al. regarding the choice of antimicrobial and optimum treatment duration or blood/bile culture should be reviewed and implemented; these papers also provide an understanding of the specific characteristics of bile duct infections 5-7. Refer to Gomi et al. on TG18 for the specific names of antimicrobials and other details 6.
We propose Lap-C for AC over open cholecystectomy. (Recommendation 2, level A)
There has been ongoing debate for many years over whether Lap-C or open cholecystectomy is the best treatment for AC. In the SAGES Guidelines published in 1993, AC was considered a relative contraindication for Lap-C 8. Since then, Lap-C has gradually been adopted for AC as surgical techniques have improved and advances have been made in optical devices and surgical instruments. TG13 states that Lap-C is preferable to open cholecystectomy 9.
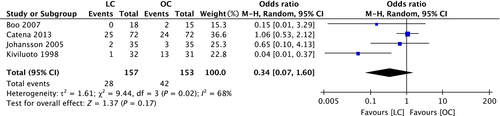
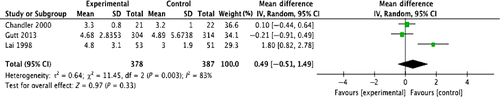
A search of the literature published between January 2013 and December 2016, after the publication of TG13, and using the keywords “acute cholecystitis,” “laparoscopic cholecystectomy,” and “open cholecystectomy” returned papers on one systematic review and one randomized controlled trial. In terms of the incidence of surgical complications, the team producing these guidelines performed a meta-analysis using a random-effects model on four randomized controlled studies 10-13 because the systematic review 14 used a fixed-effects model even though various differences in the research papers were detected. The odds ratio for the incidence of surgical complications is 0.34 (95% CI: 0.07–1.60), which suggests that laparoscopic surgery may be effective but the difference between Lap-C and open cholecystectomy is not statistically significant (Fig. 1). A meta-analysis was performed on the length of hospital stay in three of the randomized controlled trials 10-12; the results show that patients were hospitalized for shorter periods (approximately 1.7 days shorter) with laparoscopy compared with open surgery, suggesting that laparoscopy is effective, but the difference is not statistically significant (Fig. 2).

Since TG13, three population-based cohort studies on AC have been published. In a study in Ontario, Canada between 2004 and 2011, laparoscopy was chosen for 21,280 of 22,202 patients undergoing surgery for AC (95.8%) 15. According to the Swedish Registry of Gallstone Surgery and Endoscopic Retrograde Cholangiography (GallRiks), between 2006 and 2014, laparoscopy was chosen for 12,522 of 15,760 patients (79%) 16. In a multicenter joint study in Japan and Taiwan between 2011 and 2013, laparoscopy was chosen for 2,356 of 3,325 patients undergoing surgery for AC (71%) 17. Laparoscopy seems to be the treatment of choice for AC around the world, although there are some regional differences.
Compared with open surgery, laparoscopy is generally expected to result in less pain at incision sites, shorter hospital stays and recovery periods, and better quality of life. In terms of costs, laparoscopy is expected to involve higher surgery costs (cost of disposable equipment) compared with open surgery, but approximately the same overall costs (direct and indirect medical costs) given the shorter hospital stays and faster return to society 12. The choice of surgical technique should consider surgical risk to the patient, with safety as the main priority, but there are many benefits of laparoscopy if the procedure can be performed safely.
We propose that the treatment strategy be considered and chosen after an assessment has been made of cholecystitis severity, the patient's general status and underlying disease.
Grade I (mild) AC: Lap-C should ideally be performed soon after onset if the CCI and ASA-PS scores suggest the patient can withstand surgery. If it is decided that the patient cannot withstand surgery, conservative treatment should be performed at first and delayed surgery considered once treatment is seen to take effect.
Grade II (moderate) AC: Lap-C should ideally be performed soon after onset if the CCI and ASA-PS scores suggest the patient can withstand surgery and the patient is in an advanced surgical center. However, particular care should be taken to avoid injury during surgery and a switch to open or subtotal cholecystectomy should be considered depending on the findings. If it is decided that the patient cannot withstand surgery, conservative treatment and biliary drainage should be considered.
Grade III (severe) AC: The degree of organ dysfunction should be determined and attempts made to normalize function through organ support, alongside administration of antimicrobials. Doctors should investigate predictive factors, i.e. a rapid recovery in circulatory dysfunction or renal dysfunction after treatment is initiated, and CCI or ASA-PS scores; if it is decided that the patient can withstand surgery, early Lap-C can be performed by a specialist surgeon with extensive experience in a setting that allows for intensive care management. If it is decided that the patient cannot withstand surgery, conservative treatment including comprehensive management should be performed. Early biliary drainage should be considered if it is not possible to control the gallbladder inflammation. (Recommendation 2, level D)
What is the Charlson comorbidity index?
