Safety and efficacy of living donor liver transplantation for unresectable perihilar cholangiocarcinoma: A single center prospective study
Abstract
Background
The prognosis for unresectable perihilar cholangiocarcinoma (phCCA) is extremely poor. Liver transplantation in combination with neoadjuvant chemoradiation therapy has become the treatment of choice for unresectable phCCA in the USA. In 2018, we launched a prospective study to evaluate the safety and efficacy of living donor liver transplantation (LDLT) for unresectable phCCA.
Methods
A total of 10 patients were enrolled in this study between 2018 and 2024. Finally, five patients with unresectable phCCA underwent LDLT after neoadjuvant chemotherapy, radiation, and staging laparotomy, while the other five patients dropped out of the protocol.
Results
The median follow-up period was 23.7 months. The overall survival rate for the five patients who underwent LDLT was 100% after one year. Hepatic artery thrombosis and delayed gastric emptying occurred in two and three cases, respectively. The histological efficacy of preoperative treatment was grade IIb and III, according to the Evans classification, in all five patients. All surgical margins and dissected lymph nodes were negative. Four patients were alive with no evidence of disease recurrence while one patient had recurrence 10 months after LDLT.
Conclusions
LDLT is feasible and may be a last-resort treatment option for unresectable phCCA, although the long-term outcomes need to be carefully monitored.
Clinical trial register and clinical registration number
The UMIN registration number for this study is 000033348.
1 INTRODUCTION
Perihilar cholangiocarcinoma (phCCA) is a highly aggressive malignancy that has limited treatment options. Resection is the standard of care; however, even when resection is possible, the international 5-year survival rate is only 10%–40%.1-4 In Japan, resection is the only curative treatment for phCCA, and Nagino and Ebata et al. at Nagoya University reported a similar 5-year survival rate of 32.5%–38.1% for resectable phCCA.5, 6 However, owing to the usually advanced state of the disease at the time of diagnosis, resection is often not feasible. Unresectable phCCA has a poor prognosis, with a 5-year survival rate of nearly zero in both Japan and Western countries.1-6 Resection may be impossible due to distant metastasis, local progression, or insufficient function of the remnant liver, and the presence of primary sclerosing cholangitis (PSC), which may obscure the extent of the tumor.
Since the prognosis of patients with unresectable phCCA is exceedingly poor, liver transplantation has been advocated as a possible avenue for expanding the therapeutic options for unresectable phCCA. Although unresectable phCCA was initially favored as an indication for liver transplantation (LT), LT alone has shown a very high recurrence rate (53%–84%).7, 8 Thus, unresectable phCCAs have long been a contraindication for LT.
Accordingly, in 1988 at the University of Nebraska and in 1993 at the Mayo Clinic, protocols were established for pretransplant adjuvant chemotherapy, radiation therapy, and staging laparotomy for unresectable phCCA.9, 10 Strict patient selection and the combination of preoperative chemotherapy and radiation therapy improved the outcomes of LT for unresectable phCCA; therefore, LT has now become the standard of care for unresectable phCCA in the USA.11 LT for patients with unresectable phCCA, which has been widely documented to date, is typically performed following preoperative chemotherapy and radiation therapy using the Mayo protocol, with modifications made to accommodate the specific conditions at each institution. The outcomes of these transplantations are generally comparable to those reported by the Mayo Clinic.12-15
In contrast to Western countries, Japan lacks insurance coverage for living or brain-dead donor LT in cases of unresectable phCCA. Furthermore, the current treatment guidelines for biliary tract cancer in Japan only recommend chemotherapy, radiation therapy, and palliative care for unresectable phCCA.16 Therefore, we initiated a prospective clinical study in 2018 to evaluate the safety and efficacy of living donor liver transplantation (LDLT) for unresectable phCCA at a single center.
