Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study
Abstract
Background
The aim of this study was to compare the long-term outcomes and perioperative outcomes of laparoscopic liver resection (LLR) with those of open liver resection (OLR) for hepatocellular carcinoma (HCC) between well-matched patient groups.
Methods
Hepatocellular carcinoma patients underwent primary liver resection between 2000 and 2010, were collected from 31 participating institutions in Japan and were divided into LLR (n = 436) and OLR (n = 2969) groups. A one-to-one propensity case-matched analysis was used with covariates of baseline characteristics, including tumor characteristics and surgical procedures of hepatic resections. Long-term and short-term outcomes were compared between the matched two groups.
Results
The two groups were well balanced by propensity score matching and 387 patients were matched. There were no significant differences in overall survival and disease-free survival between LLR and OLR. The median blood loss (158 g vs. 400 g, P < 0.001) was significantly less with LLR, and the median postoperative hospital stay (13 days vs. 16 days, P < 0.001) was significantly shorter for LLR. Complication rate (6.7% vs. 13.0%, P = 0.003) was significantly less in LLR.
Conclusion
Compared with OLR, LLR in selected patients with HCC showed similar long-term outcomes, associated with less blood loss, shorter hospital stay, and fewer postoperative complications.
Introduction
The advancements of laparoscopic procedures in liver surgery have proceeded slowly given the inherent risks for massive bleeding associated with liver resection 1. The First International Consensus Conference on Laparoscopic Liver Surgery convened in Louisville in 2008 2, since then, the number of laparoscopic liver resection (LLR) reported has increased steadily worldwide and the greatest diffusion of LLR occurred in East Asia, North America, and Europe 3, 4. Moreover, the number of hepatocellular carcinoma (HCC) cases to which LLR is applied has increased steeply over the past 5 years, especially in Asia and Europe 5, 6. However, no randomized controlled trials (RCTs) have been published and the available data derive from multiple case series, case-control studies, reviews, and meta-analyses published over the last several years.
For new surgical procedures to become widely adopted as standard operations, they should first be compared with established procedures and shown to be superior in at least some respects 7. Despite its lateness to embrace laparoscopy, liver surgery is now gaining momentum in this paradigm shift. Following improvements in technology and equipment, LLR should now be considered a safe option, if performed by experienced surgeons. Additionally, dramatic improvements in the safety of hepatic resection, based on an increased understanding of liver anatomy and better preoperative radiologic imaging, have facilitated this transition. Thus, adoption of the laparoscopic approach for the surgical treatment of hepatic lesions is now progressively expanding. However, unfortunately, it is impossible to reach an accurate conclusion regarding benefits and risks of LLR over open liver resection (OLR) in the absence of RCTs.
Propensity score matched analysis has become increasingly used in retrospective cohorts to reduce the impact of treatment-selection bias in the comparison of treatment to a non-randomized control using observational data 8, 9. This type of evaluation has been proven to decrease selection bias in retrospective studies and allows comparison between different surgical procedures. Several studies have demonstrated that LLR for HCC is less invasive and can provide similar disease-free survival (DFS) and overall survival (OS) compared with OLR 10-17. However, most of these studies were based on retrospective analyses of case-matched studies or meta-analyses of non-randomized studies. The aim of the present study was therefore to compare the long-term oncological outcomes and the perioperative outcomes of LLR with those of OLR for HCC, using propensity score matching (PSM) of relatively large data collected from 31 institutions in Japan.
Patients and methods
This multicenter clinical study was conducted by the “Project Committee of the Endoscopic Surgery” of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. We retrospectively reviewed 3405 patients who underwent primary liver resection for HCC from 2000 to 2010, who were gathered by 31 Japanese institutions in the Endoscopic Liver Surgery Study Group. The patients were divided into LLR (n = 436) and OLR (n = 2969) groups. The diagnosis of HCC was confirmed by histologic examination of resected specimens in all patients.
This study was approved by the ethics committee of the Japanese Society of Hepato-Biliary-Pancreatic Surgery, as well as one from each Institutional Review Board, and conducted in accordance with the mandates of the Helsinki Declaration.