The CCI is a method to categorize a patient's comorbidities based on International Classification of Diseases (ICD) codes used in regulatory data such as hospital summary data 18-22. Each comorbid category is given a weighting (1–6) depending on the adjusted risk for the resources used or the mortality rate. The total of all these weightings for a patient provides a single patient comorbidity score. A score of zero shows that no comorbidities were discovered. As the score rises, the predicted mortality rate rises and treatment would require more healthcare resources (Table 1) 18.
| Assigned weights for diseases | Conditions |
|---|---|
| 1 | Myocardial infarction |
| Congestive heart failure | |
| Peripheral vascular disease | |
| Cerebrovascular disease | |
| Dementia | |
| Chronic pulmonary disease | |
| Connective tissue disease | |
| Peptic ulcer disease | |
| Mild liver disease | |
| Diabetes mellitus (uncomplicated) | |
| 2 | Hemiplegia |
| Moderate or severe chronic kidney disease | |
| Diabetes mellitus with end-organ damage | |
| Any solid tumor | |
| Leukemia | |
| Malignant lymphoma | |
| 3 | Moderate or severe liver disease |
| 6 | Metastatic solid tumor |
| Acquired immune deficiency syndrome (AIDS) |
- Assigned weights for each conditions that a patient has
- The total equals the score
- Reprinted with permission from Elsevier (No. 4183730675295)
What is the American Society of Anesthesiologists physical status classification?
The ASA-PS score is an index developed by the American Society of Anesthesiologists to provide an understanding of a patient's health status before surgery. Table 2 is a tabulated version of a chart about the ASA-PS score provided on the Society's website 23.
| ASA-PS classification | Definition | Examples, including, but not limited to: |
|---|---|---|
| ASA I | A normal healthy patient | Healthy, non-smoking, no or minimal alcohol use |
| ASA II | A patient with mild systemic disease | Mild diseases only without substantive functional limitations. Examples include (but not limited to): current smoker, social alcohol drinker, pregnancy, obesity (30 < BMI < 40), well-controlled DM/HTN, mild lung disease |
| ASA III | A patient with severe systemic disease | Substantive functional limitations; one or more moderate to severe diseases. Examples include (but not limited to): poorly controlled DM or HTN, COPD, morbid obesity (BMI ≥40), active hepatitis, alcohol dependence or abuse, implanted pacemaker, moderate reduction of ejection fraction, ESRD undergoing regularly scheduled dialysis, premature infant PCA <60 weeks, history (>3 months) of MI, CVA, TIA, or CAD/stents |
| ASA IV | A patient with severe systemic disease that is a constant threat to life | Examples include (but not limited to): recent (<3 months) MI, CVA, TIA, or CAD/stents, ongoing cardiac ischemia or severe valve dysfunction, severe reduction of ejection fraction, sepsis, DIC, ARD or ESRD not undergoing regularly scheduled dialysis |
| ASA V | A moribund patient who is not expected to survive without the operation | Examples include (but not limited to): ruptured abdominal/thoracic aneurysm, massive trauma, intracranial bleed with mass effect, ischemic bowel in the face of significant cardiac pathology or multiple organ/system dysfunction |
| ASA VI | A declared brain-dead patient whose organs are being removed for donor purposes |
- ARD acute respiratory disease, CAD coronary artery disease, COPD chronic obstructive pulmonary disease, CVA cerebral vascular accident, DIC disseminated intravascular coagulation, DM diabetes mellitus, ESRD end stage renal disease, HTN hypertension, MI myocardial infarction, PCA post-conceptual age
- Reprinted with permission from the American Association of Anesthesiologists
- The addition of “E” denotes Emergency surgery: (An emergency is defined as existing when delay in treatment of the patient would lead to a significant increase in the threat to life or body part)
The flowchart includes specific examples for application purposes.
Predictive factor
TG13 defines Grade III organ dysfunction as cardiovascular dysfunction, neurological dysfunction, respiratory dysfunction, renal dysfunction, hepatic dysfunction, or hematological dysfunction. Straightforward Lap-C is contraindicated if dysfunction occurs in these organ systems. However, in 2017, Yokoe et al. reported on joint research in Japan and Taiwan showing that Lap-C was performed fairly frequently in Grade III cases 17, 24. Furthermore, Endo et al. analyzed data on 5,329 AC patients from the same joint research in Japan and Taiwan and reported that the patients with Grade III AC accompanied by organ dysfunction included some patients who could have undergone cholecystectomy safely 25. Based on these studies, the TG18 guidelines define neurological dysfunction, respiratory dysfunction, and coexistence of jaundice (TBil ≥2 mg/dl) as negative predictive factors in Grade III AC, as multivariate analysis has shown these independent factors to be associated with a significant increase surgical mortality rates (mortality rate within 30 days of surgery). However, renal dysfunction and cardiovascular dysfunction are considered types of favorable organ system failure (FOSF) and are therefore defined as “non-negative predictive factors,” because these dysfunctions may often be reversibly improved by initial treatment and organ support.
We performed a literature search for the period after creating the TG13 guidelines (January 2013–December 2016) using the key words acute cholecystitis, severity, laparoscopic cholecystectomy, cholecystectomy, and biliary drainage. We identified two cohort research papers 26, 27 and eight case series studies 25, 28-34. In the two cohort research papers, no differences in bile duct injury and mortality rates were observed before and after the introduction of treatment strategies in line with severity grading, but overall hospital stays were shorter and medical costs lower following the introduction of this method. In some of the case series studies, survival rates and complication rates differed for each severity grading, so the authors were in agreement with the TG13 treatment strategies that are based on severity 26-30. In other case series studies, surgical outcomes were equivalent across the cholecystitis severity gradings for patients assessed as capable of withstanding surgery and who underwent early surgery; so, other authors considered TG13 to be too restrictive 33, 34.