2 METHODS
This was a single-center, prospective study. The criteria for evaluating patients with unresectable phCCA involved four specific conditions: (1) An insufficient future liver remnant, (2) locally advanced disease that precludes vascular reconstruction, (3) longitudinal extension that makes R0 resection impossible, and (4) concomitant PSC, which complicates the determination of the extent of cancer. Patients with unresectable phCCA due to any of the aforementioned conditions were eligible for treatment, whereas those with distant metastasis or lymph node metastasis were excluded. The determination of unresectability was reached following a multidisciplinary conference involving the Gastroenterology, Radiology, Oncology, and Hepato-Biliary-Pancreatic Surgery departments at our institution's Cancer Board. Comprehensive evaluation is conducted utilizing dynamic computed tomography (CT), Gd-EOB-DTPA magnetic resonance imaging (MRI), 18F-fluorodeoxyglucose positron emission tomography (PET), and endoscopic retrograde cholangiopancreatography (ERCP) biopsy. Similarly, PSC cases were determined to be unresectable based on magnetic resonance cholangiopancreatography (MRCP) and ERCP biopsy results.
2.1 Study protocol
Our treatment protocol was a modified version of the Mayo protocol for LDLT (Figure 1). The current Mayo protocol initially involves diagnosing unresectable phCCA and confirming the absence of distant metastases or lymph node metastasis via imaging studies. Subsequently, chemotherapy and radiation therapy (including external radiation) are administered followed by a subsequent round of radiation therapy (focused on internal radiation). Following this, staging laparotomy is performed to verify the absence of any intra-abdominal dissemination, and lymph node sampling is conducted to confirm the absence of metastasis. The patient is then added to the waiting list for brain-dead LT; until a suitable brain-dead liver donor becomes available, chemotherapy is continued throughout the waiting period.11
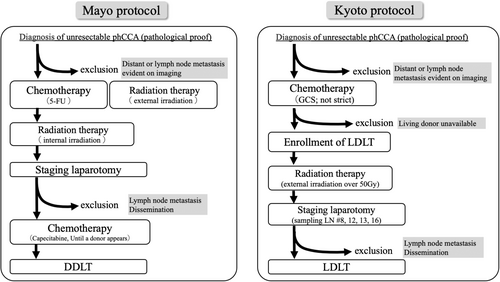
Our modified protocol is specifically designed for LDLT. If the patient was initially diagnosed with unresectable phCCA and chose to undergo LDLT, chemotherapy was initiated. Our chemotherapy protocol consisted of gemcitabine (G) + cisplatin (C) + tegafur gimeracil oteracil potassium (S) for over 2 months. The prescribed dosage regimen for the drug is as follows: G; 1000 mg/m2 as a single dose, C; 25 mg/m2 as a single dose, S; 80 mg/day for body surface area (BSA) <1.25, 100 mg for BSA 1.25–1.5, 120 mg for BSA >1.5 administered for 7 days, with a dosing cycle repeated every 2 weeks. However, some patients may already be undergoing chemotherapy upon referral to our department. We do not specify the type of drug, but we generally administer G, C, and S in principle. The occurrence of cholangitis during chemotherapy necessitates significant attention. The management and potential biliary stent (internal or external) replacement for cholangitis are under consideration in collaboration with department of gastroenterology or referral hospitals. After chemotherapy, if there was no evidence of tumor progression, distant metastasis, intrahepatic metastasis, or lymph node metastasis as determined via dynamic CT, 18F-fluorodeoxyglucose PET, or Gd-EOB-DTPA MRI, a living donor was evaluated. If no living donors were available, patients were excluded. If there were no issues with the living donor, the schedule for LDLT was adjusted, and the patient underwent external radiation therapy. Radiation therapy is administered via external irradiation, basically total 56 Gy (2 Gy/day for 28 days), targeting the perihilar region, pancreatic head region, hepatoduodenal ligament, and para-aortic region and in the adverse events, the dose is reduced, or the treatment duration is abbreviated. Following the completion of radiation therapy, CT, MRI, and PET are utilized to assess tumor progression, the presence of distant metastases, and the occurrence of intrahepatic metastases. Approximately 2–3 weeks before LDLT, staging surgery was performed under open laparotomy to confirm the absence of peritoneal dissemination and lymph node metastasis. During laparotomy or laparoscopic surgery, lymph nodes 8, 12, 13, and 16 were sampled and examined pathologically to confirm the presence or absence of metastases. After confirming the absence of metastases, LDLT was performed.