Propensity score analysis
To avoid confounding differences due to baseline varieties between laparoscopic and open approaches, we performed a propensity score-matched subset. Propensity score analysis was used to build a matched group of patients for comparison of oncological and short-term outcomes between LLR and OLR groups. The propensity scores were generated with the preoperative characteristics, including sex, age, underlying liver disease (hepatitis B surface antigen (HBs-Ag) and anti-hepatitis C virus antibody positivity), tumor size, tumor number, serum α-fetoprotein and des-gamma-carboxy prothrombin levels, indocyanine green retention rate at 15 min (ICGR 15 min), extent of liver damage (decide according to the Criteria of the Liver Cancer Study Group of Japan) 18, 19, Child–Pugh score, difficult tumor location (yes, no), and distant metastasis (yes, no). Difficult tumor location was defined as postero-superior segments of the liver (segment 1, 7, 8 and the superior part of segment 4) 20. Surgical procedures were classified according to the Brisbane 2000 nomenclature of liver resection 21. In this study, hemihepatectomy, trisectionectomy, central bisectionectomy, right anterior sectionectomy, right posterior sectionectomy, and medial sectionectomy were defined as major hepatectomy, while wedge resection and left lateral sectionectomy were as minor hepatectomy. The wedge resection was only non-anatomical resection. The predicted probability of preprocedural stains was calculated by fitting a logistic regression model, using all preoperative relevant clinical variables as shown in Table 1. PSM was performed using a 1:1 ratio without replacement by caliper-matching on the estimated propensity score. The value of the caliper was calculated by 0.25 × (the standard deviation (SD) of log (the propensity score (PS)/ 1-PS)). Receiver operating characteristic (ROC) curves were used to assess the accuracy of PSM, as a predictor of LLR indicated by a propensity score.
| Covariates | LLR (n = 436) | OLR (n = 2969) | P | Matched-LLR (n = 387) | Matched-OLR (n = 387) | P |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 142 (32.6%) | 644 (21.7%) | 125 (32.30%) | 126 (32.56%) | ||
| Male | 294 (67.4%) | 2325 (78.3%) | <0.001 | 262 (67.70%) | 261 (67.44%) | 0.939 |
| Age (year) | 66.48 ± 9.87 | 66.68 ± 9.64 | 0.69 | 66.42 ± 9.84 | 66.19 ± 9.96 | 0.741 |
| Height | 160.2 ± 9.12 | 161.0 ± 8.67 | 0.073 | 160 ± 9.19 | 160.9 ± 8.72 | 0.21 |
| Weight | 59.0 ± 11.25 | 60.5 ± 11.18 | 0.012 | 59.0 ± 10.92 | 60.0 ± 11.11 | 0.203 |
| HBV positive | 99 (22.7%) | 663 (23.1%) | 0.886 | 91 (23.51%) | 100 (25.84%) | 0.453 |
| HCV positive | 222 (51.0%) | 1473 (51.3%) | 0.932 | 195 (50.39%) | 198 (51.16%) | 0.829 |
| Liver damage | ||||||
| A | 347 (80.51%) | 2244 (75.94%) | 312 (80.62%) | 311 (80.36%) | ||
| B | 73 (16.94%) | 533 (18.04%) | 65 (16.80%) | 70 (18.09%) | ||
| C | 11 (2.55%) | 178 (6.02%) | 0.009 | 10 (2.58%) | 6 (1.55%) | 0.552 |
| Child–Pugh | 5.34 ± 0.66 | 5.34 ± 0.66 | 0.944 | 5.33 ± 0.64 | 5.32 ± 0.61 | 0.774 |
| ICG R15 | 15.8 ± 10.9 | 15.8 ± 9.12 | 0.949 | 15.7 ± 11.0 | 16.5 ± 9.93 | 0.292 |
| Number | 1.16 ± 0.50 | 1.47 ± 1.09 | <0.001 | 1.17 ± 0.52 | 1.21 ± 0.54 | 0.246 |
| Size (mm) | 28.7 ± 15.2 | 40.2 ± 26.0 | <0.001 | 28.8 ± 15.1 | 28.8 ± 15.0 | 0.992 |
| Difficult location | 96 (22.1%) | 1447 (50.2%) | <0.001 | 82 (21.19%) | 80 (20.67%) | 0.86 |
| Distant meta | 3 (0.69%) | 11 (0.37%) | 0.332 | 3 (0.78%) | 1 (0.26%) | 0.316 |
| AFP (ng/ml) | 9.45 (4.35, 65.25) | 14.3 (5.1, 133) | 0.027 | 9.3 (4.3, 61.9) | 13.5 (5, 100) | 0.067 |
| DCP (mAU/ml) | 47 (23, 210) | 81 (25, 637) | 0.206 | 48 (24, 225) | 42 (21, 195.9) | 0.206 |
| Major hepatectomy | 46 (10.55%) | 952 (32.21%) | <0.001 | 42 (10.85%) | 36 (9.30%) | 0.474 |
| Minor hepatectomy | 341 (78.21%) | 1384 (46.82%) | <0.001 | 299 (77.26%) | 305 (78.81%) | 0.602 |
- AFP α-fetoprotein, DCP des-gamma-carboxy prothrombin, HBV hepatitis B virus, HCV hepatitis C virus, ICG indocyanine green
Comparison between the two matched groups
The study criteria for comparing the two matched groups were the following: (i) clinicopathologic data of each matched group; (ii) intraoperative and surgical results. Morbidity was graded according to the Clavien-Dindo classification and Grade IIIa or greater complications were counted between the two matched groups. Further, we investigated each perioperative outcome of patients who underwent major hepatectomy or minor hepatectomy in each matched group; and (iii) long-term oncologic outcomes in aspects of OS and DFS.