A study on the usefulness of biliary drainage according to severity showed that this method was effective in alleviating symptoms and reducing the inflammatory response in blood tests 35. However, two retrospective analyses showed that patients undergoing biliary drainage had longer operating times, longer hospital stays, and higher mortality rates than patients not undergoing biliary drainage, with the same percentage of patients being switched to open surgery; these studies therefore showed biliary drainage did not have an useful effect on surgical outcomes 36, 37.
The introduction of systems to select treatment strategies according to severity grading is expected to have many benefits, as this method should allow doctors to choose treatments more accurately according to patient status, shorten overall hospital stays, and decrease medical costs 25, 38. We expect large-scale clinical studies will be performed to produce high-level evidence on the optimum treatment strategy for each severity grade and for this evidence to be used to further improve these guidelines.
Patient factors like predictive factors and CCI or ASA-PS scores can be used to decide whether surgery is possible. See CQ5 for more details.
At the Consensus Meeting, some participants stated that the guidelines should stress that surgical procedures should be performed only at facilities where advanced laparoscopic surgeons practice, in order to ensure that surgery was safe for patients with Grade II or Grade III AC.
If a patient is deemed capable of withstanding surgery for AC, we propose early surgery regardless of exactly how much time has passed since onset. (Recommendation 2, level B)
TG07 recommended that surgery for AC be performed soon after hospital admission, whereas TG13 recommended that surgery be performed soon after admission and within 72 h after onset. When managing AC, it is difficult to determine precisely how many hours have passed since disease onset. Some patients only present after 72 h have already passed since onset. For “early surgery” as described in TG07 and TG13, we have added further considerations on whether the “within 72 h” rule should be strictly observed and what is the optimal timing for surgery.
We based our considerations on a search of the literature after the publication of the TG13 guidelines (using the key words: acute cholecystitis, laparoscopic cholecystectomy, early cholecystectomy, delayed cholecystectomy, timing), which returned 17 randomized controlled trials, six meta-analyses, and three systematic reviews.
Lap-C was performed in the studies described by all of these papers. Diagnosis of AC was based on TG13 in one paper 39, and biochemical data, diagnostic imaging, and subjective/objective symptoms in the remaining 14 papers. Surgery timing was indicated as early cholecystectomy or delayed cholecystectomy. Early was defined as within 72 h since onset (as recommended in TG13) in two papers 40, 41; within 24 h of hospital admission in two papers 42, 43; within 24 h since the study began in one paper 44; within 72 h since patient presentation (or admission) or the study start in six papers 45-50; within 4 days in one study 51; within 1 week since onset in one study 52; and as soon as possible after patient presentation (with the actual timing not recorded) in two studies 39, 53. Delayed was defined in various different ways, including after diagnosis or after the symptoms diminished, but was most commonly defined as after at least 6 weeks. We therefore identified two sub-categories of early: within 72 h (of onset, presentation, or admission) and within one week including within 72 h (including those studies that stated “as early as possible”). Of the 17 randomized controlled trials, we excluded one study for which data could not be extracted 54. We also excluded another study where we thought there might be some bias, because the incidence of bile duct injury was higher than in normal clinical practice 55. We performed a meta-analysis on the remaining 15 studies.
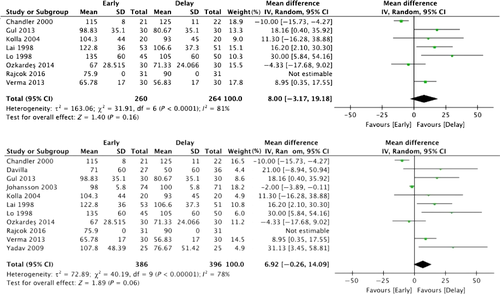
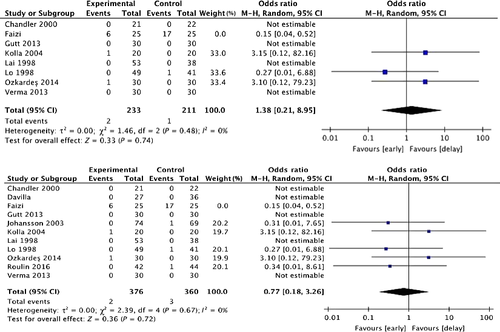
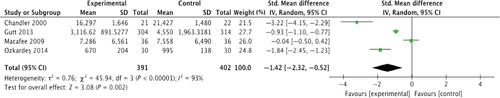
Meta-analysis