The study protocol was approved by the Ethics Committee of Kyoto University (no. C1387-4) and conducted in accordance with the institutional and ethical guidelines mandated by the Declaration of Helsinki (2013) and the Declaration of Istanbul (2018). Written informed consent was obtained from each patient and donor. The UMIN registration number for this study is 000033348. In statistical analyses, patients' survival rate was evaluated using the log-rank test. This statistical analysis was performed using JMP Pro software version 17.0 (SAS Institute, Cary, NC, USA), with statistical significance set at p < .05.
2.2 Surgery of living donor liver transplantation
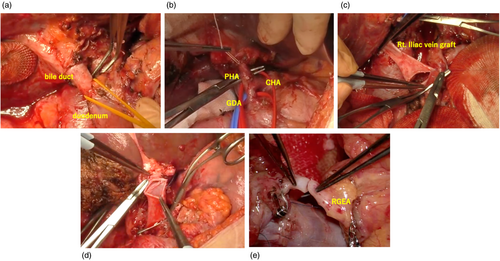
The indications for LDLT, donor selection criteria, perioperative patient care, surgical procedures, and immunosuppression regimens have been previously detailed.17 The immunosuppression protocol for unresectable phCCA employed in our practice utilizes tacrolimus and mycophenolate mofetil. For autoimmune diseases, such as PSC, prednisone is administered in addition to the immunosuppression. About 1 month after LDLT, the protocol involves transitioning from mycophenolate mofetil to everolimus. The liver transplant procedure at hand varied substantially from a conventional procedure, as it entailed complete removal of the hepatoduodenal ligament and thorough lymph node dissection. During the process of total hepatectomy, meticulous regional lymph node dissection (8a, 8p, 12a, 12b, 12c, 12 h, 12p, 13a) for cholangiocarcinoma surgery was performed in conjunction with (1) resection of the intrapancreatic bile duct and confirmation of negative margins (in cases of positive margins for the intrapancreatic bile duct, additional pancreatoduodenectomy (PD) should be considered), (2) removal of the hepatoduodenal ligament at the superior border of the duodenum, (3) excision of the hepatoduodenal ligament and the root of the proper hepatic artery and (4) substitution of the portal vein with a graft from the right external iliac vein (Figure 2). In the field of LT, anastomosis is a critical step, in which the graft liver is connected to the recipient's blood vessels. This connection is essential for the successful outcomes of the procedure. In our study, the portal vein of the graft liver was anastomosed with the replaced right external iliac vein graft, whereas the graft hepatic vein was anastomosed normally. In the first two cases, the graft hepatic artery was anastomosed with the proper hepatic artery. In the subsequent three cases, it was anastomosed to the middle colic artery in one case and to the right gastroepiploic artery in two cases. A Roux-en-Y lib was created to reconstruct the bile duct of the graft.
3 RESULTS
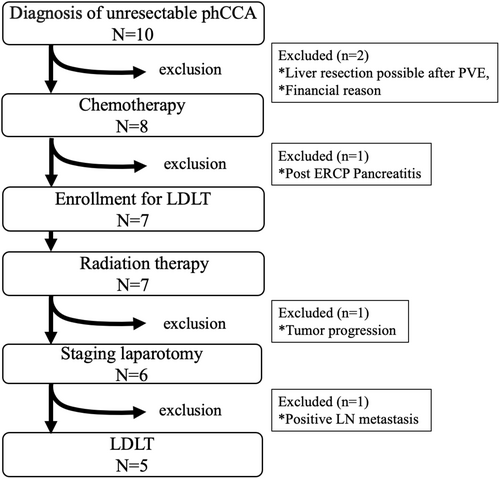
We enrolled 10 patients in this prospective study at our single center from 2018 to 2024 (Figure 3). One patient eventually underwent hepatic resection after portal vein embolization and the other refused LDLT for financial reasons. Eight patients underwent chemotherapy; one was excluded during chemotherapy because of severe pancreatitis after post-ERCP. Seven patients received radiation therapy after completing chemotherapy, and one patient was excluded after chemotherapy due to tumor progression. After radiation therapy, one patient had a positive lymph node (lymph node 8) biopsy on staging laparotomy and dropped out of the protocol. In each case, 3–10 (median 3) lymph nodes were sampled. Finally, the present study analyzed five cases of unresectable phCCA that underwent LDLT.