Statistical analysis
PSM and the other statistical analyses after PSM were performed by Stata 13 (Stata Corporation, College Station, TX, USA). In analyses and comparisons of preoperative covariates and clinical parameters after PSM, Student's t-test or Wilcoxon rank sum test for continuous variables, and χ2 test or Fisher's exact test for categorical variables were used. All categorical data were expressed as number or frequency (%), and all continuous data were as mean ± standard deviation, or median (25, 75% quartile deviation). The DFS period was calculated from the date of surgery to the recurrence of HCC. Survival rates were estimated using the Kaplan–Meier methods and the log-rank test for the P-value for OS and DFS. The Cox proportional hazards regression was used to calculate the hazard ratio (HR) and 95% confidence interval for univariate and multivariate analyses. A P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 summarizes the baseline characteristics of the overall cohort and that selected after PSM. In the overall cohort, most of the LLR patients were females (32.6% vs. 21.7%), the mean height of the LLR patients was lower than that of the OLR patients, most of the LLR patients had Liver damage A (80.51% vs. 75.94%), the number and size of the tumor in the LLR patients were significantly less and smaller than in the OLR patients, most of the LLR patients had non-difficult location of the tumor (77.9% vs. 49.8%), and minor hepatectomy had been performed in most of the LLR patients (78.21% vs. 46.82%). After PSM both groups were well balanced for all variables, as shown in Table 1. The ROC area under the curve of the propensity score for undergoing LLR was 0.786 (Figs S1,S2).
Clinicopathological outcomes
Between the LLR and OLR groups after PSM, the background of the liver about the staging of the fibrosis according to new Inuyama classification of chronic hepatitis 22, microvascular invasion, positive pathological surgical margin, and the tumor stage according to the General Rules for the Clinical and Pathological Study of Primary Liver Cancer, were almost similar (Table 2).
| Matched–LLR (n = 387) | Matched–OLR (n = 387) | p | |
|---|---|---|---|
| Background of liver | |||
| F3–F4 | 232 (61.7%) | 211 (59.6%) | 0.562 |
| A2– | 90 (33.21%) | 99 (43.23%) | 0.021 |
| Differentiation | |||
| Well | 59 (15.57%) | 85 (22.49%) | |
| Moderately | 266 (70.18%) | 236 (62.43%) | |
| Poorly | 44 (11.61%) | 53 (14.02%) | 0.031 |
| Vascular invasion | |||
| va1–va2 | 4 (1.04%) | 1 (0.26%) | 0.18 |
| vp1–vp2 | 48 (12.5%) | 58 (15.03%) | |
| vp3 | 2 (0.52%) | 1 (0.26%) | 0.51 |
| vv1–vv2 | 12 (3.13%) | 21 (5.47%) | 0.111 |
| b1–b2 | 3 (0.79%) | 1 (0.26%) | 0.311 |
| Pathological surgical margin (+) | 18 (4.68%) | 17 (4.43%) | 0.869 |
| TNM classification | |||
| Stage I | 97 (25.06%) | 96 (24.81%) | |
| Stage II | 227 (58.66%) | 210 (54.26%) | |
| Stage III | 55 (14.21%) | 73 (18.86%) | |
| Stage IVa or IVb | 8 (2.06%) | 8 (2.06%) | 0.269 |
Long-term oncologic outcomes
We performed Kaplan–Meier analyses for OS and DFS curves, as shown in Figures 1 and 2. The median observation period in the LLR group was 46.7 months (25% quartile deviation: 31.4 months, 75%: 60.5 months), and one in the OLR group was 51.7 months (25%: 31.8 months, 75%: 75.8 months). The cumulative 1-, 3- and 5-year OS rates were 95.8, 86.2 and 76.8% in the LLR group, 95.8 and 84.0, and 70.9% in the OLR group. On the other hand, the cumulative 1-, 3- and 5-year DFS rates were 83.7, 58.3 and 40.7% in the LLR group, 79.6 and 50.4, and 39.3% in the OLR group, respectively. There were no significant differences in OS (P = 0.358) and DFS (P = 0.422) between the matched two groups.