We compared early cholecystectomy (early surgery within 1 week or within 72 h) with delayed cholecystectomy. Key outcomes were operating times, incidence of bile duct injury, length of hospital stay, and overall cost of treatment. Operating times for delayed cholecystectomy tended to be shorter than for early cholecystectomy (both within 72 h and within 1 week), although the difference is not statistically significant (P = 0.16, P = 0.06) (Fig. 3). The incidence of bile duct injury did not differ between early (both within 72 h and within 1 week) and delayed cholecystectomy (P = 0.45, P = 0.72) (Fig. 4). However the total number of patients in the meta-analysis is much too low to draw any conclusions in this regard (“Absence of evidence is not evidence of absence”). Length of hospital stay was shorter for early cholecystectomy (both within 72 h and within 1 week) than delayed cholecystectomy (P < 0.0001, P < 0.00001) (Fig. 5). However, there was no difference in length of hospital stay after surgery (P = 0.33) (Fig. 6). Overall cost of treatment was lower for early cholecystectomy within 72 h than delayed cholecystectomy (P = 0.002) (Fig. 7). This meta-analysis on 15 randomized controlled trials shows that early cholecystectomy was not inferior to delayed cholecystectomy in terms of mortality rates and incidence of complications. There was no difference in length of hospital stay after surgery, but total hospital stays were shorter for early cholecystectomy and therefore overall cost of treatment was also lower. The five studies in these randomized controlled trials excluded the cases in which symptom onset began more than 72 h–1 week previously, and those whose symptoms suddenly recurred during the waiting period such that emergency Lap-C had to be performed were also discontinued from consideration for delayed surgery. Therefore, it is not clear how many of the AC cases included cases with chronic inflammation and acute exacerbations. In the 15 randomized controlled trials, 6–23% of patients underwent emergency Lap-C when symptoms suddenly recurred during the waiting period. With delayed cholecystectomy, AC can flare up again during the waiting period. Tissues become progressively more scarred with repeated episodes of inflammation, making surgery more difficult. From this perspective, delayed cholecystectomy is associated with greater risk. The TG13 guidelines basically recommended early surgery as the treatment for AC, with a specific recommendation for cholecystectomy soon after hospitalization if no more than 72 h has passed since symptom onset. Two randomized controlled trials compared delayed cholecystectomy versus early cholecystectomy in patients where symptoms started no more than 72 h previously 40, 41. In both of these trials, the early surgery group had shorter total hospital stays and shorter operating times. No mention was made of the incidence of bile duct injury.
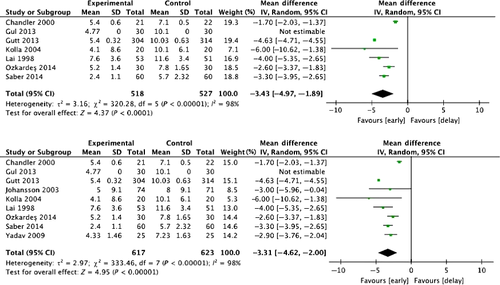
The meta-analysis of the case study reports found that, compared with delayed cholecystectomy, early cholecystectomy for cases within 72 h of patient presentation or symptom onset was associated with lower mortality rates, complication rates, incidence of bile duct injury, and switching to open surgery. Similar results were also obtained with early cholecystectomy for cases where patient presentation/symptom onset occurred 72 h–1 week previously 56. Therefore, for AC patients for whom more than 72 h has passed since symptom onset, there still are benefits to performing surgery early.
A comparison of early surgery performed within 24 h of symptom onset and early surgery performed within 72 h shows that the outcomes from the former group were not superior to those in the latter group 57. Even if there are benefits to early surgery, this does not mean that urgent surgery after hours should be performed. Ideally, surgery should be performed by surgeons experienced in laparoscopy or at facilities with a long history of laparoscopic procedures 58.
Compared with delayed cholecystectomy, early cholecystectomy performed within 72 h if possible and even within 1 week may reduce costs, as the overall hospital stays are shorter and there is less chance the patient will require additional treatments or emergency surgery due to symptoms suddenly recurring during the waiting period.
There are no reports providing quality scientific evidence on the best timing for surgery after percutaneous transhepatic gallbladder drainage (PTGBD; also called cholecystostomy), so a consensus has not been reached. (Level C)
There are no randomized controlled trials on the best time for Lap-C after PTGBD. Four observational studies featured various different times before surgery after PTGBD, and we assign these studies as evidence level C. Table 3 provides a summary of these studies 59-62.