3.1 Patient characteristics
The clinical characteristics of the patients are summarized in Table 1. The age range of the recipients was 30–52 years, with two men and three women. The reasons for unresectability were local advancement and longitudinal extension that makes R0 resection impossible in three patients and PSC in two. Among the three patients with normal liver, in case no. 2, CT and MRI revealed tumor involvement of the hepatic artery and portal vein, rendering reconstruction unfeasible. In the remaining two cases, the hepatic artery and portal vein were similarly affected by tumors, and step biopsy with ERCP demonstrated tumor presence beyond the limit of bile duct resection, precluding the possibility of R0 resection. All the patients underwent 7–15 cycles of neoadjuvant chemotherapy, with only patient 1 receiving G and C and the others receiving G, C, and S. Additionally, all the patients received 50–56 Gy of radiation therapy. Pretreatment and preoperative tumor markers (carcinoembryonic antigen/carbohydrate antigen 19–9) decreased in all five patients following neoadjuvant chemotherapy and radiation therapy. The interval duration between the completion of chemotherapy and LDLT ranged from 85 to 196 (median 104) days, while the interval duration between the completion of radiation therapy and LDLT ranged from 20 to 50 (median 36) days.
| Patient No. | Recipient age/sex | Background liver | Reasons why phCCA was unresectable | Chemotherapy cycle | Radiation therapy (Gy) | CEA Pretreatment/Preoperative | CA19-9 Pretreatment/Preoperative |
|---|---|---|---|---|---|---|---|
| 1 | 30/male | PSC | PSC | 15 | 50 | 5.9/3.2 | 79.7/34.4 |
| 2 | 41/male | Normal | locally advanced (HA, PV) | 16 | 56 | 2.1/1.2 | 24.7/21.9 |
| 3 | 48/female | PSC | PSC | 7 | 56 | 3.4/1.8 | 46.2/31.3 |
| 4 | 52/female | Normal |
longitudinal extension, Locally advanced (HA, PV) |
7 | 56 | 0.5/0.3 | 23.6/9.7 |
| 5 | 44/female | Normal | Longitudinal extension, locally advanced (HA, PV) | 9 | 56 | 6.1/0.4 | 351.0/28.5 |
- Abbreviations: CA19-9, carbohydrate antigen (U/mL); CEA, carcinoembryonic antigen (ng/mL); HA, hepatic artery: phCCA, perihilar cholangiocarcinoma; PSC, primary sclerosing cholangitis; PV, portal vein.
3.2 Living donors and operative findings
Table 2 presents the living donors and operative factors. The donors were aged 37–47 years, with one woman and four men. Four donors were the patients' siblings, and one donor was spouse. Their blood groups were identical in four and compatible in one case. Liver transplants consisted of two right and three left lobes. Our indication criteria for LDLT were a graft recipient weight ratio (GRWR) >0.6; the actual GRWRs in this study ranged from 0.7 to 1.27. The operative time for each transplant ranged from 694 to 868 min, and blood loss varied from 360 to 2380 g. Cold ischemic time ranged from 40 to 232 min and warm ischemic time, 24 to 43 min. In one PSC case, the tumor extended to the intrapancreatic bile duct and required simultaneous LDLT and PD.18
| Patient No. | Donor age/sex | Relationship | ABO combination | Graft type | GRWR (%) | Operation time (min) | Blood loss (g) | CIT/WIT (min) | Additional operation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37/male | Siblings | Identical | Right | 1.25 | 834 | 1300 | 232/32 | - |
| 2 | 46/female | Siblings | Identical | Left | 0.70 | 797 | 2380 | 78/40 | - |
| 3 | 47/male | Spouse | Compatible | Left | 0.83 | 828 | 1350 | 90/24 | PD |
| 4 | 46/male | Siblings | Identical | Left | 0.95 | 694 | 360 | 40/43 | - |
| 5 | 41/male | Siblings | Identical | Right | 1.27 | 868 | 1130 | 148/38 | - |
- Abbreviations: CIT, cold ischemia time; GRWR, graft recipient weight ratio; PD, pancreatoduodenectomy; WIT, warm ischemia time.