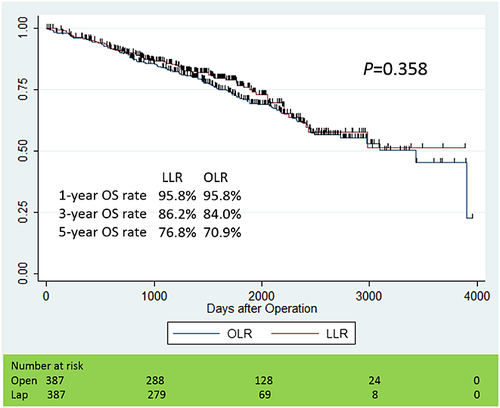
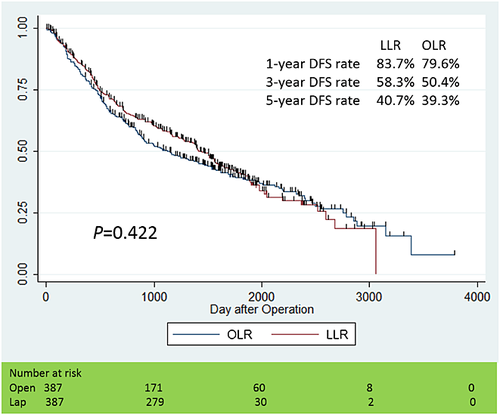
Perioperative outcomes
In the LLR group after PSM, the median blood loss (158 g) was significantly less (P < 0.001) than in the OLR group (400 g), and the median postoperative hospital stay (13 days) for the LLR patients was significantly shorter (P < 0.001) than for the OLR patients (16 days), while the operation time in the LLR group (294.4 ± 158.8 min) was significantly longer than in the OLR group (271.0 ± 130.0) (P = 0.025). Conversion from LLR to OLR or Hybrid or hand-assisted laparoscopic surgery occurred in 25 patients (6.5%). Complications over Grade IIIa according to the Clavien-Dindo classification after LLR included ascites (n = 7), intraperitoneal abscess (n = 4), pleural effusion (n = 2), bile leak (n = 5), and liver failure (n = 2), while ones after OLR included ascites (n = 12), intraperitoneal abscess (n = 4), pleural effusion (n = 5), bile leak (n = 9), and liver failure (n = 7). Postoperative complication rates in the LLR group were significantly lower than in the OLR group (6.7% vs. 13.0%, P = 0.003). The frequency of intraoperative accident was almost the same between the two groups. Mortality at 30 postoperative days was none, at 90 days was 0.26% (n = 1) in the LLR group, while at 30 days it was 0.26% (n = 1), and at 90 days it was 1.03% (n = 4) in the OLR group (Table 3).