| Author | Time until surgery after PTGBD | Summary of outcomes | |
|---|---|---|---|
| Early surgery group (n) | Delayed surgery group (n) | ||
| Han et al. 2012 59 | <72 h (21) | ≥72 h (46) | Early group had higher incidence of postoperative complications, longer operating times. Percentage of patients switched to open surgery was the same in the two groups. Early group had shorter total hospital stays |
| Choi et al. 2012 60 | <72 h (63) | ≥5 days (40) | Early group had higher bleeding volumes and longer operating times |
| Jung et al. 2015 62 | <10 days (30) | ≥10 days (44) | Equivalent rates between the two groups for postoperative complication rates, operating times, percentage of patients switched to open surgery, and total hospital stays |
| Tanaka et al. 2016 61 | <14 days (16) | ≥14 days (47) | Higher bleeding volumes in the early group |
- PTGBD percutaneous transhepatic gallbladder drainage
PTGBD is used for therapeutic purposes if the patient has problematic complications or comorbidities. In a large-scale case series study in Japan and Taiwan, mortality risk with urgent surgery was higher in patients scoring CCI ≥6 or body mass index (BMI) ≤20 if they had Grade I or II AC according to the TG13 severity grading and in patients with jaundice (TBil ≥2.0 mg/dl), cranial neuropathy, or respiratory dysfunction if they had Grade III AC 25. For such high-risk patients, early/urgent surgery is not recommended and PTGBD is indicated. When PTGBD is performed for high-risk patients, it is assumed that it would be difficult to perform surgery immediately after the PTGBD procedure. In practice, studies have shown various outcomes in high-risk patients who underwent PTGBD followed by early/urgent surgery, including longer operating times and increased bleeding 60, 61. That said, one study reported that the differences were not substantial between the two approaches 62. Furthermore, two studies comparing surgery after PTGBD to early surgery without PTGBD (one randomized controlled trial 63 and one cohort study 64) both reported good outcomes when Lap-C was performed after waiting 4–6 weeks after PTGBD for the factors bleeding volume, operating times, percentage of patients switched to open surgery, and incidence of complications. These results suggest that risks may be increased further when Lap-C is performed at a relatively early stage after PTGBD in high-risk patients. From a cost perspective, however, another study reported that costs were lower in patients treated with early Lap-C after PTGBD 59. At this stage, a consensus has yet to be reached on the timing of surgery after PTGBD. Ideally, the physician treating the patient will determine the optimum timing for managing the patient while bearing in mind patient risk. We look forward to more studies like the CHOCOLATE trial currently underway 65 to build up a body of quality evidence.
For Grade I and II patients, we propose scores of CCI ≥6 and ASA-PS ≥3 as surgical risk factors.
For risk factors for Grade III patients, we propose the negative predictive factors of neurological dysfunction, respiratory dysfunction, and coexistence of jaundice (TBil ≥2 mg/dl). We propose scores of CCI ≥4 and ASA-PS ≥3 as risk factors indicating that the patient might not withstand surgery. (Level C)
In a cohort analysis by Endo et al. of 5,459 AC patients in Japan and Taiwan, multivariate analysis showed a statistically significant increase in 30-day mortality patients with Grade I or II AC who had CCI ≥6 (Table 4) 25. Multivariate analysis was also used to analyze 30-day mortality risk factors in Grade III patients (Table 5) 25. Grade III patients of AC have at least one organ failure. Among prescribed organ disorders in TG13, neurological and respiratory failure were predictive factors. Furthermore, coexistence of jaundice is another predictive factor in addition to one or more organ dysfunction regulated by TG13. Predictive factors for 30-day and 90-day mortality were also investigated in Grade III patients undergoing straightforward cholecystectomy and Grade III patients undergoing cholecystectomy after PTGBD (Table 6) 25. The top of Table 6 shows the 30-day mortality rate and the bottom shows the 90-day mortality rate. In group A, straightforward cholecystectomy is performed, and in group B, surgery is performed after drainage. There is no significant 30-day and 90-day mortality rate between A and B in Grade III without predictive factors (neurological dysfunction, respiratory failure, coexistence of jaundice) 25.
| Survivor (n = 2,677) | Non-survivor (n = 21) | Univariate P-value | Multivariate P-value | Odds ratio | 95% CI | |
|---|---|---|---|---|---|---|
| Body mass index | ||||||
| <20 | 349 | 9 | <0.01 | 0.011 | ||
| >20 to <25 | 1,360 | 7 | <0.01 | 0.241 | 0.088–0.659 | |
| >25 | 968 | 5 | 0.032 | 0.290 | 0.094–0.898 | |
| PS | ||||||
| 0–2 | 2,571 | 17 | <0.01 | 0.054 | ||
| 3–4 | 106 | 4 | ||||
| CCI | ||||||
| 0–5 | 2,140 | 9 | <0.01 | <0.01 | 4.433 | 1.816–10.822 |
| ≥6 | 537 | 12 | ||||