3.3 Pathological findings of the explant liver
The preoperative histological diagnosis in all the patients was tubular adenocarcinoma. All specimens were retrieved via ERCP and none of the patients underwent transperitoneal biopsy. Upon pathological examination of the removed whole liver, two cases (Nos. 1 and 3) showed the presence of PSC, one case had suspected of PSC (No. 5), one case (No. 2) revealed sinusoidal obstruction syndrome, and one case (No. 4) showed a normal liver. Tumor differentiation varied from biliary intraepithelial neoplasia-3 to poorly differentiated tumors, and the size ranged from 0.5 to 4.2 cm. One patient had portal vein involvement, but all the patients had negative lymph nodes. Additionally, the resection margins were negative in all the patients. Despite adjuvant chemotherapy and radiation therapy, the Evans classification remained IIb to III, and the tumor persisted (Table 3).
| Patient No. | Background liver | Differentiation | Size (cm) | T | pPV | pA | ly | v | ne | N | Stage | Margin | R | Evans classification |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
PSC A2F2 |
BillN3 | - | Tis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Negative | 0 | III |
| 2 |
Normal SOS |
Moderate-poor | 4.2 | T2b | 0 | 0 | 0 | 0 | 1c | 0 | 2 | Negative | 0 | IIb |
| 3 |
PSC A1F2 |
Moderate | 0.5 | T1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | Negative | 0 | IIb |
| 4 | Normal | Well | 2.5 | T4 | 1 | 0 | 0 | 0 | 1a | 0 | 3b | Negative | 0 | IIb |
| 5 |
PSC s/o A2F1 |
Moderate | 3.5 | T2a | 0 | 0 | 0 | 0 | 0 | 0 | 2 | Negative | 0 | IIb |
- Abbreviations: BilIN, biliary intraepithelial neoplasia; PSC, primary sclerosing cholangitis; s/o, suspected of; SOS, sinusoidal obstruction syndrome.
3.4 Patient complications and prognosis
Table 4 shows the postoperative complications and prognosis. The duration of hospitalization after surgery for each patient varied from 26 to 58 days (median, 49 days). According to the Clavien-Dindo classification, all the patients experienced complications, with three cases classified as II, one case as IIIa, and one case as IIIb. Only one case of T cell mediated rejection was observed and treated with steroid pulse therapy, while no other cases developed. A few notable postoperative complications were also observed. Notably, hepatic artery thrombosis (HAT) and delayed gastric emptying (DGE) occurred in two and three cases, respectively. In patient 2, HAT developed on postoperative day 4, necessitating reanastomosis. In patient 1, a CT scan 3 months after LDLT showed occlusion of the hepatic artery following a subclinical course. DGE developed approximately 1 week postoperatively and persisted for approximately 3 months at the longest. In one case of PSC coexistence, the tumor extended into the intrapancreatic bile duct; therefore, PD was performed at the same time as LDLT. Therefore, the possibility of DGE secondary to PD could not be ruled out. However, all three patients gradually improved, and the DGE disappeared.
| Patient No. | Postoperative stay | Clavien-Dindo classification | Complication | Prognosis |
|---|---|---|---|---|
| 1 | 39 | II | TCMR, HAT | Alive 39.6 months |
| 2 | 56 | IIIb | HAT, CMV infection |
Dead 23.7 months (Bone metastasis 10 months after LDLT) |
| 3 | 49 | II | DGE, chylous ascites | Alive 28.4 months |
| 4 | 26 | II | DGE | Alive 12.9 months |
| 5 | 58 | IIIa | DGE, bile leakage | Alive 10.3 months |
- Abbreviations: CMV, cytomegalovirus; DGE, delayed gastric emptying; HAT, hepatic artery thrombosis; LDLT, living donor liver transplantation; TCMR, T cell mediated rejection.
The median follow-up period was 23.7 months (range, 10.3–39.6 months) after LDLT. The overall survival rate for all five patients with unresectable phCCA was 100% at 1-year postoperatively. One patient experienced recurrence with multiple bone metastases 10 months after the LDLT. Following recurrence, treatment involved radiation therapy and chemotherapy, which maintained a partial response for 8 months. Nevertheless, chemotherapy proved ineffective thereafter, and the patient died approximately 24 months post-transplantation due to tumor progression. The remaining four patients exhibited no signs of recurrence for the duration of the observational period. None of the donors experienced any complications.