| Matched–LLR (n = 387) | Matched–OLR (n = 387) | P | |
|---|---|---|---|
| Blood loss (ml) | 158 (50, 450%) | 400 (170, 675%) | <0.001 |
| RCC transfusion | 28 (7.24%) | 38 (9.82%) | 0.198 |
| FFP transfusion | 17 (4.44%) | 30 (7.85%) | 0.049 |
| Operation time (min) | 294.4 ± 158.8 | 271.0 ± 130.0 | 0.025 |
| Hospital stay (days) | 13 (9, 18) | 16 (11, 25) | <0.001 |
| Conversion | |||
| Pure → Hybrid or HALS | 7 (1.81%) | – | |
| Pure → Open | 7 (1.81%) | – | |
| Hybrid or HALS → Open | 11 (2.84%) | – | |
| Accident | |||
| Bleeding | 9 (2.33%) | 14 (3.79%) | |
| Injury of other organs | 0 | 0 | |
| Others | 1 (0.26%) | 0 | 0.313 |
| Complications | 26 (6.72%) | 50 (12.99%) | 0.003 |
| Ascites | 7 | 12 | |
| Intraperitoneal abscess | 4 | 4 | |
| Pleural effusion | 2 | 5 | |
| Bile leak | 5 | 9 | |
| Liver failure | 2 | 7 | |
| Wound infection | 1 | 4 | |
| Bleeding | 1 | 1 | |
| Others | 4 | 8 | |
| 30 days mortality | 0 | 1 (0.26%) | 0.317 |
| 90 days mortality | 1 (0.26%) | 4 (1.03%) | 0.178 |
- FFP fresh frozen plasma, HALS hand-assisted laparoscopic surgery, RCC red cell concentration
Discussion
A comprehensive meta-analysis of 26 studies comparing LLR with OLR revealed that there were advantages associated with LLR, such as reduced blood loss, decreases in overall and liver-specific complications, and shorter postoperative hospital stays, although LLR procedures were associated with longer operating time, and moreover, it found that the oncological outcomes were not different from OLR 23. Likewise the large study analyzing 31 papers comparing LLR (1146 patients) to OLR (1327 patients) came to the same conclusions for benefits of LLR over OLR, with equivalent cancer outcomes 24. Another meta-analysis about surgical and oncological outcomes following LLR versus OLR for HCC included 10 studies comprising 627 patients 25. The 10 studies were six case-control and four retrospective analyses; no RCTs were included. The laparoscopic group had significantly less blood loss by 223.17 ml (P < 0.001), less need for transfusions (P = 0.007), shorter hospital stay by 5.05 days (P < 0.001) and fewer postoperative complications (P = 0.002). However, these results were not produced by RCTs and were affected more or less by the selection-bias of LLR. To date, several reports have been published using PSM, confirming short-term advantages and comparable oncological outcomes in LLR patients compared with OLR patients for HCC. Common short-term advantages by LLR were less intraoperative blood loss and a shorter hospital stay 26, 27. However, these studies included a relatively small number of patients after PSM.
Although we can easily assess the malignancy of HCC using tumor size, number, and the levels of serum tumor markers, and the liver functional reserve examining the extent of liver damage and Child–Pugh score, it is difficult to evaluate the complexity of the hepatic resection. We evaluated the difficulty of hepatic resection by finding difficult tumor locations for LLR demonstrated by Cho et al. 20, and additionally we divided the hepatic resection into the major hepatectomy, minor hepatectomy and others. Practically in the overall cohort, there were significant differences not only in tumor size and tumor number but also in the difficult location and in the frequency of the major or minor hepatectomy between LLR and OLR for HCC (Fig. S3).
In each institution there are various selection criteria of LLR for HCC, and the criteria might be affected by the learning curve of the surgical skill of LLR. And so, we intend to treat all preoperative relevant clinical variables as the covariates to build the propensity score. Forty-nine LLR patients were not matched because PSM was statistically performed using a 1:1 ratio on the estimated propensity score. Nevertheless, our study includes the large number of 387 patients and 387 patients for HCC in LLR and OLR, respectively, after the background characteristics of each patient were almost identical.
Regarding the long-term survival of two matched groups, median observation periods were not comparable between the two groups because the timing that LLR had been introduced was different in each institution. Regarding the histopathological outcomes, the degree of inflammation of the background liver and the differentiation of the tumor in the matched-open group were worse than those in the matched-lap group. These results were limitations in our retrospective study. However, it is difficult to assess the impact of primary hepatic resection on the OS in HCC treatment. The result that DFS as well as OS in the well-matched groups was statistically even in the comparison of the oncological outcomes, was noteworthy.
Further, in regard to the perioperative outcomes between the well-matched groups, the LLR patients had less blood loss, shorter hospital stay, and less morbidity than the OLR patients as shown in Table 3. These results are consistent with the recommendations in the 2nd International Consensus Conference on LLR held in Morioka, Japan, from 4 to 6 October 2014 28. However, in the subgroup analysis, major LLR failed to show less blood loss, although it still had shorter hospital stay and less morbidity (Table S1). These significances cannot be blindly accepted as the patient number of each matched group was small. It is also likely that major LLR was in an introduction phase and the learning curve must have an impact during our study period.