- Source: Endo et al. 25, reprinted with permission from John Wiley & Sons (No. 4177091307865)
| Survivor (n = 591) | Non-survivor (n = 20) | Univariate P-value | Multivariate P-value | Odds ratio | 95% CI | |
|---|---|---|---|---|---|---|
| PS | ||||||
| 0–2 | 532 | 14 | <0.01 | 0.156 | ||
| 3–4 | 59 | 6 | ||||
| CCI | ||||||
| 0–5 | 304 | 7 | 0.148 | 0.380 | ||
| 26 | 287 | 13 | ||||
| Jaundice | ||||||
| − | 477 | 9 | <0.01 | <0.01 | 6.470 | 2.446–17.110 |
| + | 114 | 11 | ||||
| Cardiovascular | ||||||
| − | 457 | 13 | 0.198 | 0.493 | ||
| + | 134 | 7 | ||||
| Neurological | ||||||
| − | 518 | 12 | <0.01 | <0.01 | 4.346 | 1.640–11.515 |
| + | 73 | 8 | ||||
| Respiratory | ||||||
| − | 528 | 13 | <0.01 | <0.01 | 5.843 | 2.052–16.635 |
| + | 63 | 7 | ||||
| Renal | ||||||
| − | 385 | 10 | 0.164 | 0.073 | ||
| + | 206 | 10 | ||||
| Hepatic | ||||||
| − | 371 | 14 | 0.510 | 0.360 | ||
| + | 220 | 6 | ||||
| Hematological | ||||||
| − | 459 | 17 | 0.437 | 0.513 | ||
| + | 132 | 3 | ||||
- Source: Endo et al. 25, reprinted with permission from John Wiley & Sons (No. 4177091307865)
| Group A (n = 260) | Group B (n = 180) | Group C (n = 93) | Group B + C (n = 273) | P-value | ||
|---|---|---|---|---|---|---|
| 30-day mortality | ||||||
| No positive | 0 | 0 | 2 | 2 | NA | (A vs. B) |
| PF | 0.00 | 0.00 | 4.55 | 1.27 | 0.040 | (A vs. C) |
| 0.226 | (A vs. B+C) | |||||
| Any positive | 8 | 0 | 7 | 7 | 0.010 | (A vs. B) |
| PFs | 9.30 | 0.00 | 14.29 | 6.09 | 0.403 | (A vs. C) |
| 0.426 | (A vs. B+C) | |||||
| Group A (n = 219) | Group B (n = 168) | Group C (n = 74) | Group B + C (n = 242) | P-value | ||
|---|---|---|---|---|---|---|
| 90-day mortality | ||||||
| No positive | 2 | 0 | 6 | 6 | 0.513 | (A vs. B) |
| PF | 1.31 | 0.00 | 16.22 | 4.14 | 0.001 | (A vs. C) |
| 0.164 | (A vs. B+C) | |||||
| Any positive | 7 | 0 | 9 | 9 | 0.014 | (A vs. B) |
| PFs | 10.61 | 0.00 | 24.32 | 9.28 | 0.089 | (A vs. C) |
| 0.794 | (A vs. B+C) | |||||
- Group A: cholecystectomy, Group B: cholecystectomy after PTGBD
- NA statistical value could not be analyzed, PF jaundice, neurological dysfunction, respiratory dysfunction
- Source: Endo et al. 25, reprinted with permission from John Wiley & Sons (No. 4177091307865)
The ASA-PS is also reported as a risk factor in AC in several articles. ASA-PS 3 or over is high risk for emergency cholecystectomy 66-69. ASA-PS score (from 2 to 5) was a significant risk factor for death 70. Based on the above, ASA-PS was also adopted. However, one study reported no deaths after cholecystectomy when patients with ASA-PS ≥3 were operated on at advanced centers (where experienced surgeons practice) 67. We hope that more case series data will be gathered for future analysis.
Flowchart for the management of acute cholecystitis
Grade I
Figure 8 shows a treatment flowchart for Grade I AC. There are no substantial differences with the TG13 guidelines, but the flowchart does include additional considerations on patient risk factors.
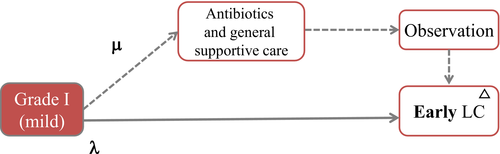
Explanation of flowchart of Grade I AC (Fig. 8)
In principle, early Lap-C is the first-line treatment for the cases of Grade I. However, in patients with surgical risk (broken line) using CCI and ASA-PS, antibiotics and general supportive care are firstly necessary. Then, after improvement with initial medical treatment, they could be indicated to Lap-C.
The patient's status should be fully understood and surgery performed with a focus on safety. For information on early treatment, doctors should refer to the description of initial treatment for bile duct inflammation from Miura et al. 4 and to guidelines on antimicrobials from Gomi et al. 6.
Grade II
Figure 9 shows a treatment flowchart for Grade II AC.
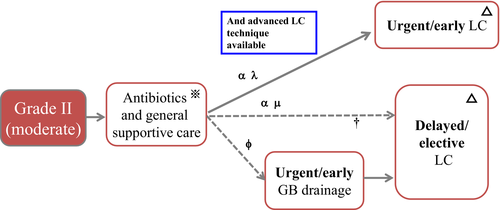
Explanation of flowchart of Grade II AC (Fig. 9)
Grade II (moderate) AC is often accompanied by severe local inflammation. Therefore, surgeons should take the difficulty of cholecystectomy into consideration in selecting a treatment method.
Early Lap-C could be first indicated if advanced laparoscopic techniques are available. When the judgment of cholecystectomy is made, general condition should be evaluated using CCI and ASA-PS. Elective cholecystectomy after the improvement of the acute inflammatory process could be indicated in the poor conditional patients (broken line). If a patient does not respond to initial medical treatment, urgent or early gallbladder drainage is required (broken line). CCI 6 or greater and ASA-PS 3 or greater are high risk. If not, transfer to advanced center should be considered.
The patient's risk factors should be fully understood and it is essential that surgery be performed in a facility capable of conducting such procedures safely. If the medical facility is not capable of providing treatment such as early cholecystectomy or biliary drainage, the patient should be transferred to an appropriate medical facility as soon as possible. For biliary drainage, PTGBD is currently recommended 38 and doctors should refer to the paper by Mori et al. 71.