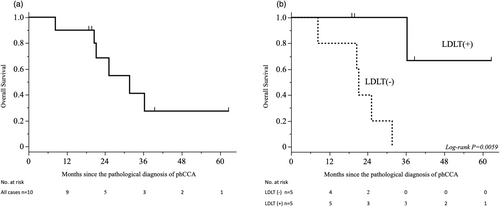
In the intention-to-treat all cases (n = 10), six deaths had been reported during a median follow-up of 23.3 (range 8.4–62.8) months from the date of diagnosis to the database cutoff. The 5-year overall survival rate was 27.4% for all patients enrolled in the study (Figure 4a). Subsequently, as a per-protocol analysis, the cohorts (n = 5) that underwent LDLT were compared with those (n = 5) that did not receive LDLT. In five cases that did not result in LDLT, all available therapeutic interventions, including chemotherapy, radiation therapy and surgical procedures, were implemented. However, the median survival time following pathological diagnosis of phCCA was 21.9 months, representing a significantly inferior prognosis compared to this cohorts that underwent LDLT as a per-protocol cases (p = .0059) (Figure 4b). In all cases within the non-LDLT cohort, the primary cause of mortality was attributed to the progression of the underlying neoplastic disease (phCCA).
4 DISCUSSION
Unresectable phCCA is a very aggressive malignancy that is limited to chemotherapy; however, the treatment outcome is extremely poor. In a large Japanese study,6 cases that were resected with R0 had 87.5 months of mean survival time, which compares favorably with Western reports. However, the prognosis is extremely poor in cases of unresectable lymph node metastasis and R1 and R2 resection.6 In comparison with resectable phCCA, the results of LT for unresectable phCCA in Western countries, when combined with preoperative chemotherapy and radiation therapy, demonstrated similar or superior survival rates.11, 12 Moreover, an analysis of the impact of neoadjuvant therapy followed by LT for unresectable phCCA as opposed to curative liver resection for resectable phCCA on overall survival in an intention-to-treat study using a substantial 10-center database in the USA revealed that neoadjuvant therapy followed by LT was associated with improved overall survival compared to resection.19 Consequently, it was established that neoadjuvant chemotherapy and radiation therapy enhances the outcome of LT in patients with unresectable phCCA.
There has recently been an improvement in the perioperative results of LT for end-stage liver cirrhosis in Japan,20 which has led to the expansion of the indications for this surgical procedure to include other medical conditions. Our primary goal was to evaluate the safety and efficacy of LDLT as a therapeutic option for unresectable phCCA in Japan, considering the established outcomes that were documented globally. Our study demonstrated positive outcomes in a prospective study on LDLT in patients with unresectable phCCAs. The findings of the LT surgical method were reflected in the 100% 1-year survival rate for all five patients, highlighting the perioperative safety of the surgery. However, in case No. 2, distant metastasis was detected 10 months after LDLT. The etiology of distant metastasis remains undetermined; nevertheless, it has been reported that tumor size exceeding 3 cm is a risk factor for lymph node metastasis and distant metastasis. And elevated tumor markers are associated with higher dropout rates.11 These findings remain in the investigative stage and is subject to ongoing debate in current global studies. In our study, we did not specify tumor size criteria, acknowledging the potential to extend life-saving interventions to a broader patient population in Japan. We posit that further investigation is warranted as risk factors become elucidated through the accumulation of future clinical studies.
Furthermore, the high frequency of a few characteristic postoperative complications, HAT and DGE, are a matter of concern. HAT is a severe complication that can occur after LT. In our department, the incidence of HAT in LDLT for cirrhotic patients is 6.4%, which is relatively low compared with other reports.21 However, when it occurs, it can have serious consequences and necessitate postoperative attention.
In this study, we observed a high frequency of HAT. In the first two cases, we used the proper hepatic artery and graft artery as in the usual LT for arterial anastomosis. However, the proper hepatic artery is located within the hepatoduodenal ligament and within the range of preoperative irradiation. The postoperative coagulation function improved immediately, suggesting that HAT developed because of radiation and immediate improvement in coagulation function. In the following three cases, we anastomosed the graft artery with the middle colic artery in one case and the right gastroepiploic artery in two cases, which did not result in HAT.