With respect to the contents of the complications, the frequency of the liver failure after LLR was lower than after OLR. This result might be explained by less destruction of the collateral blood/lymphatic flow by LLR during mobilization of the liver. Reduction of surgery-induced injury with LLR may lower the risk of liver failure after LLR for HCC patients with severe cirrhosis 29, 30.
In conclusion, compared with OLR, LLR in selected patients with HCC showed similar long-term outcomes, associated with less blood loss, shorter hospital stay, and fewer postoperative complications. Especially, the minor LLR in selected patients is confirmed to be one good option as a standard practice for the treatment of HCC.
Acknowledgments
We are indebted to the surgeons for their excellent collection and management of data, and any other support in the institutions of the Endoscopic Liver Surgery Study Group; Fumitoshi Hirokawa, Kazuhisa Uchiyama, Satoru Imura, Mitsuo Shimada, Katsunori Sakamoto, Goro Honda, Hirokatsu Katagiri, Hiroyuki Nitta, Yutaka Takeda, Masaaki Hidaka, Susumu Eguchi, Norihiro Kishida, Osamu Itano, Akihiro Takai, Yasutsugu Takada, Morihiko Ishizaki, Masaki Kaibori, Hiroki Uchida, Hiroshi Uchinami, Takanori Morikawa, Yutaka Sunose, Naokazu Chiba, Hiroshi Nakajima, Takashi Shirobe, Hiroshi Wada, Hiroaki Nagano, Tadashi Tsukamoto
Conflict of interest
None declared.
Appendix: author's affiliations
Takeshi Takahara and Go Wakabayashi, Department of Surgery, Iwate Medical University School of Medicine, Iwate, Japan; Toru Beppu and Hideo Baba, Department of Gastroenterological Surgery, Graduate School of Life Sciences, Kumamoto University, Kumamoto, Japan; Arihiro Aihara and Minoru Tanabe, Department of Hepatobiliary and Pancreatic Surgery, Graduate School of Medicine, Tokyo Medical and Dental University, Tokyo, Japan; Kiyoshi Hasegawa and Norihiro Kokudo, Hepato-Biliary-Pancreatic Surgery Division, Department of Surgery, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan; Naoto Gotohda and Masaru Konishi, Department of Hepatobiliary and Pancreatic Surgery, National Cancer Center Hospital East, Kashiwa, Chiba, Japan; Etsuro Hatano and Shinji Uemoto, Department of Surgery, Graduate School of Medicine, Kyoto University, Kyoto, Japan; Yoshinao Tanahashi and Atsushi Sugioka, Department of Surgery, Fujita Health University School of Medicine, Aichi, Japan; Toru Mizuguchi and Koichi Hirata, Department of Surgery, Surgical Oncology and Science, Sapporo Medical University School of Medicine, Sapporo, Japan; Toshiya Kamiyama and Akinobu Taketomi, Department of Gastroenterological Surgery I, Hokkaido University Graduate School of Medicine, Sapporo, Japan; Tetsuo Ikeda and Yoshihiko Maehara, Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan; Shogo Tanaka and Shoji Kubo, Department of Hepato-Biliary-Pancreatic Surgery, Osaka City University Graduate School of Medicine, Osaka, Japan; Nobuhiko Taniai and Eiji Uchida, Department of Gastrointestinal Hepato-Biliary-Pancreatic Surgery, Nippon Medical School, Tokyo, Japan; Hiroaki Miyata, Department of Healthcare Quality Assessment, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan; Masafumi Nakamura, Department of Digestive Surgery, Kawasaki Medical School, Okayama, Japan; Hironori Kaneko, Department of Surgery, Toho University Faculty of Medicine, Tokyo, Japan; Hiroki Yamaue, Second Department of Surgery, Wakayama Medical University School of Medicine, Wakayama, Japan; Masaru Miyazaki, Department of General Surgery, Chiba University Graduate School of Medicine, Chiba, Japan; Tadahiro Takada, Japanese Society of Hepato-biliary-Pancreatic Surgery, Department of Surgery, Teikyo University School of Medicine, Tokyo, Japan.