When surgery is performed, it is important to be aware that the degree of surgical difficulty can vary widely depending on the level of inflammation and fibrosis. During surgery, findings on the difficulty index should be confirmed and Lap-C should be undertaken safely making sure to avoid risks 72-76. In case of serious operative difficulty, bail-out procedures including conversion should be used 76.
Grade III
Figure 10 shows a treatment flowchart for Grade III AC.

Explanation of flowchart of Grade III AC (Fig. 10)
Grade III AC is accompanied by organ dysfunction. Appropriate organ support such as ventilatory/circulatory management (noninvasive/invasive positive pressure ventilation and use of vasopressors, etc) in addition to initial medical treatment is necessary. Early or urgent cholecystectomy can be possible under intensive care, when the judgment of cholecystectomy is made using predictive factor, FOSF, CCI and ASA-PS. The predictive factors in Grade III are jaundice (TBil ≥2), neurological dysfunction, and respiratory dysfunction. As early operation is best in those patients who have rapidly reversible failure of cardiovascular and/or renal failure, we advocate FOSF. FOSF means cardiovascular or renal organ system failure which is rapidly reversible after admission and before early Lap-C in AC. Because Grade III patients have one or more organ dysfunction, CCI 6 is too high score and not cutoff value of high risk for cholecystectomy. CCI 4 or greater and ASA-PS 3 or greater are eligible high risk factor for cholecystectomy in Grade III. If not, urgent or early gallbladder drainage should be performed. Elective cholecystectomy may be performed after the improvement of acute illness has been achieved by gallbladder drainage. Lap-C in Grade III of AC should be performed by expert surgeon who often completed additional training beyond their basic general surgical education under intensive care. If not, transfer to advanced center should be considered.
With Grade III AC, the patient's overall status has deteriorated significantly and treatment should be chosen based on full and careful consideration of the patient's background, including complications and comorbidities (organ failure). When Lap-C is chosen, we stress that it is absolutely vital for this to be performed by someone with advanced skills. Ideally the patient should be transferred quickly to a suitable medical facility if the initial medical facility is not capable of providing complete intensive care and treatments like early cholecystectomy and biliary drainage. PTGBD is recommended for biliary drainage, as with Grade II patients 38; for more details on the method, doctors should refer to the paper by Mori et al. 71.
After considering predictive factors and FOSF, even when surgery is performed on patients whose overall status allows resection, rigorous whole-body management is vital to manage organ dysfunction and other issues, and surgeons need to bear in mind the possibility that the surgery may be extremely difficult, so difficulty indicators should be monitored during surgery and every effort should be made to avoid risks to ensure the Lap-C is performed safely 72-76. If the cholecystectomy proves difficult, surgeons should not hesitate to perform bail-out surgery 76.
Criteria for transfer to an “advanced center” (Table 7)
| Severe acute cholecystitis (Grade III) |
| When a patient meets certain conditions defined by the AC flowchart, Lap-C can be performed only by an expert laparoscopic surgeon at a specialized center that provides intensive care. Otherwise, transfer to advanced facilities should be considered |
| Moderate acute cholecystitis (Grade II) |
| Patients should be treated at centers that can provide emergent drainage of the gallbladder or early Lap-C. Otherwise, transfer to advanced facilities should be considered |
| Mild acute cholecystitis (Grade I) |
| In the case of patients whose operation is delayed because of existing serious comorbidity transfer to advanced facilities that can provide emergent drainage of the gallbladder or early Lap-C should be considered |
The Statement
Surgical skill and experience in advanced MIS surgery vary.
The selection of a particular pathway of care should take this factor into account.
When skill and experience are high, early LC in AC may be appropriate in all Grade of AC as indicated in the flowcharts.
The application of patient selection criteria is other key factor predictive of success (predictive factor, FOSF, CCI, ASA-PS etc).
Acknowledgments
We would like to express our deep gratitude to the Japanese Society for Abdominal Emergency Medicine, the Japan Biliary Association, Japan Society for Surgical Infection, and the Japanese Society of Hepato-Biliary-Pancreatic Surgery, which provided us with great support and guidance in the preparation of the Guidelines.
Conflict of interest
Goro Honda has received honoraria from Johnson and Johnson and Medtronic.