We observed three instances of DGE, although the reason for one case remains uncertain because it was a combination of LDLT and PD. It is possible that the level of radiation exposure has been affected. The other two cases were likely influenced by the intensity of radiation therapy. Therefore, reassessment of the range of irradiation to prevent HAT and DGE took place, and it was observed that no instances of DGE arose following these five cases. Through an examination of preventive measures, we currently hold the conviction that it is feasible to execute LT with even greater safety.
Long-term recurrence-free survival is also likely to be achieved by a combination of preoperative chemotherapy and radiation therapy, and reports from the USA have shown good results.11, 18 In our study, chemotherapy and radiation therapy before LT were very effective, and the results after LT were very good, despite the characteristic complications. Our chemotherapy regimen was structured according to the treatment for unresectable phCCA, adhering to the Japanese biliary tract cancer treatment guidelines. In Japan, three-drug combination therapy, known as GCS, has demonstrated a favorable response compared to GC and S.22, 23 The efficacy of the immune checkpoint inhibitor durvalumab (D) was recently confirmed in a phase III clinical trial (TOPAZ-1).24 The trial results demonstrated that GCD therapy was more effective in achieving the primary endpoint of overall survival. Similarly, a phase III trial (KEYNOTE-966) of GC plus pembrolizumab (P), an anti-PD-1 antibody, in combination with a triple-drug regimen (GCP) and GC plus placebo, demonstrated the superiority of GCP.25 Progress in immune checkpoint inhibitors could lead to improvements in chemotherapy for phCCA, which may result in a reduced incidence of post-LDLT recurrence compared with cases treated with the currently accepted GCS regimen.
Considering the current state of knowledge, it may be possible for physicians to examine the potential benefits of incorporating GCD or GCP therapy in conjunction with CGS therapy as a preoperative chemotherapy regimen. Nevertheless, instances of severe rejection following LT were documented when immune checkpoint inhibitors were used before LT.26 Therefore, caution should be exercised when using these therapies. Immunosuppression protocols for LT following the use of immune checkpoint inhibitors have not yet been established, and the outcomes of future investigations are anticipated. In addition, it is vital to determine the appropriate cases for postoperative adjuvant chemotherapy to prevent recurrence. One possible approach is to employ the latest biomarkers, including lymph node-positive cases, following LT and circulating tumor DNA.27
Although chemotherapy has demonstrated considerable progress in the realm of antitumor effects, it is unfortunate that none of the five cases studied in this investigation resulted in pathologically complete responses following LDLT. Nonetheless, LDLT continues to be the preferred treatment choice for patients who are unable to undergo resection because of local advancement. This is because the procedure has proven beneficial when used in combination with effective chemotherapy prior to transplantation. The medical community widely acknowledges that LT is the preferred treatment option for individuals who are unresectable due to local progression, regardless of the efficacy of pretransplant chemotherapy. It is difficult to determine whether R0 resection is possible even if preoperative therapy is effective when unresectable phCCAs are considered for LT. Therefore, LT should be actively considered because of its enormous impact if reconstruction is not possible, or if there is a positive margin of malignancy.
The limitations of this study are the small sample size, short period of observation, and single-center design. A nationwide survey is required to validate our findings. Currently, a multicenter, prospective clinical trial of LDLT for unresectable phCCA is ongoing in Japan (jRCT1070220052). It is classified as an advanced medical care B aiming for future insurance coverage and revision of clinical practice guidelines.28 Thus, our goal is to secure insurance coverage for this procedure.
In conclusion, LDLT is a feasible and effective treatment for phCCA in Japan. Liver transplantation may be the last-resort treatment option for unresectable phCCA. Therefore, the long-term outcomes should be carefully monitored.
AUTHOR CONTRIBUTIONS
T.I. conducted data analysis and composed the main body of the manuscript. K.T. designed the study. K.F., S.O., S.O., T.A., K.N., Y.U., and T.I. interpreted results and critically reviewed the manuscript. E.H. supervised the reliability of the manuscript.
ACKNOWLEDGMENTS
The authors thank the members of the Transplantation Counseling Office at Kyoto University for arranging all the liver transplantations.
FUNDING INFORMATION
This study was supported by the Japan Society for the Promotion of Science KAKENHI (grant no. 22 K08750). The funding source was not involved in the study design; data collection, analysis, or interpretation; manuscript preparation, review, or approval; or decision to submit the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