Appendix: author's affiliations
Kohji Okamoto, Department of Surgery, Center for Gastroenterology and Liver Disease, Kitakyushu City Yahata Hospital, Fukuoka, Japan; Kenji Suzuki, Department of Surgery, Fujinomiya City General Hospital, Shizuoka, Japan; Tadahiro Takada, Fumihiko Miura and Keita Wada, Department of Surgery, Teikyo University School of Medicine, Tokyo, Japan; Steven M. Strasberg, Section of Hepato-Pancreato-Biliary Surgery, Washington University School of Medicine in St. Louis, St. Louis, MO, USA; Horacio J. Asbun, Department of Surgery, Mayo Clinic College of Medicine, Jacksonville, FL, USA; Itaru Endo, Department of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine, Kanagawa, Japan; Yukio Iwashita and Masafumi Inomata, Department of Gastroenterological and Pediatric Surgery, Oita University Faculty of Medicine, Oita, Japan; Taizo Hibi, Department of Surgery, Keio University School of Medicine, Tokyo, Japan; Henry A. Pitt, Lewis Katz School of Medicine at Temple University, Philadelphia, PA, USA; Akiko Umezawa, Minimally Invasive Surgery Center, Yotsuya Medical Cube, Tokyo, Japan; Koji Asai, Department of Surgery, Toho University Ohashi Medical Center, Tokyo, Japan; Ho-Seong Han and Yoo-Seok Yoon, Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seoul, Korea; Tsann-Long Hwang, Keng-Hao Liu and Miin-Fu Chen, Division of General Surgery, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan; Yasuhisa Mori and Masafumi Nakamura, Department of Surgery and Oncology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan; Wayne Shih-Wei Huang, Department of Surgery, Show Chwan Memorial Hospital, Changhua, Taiwan; Giulio Belli, Department of General and HPB Surgery, Loreto Nuovo Hospital, Naples, Italy; Christos Dervenis, First Department of Surgery, Agia Olga Hospital, Athens, Greece; Masamichi Yokoe and Yoshinori Noguchi, Department of General Internal Medicine, Japanese Red Cross Nagoya Daini Hospital, Aichi, Japan; Seiki Kiriyama, Department of Gastroenterology, Ogaki Municipal Hospital, Gifu, Japan; Takao Itoi, Department of Gastroenterology and Hepatology, Tokyo Medical University Hospital, Tokyo, Japan; Palepu Jagannath, Department of Surgical Oncology, Lilavati Hospital and Research Centre, Mumbai, India; O James Garden, Clinical Surgery, University of Edinburgh, Edinburgh, UK; Akihiko Horiguchi, Department of Gastroenterological Surgery, Fujita Health University School of Medicine, Aichi, Japan; Go Wakabayashi, Department of Surgery, Ageo Central General Hospital, Saitama, Japan; Daniel Cherqui, Hepatobiliary Center, Paul Brousse Hospital, Villejuif, France; Eduardo de Santibañes, Department of Surgery, Hospital Italiano, University of Buenos Aires, Buenos Aires, Argentina; Satoru Shikata, Director, Mie Prefectural Ichishi Hospital, Mie, Japan; Tomohiko Ukai, Department of Family Medicine, Mie Prefectural Ichishi Hospital, Mie, Japan; Ryota Higuchi and Masakazu Yamamoto, Department of Surgery, Institute of Gastroenterology, Tokyo Women's Medical University, Tokyo, Japan; Goro Honda, Department of Surgery, Tokyo Metropolitan Komagome Hospital, Tokyo, Japan; Avinash Nivritti Supe, Department of Surgical Gastroenterology, Seth G S Medical College and K E M Hospital, Mumbai, India; Masahiro Yoshida, Department of Hemodialysis and Surgery, Ichikawa Hospital, International University of Health and Welfare, Chiba, Japan and Department of EBM and Guidelines, Japan Council for Quality Health Care, Tokyo, Japan; Toshihiko Mayumi, Department of Emergency Medicine, School of Medicine, University of Occupational and Environmental Health, Fukuoka, Japan; Dirk J. Gouma, Department of Surgery, Academic Medical Center, Amsterdam, The Netherlands; Daniel J. Deziel, Department of Surgery, Rush University Medical Center, Chicago, IL, USA; Kui-Hin Liau, Liau KH Consulting PL, Mt Elizabeth Novena Hospital, Singapore, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore; Kazunori Shibao, Department of Surgery 1, School of Medicine, University of Occupational and Environmental Health, Fukuoka, Japan; Cheng-Hsi Su, Department of Surgery, Cheng Hsin General Hospital, Taipei, Taiwan; Angus C. W. Chan, Surgery Centre, Department of Surgery, Hong Kong Sanatorium and Hospital, Hong Kong, Hong Kong; Dong-Sup Yoon, Department of Surgery, Yonsei University Gangnam Severance Hospital, Seoul, Korea; In-Seok Choi, Department of Surgery, Konyang University Hospital, Daejeon, Korea; Eduard Jonas, Surgical Gastroenterology/Hepatopancreatobiliary Unit, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa; Xiao-Ping Chen, Hepatic Surgery Centre, Department of Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Sheung Tat Fan, Director, Liver Surgery Centre, Hong Kong Sanatorium and Hospital, Hong Kong, Hong Kong; Chen-Guo Ker, Department of Surgery, Yuan's General Hospital, Kaohsiung, Taiwan; Mariano Eduardo Giménez, Chair of General Surgery and Minimal Invasive Surgery “Taquini”, University of Buenos Aires, DAICIM Foundation, Buenos Aires, Argentina; Seigo Kitano, President, Oita University, Oita, Japan; Koichi Hirata, Department of Surgery, JR Sapporo Hospital, Hokkaido, Japan; Kazuo Inui, Department of Gastroenterology, Second Teaching Hospital, Fujita Health University, Aichi, Japan; Yoshinobu Sumiyama, Director, Toho University, Tokyo, Japan.




