Initial experience of endoscopic ultrasound-guided antegrade covered stent placement with long duodenal extension for malignant distal biliary obstruction (with video)
Hoonsub So and Dongwook Oh equally contributed to the study.
Abstract
Background/Purpose
This study aimed to evaluate the feasibility of endoscopic ultrasound (EUS)-guided antegrade covered stent placement with long duodenal extension (EASL) for malignant distal biliary obstruction (MDBO) with duodenal obstruction (DO) or surgically altered anatomy (SAA) after failed endoscopic retrograde cholangiopancreatography (ERCP).
Methods
Outcomes were technical and clinical success, reintervention rate, adverse events, stent patency, and overall survival. Inverse probability of treatment weighting (IPTW) and competing-risk analysis were performed to compare with conventional EUS-BD.
Results
Twenty-five patients (DO, n = 18; SAA, n = 7) were included. The technical and clinical success rates were 96% and 84%, respectively. Reintervention occurred in two patients (8.3%). Adverse events occurred in six patients (24%; two cholangitis, 16%; four mild postprocedural pancreatitis [24% (n = 4/17) in patients with non-pancreatic cancers]). The median patency was 9.4 months, and the overall survival was 2.73 months. After IPTW adjustment, the median patency in the EASL (n = 25) and conventional EUS-BD (n = 29) were 10.1 and 6.5 months, respectively (P = .018).
Conclusions
EASL has acceptable clinical outcomes with a low reintervention rate but higher rate of postprocedural pancreatitis in patients with non-pancreatic cancers. Randomized trials comparing EASL and conventional EUS-BD for MDBO with pancreatic cancers and DO/SAA after failed ERCP are needed to validate our findings.
1 INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) with self-expanding metal stent (SEMS) placement has been the primary choice for the palliation of malignant distal biliary obstruction (MDBO) owing to its long patency duration.1 However, ERCP with transpapillary metal stenting is not always successful in patients with duodenal obstruction (DO) or a surgically altered anatomy (SAA). Conventionally, the percutaneous approach has been used after a failed ERCP; however, it is associated with considerable morbidities and an adverse event rate of up to 33%.2 Endoscopic ultrasound (EUS)-guided biliary drainage (EUS-BD) may be preferred for its better clinical success, lower adverse event rate, and fewer reinterventions than the percutaneous approach after a failed ERCP.3
EUS-guided hepaticogastrostomy (EUS-HGS) with transmural or antegrade stenting has been suggested as a practical alternative for patients with DO or SAA after a failed ERCP.4 However, stent dysfunction related to sludge impaction in EUS-HGS with transmural covered metal stenting and tumor ingrowth in EUS-guided antegrade uncovered metal stenting (EUS-AGUS) are not uncommon. Furthermore, reflux of gastroduodenal contents such as food material can lead to stent dysfunction induced by sludge formation or ascending infection when the stent crosses the main duodenal papilla.5 To simultaneously prevent reflux cholangitis and tumor ingrowth, percutaneous antegrade placement of the distal end of the stent at the third portion of the duodenum has been proposed for MDBO.6 This study aimed to evaluate whether EUS-guided antegrade covered metal stent with long duodenal extension (EASL) in patients with unresectable MDBO after a failed ERCP can reduce the reintervention rate for stent dysfunction due to reflux cholangitis and tumor ingrowth without increasing the adverse events.
2 METHODS
2.1 Patients
This was a retrospective pilot study with single participating center for EASL (Asan Medical Center, Korea). From September 2016 to June 2018, patients with unresectable MDBO with failed ERCP owing to DO or SAA who were unsuitable for EUS-guided choledochoduodenostomy were consecutively enrolled in this study. The patients were treated using EASL (with a fully covered metal stent measuring 8 mm in diameter and 11-13 cm in length). Patients with coagulopathy (international normalized ratio ≥3, platelet count ≤50 000/mm3) or age <18 years were excluded. The primary outcome was technical success. The secondary outcomes were clinical success, reintervention rate, adverse events, stent patency, and overall survival. The result was compared with conventional EUS-BD performed during the same period in three centers (Gifu University, Japan; Kindai University, Japan; and The University of Tokyo, Japan). This study was approved by the institutional review board (IRB) of each hospital (Asan medical center IRB approval number: 2018-0562, Kindai University IRB approval number: 30-149, Gifu University: 2018-084, University of Tokyo: 2018125NI).
2.2 Materials
For EASL, a fully covered SEMS (commercially available, silicone-covered, and nitinol-wired with both ends flared, 8 mm in diameter, 11-13 cm in length; Standard Sci Tech, Seoul, Korea) was placed using an 8-Fr-diameter stent introducer (Figure 1).

2.3 Procedure
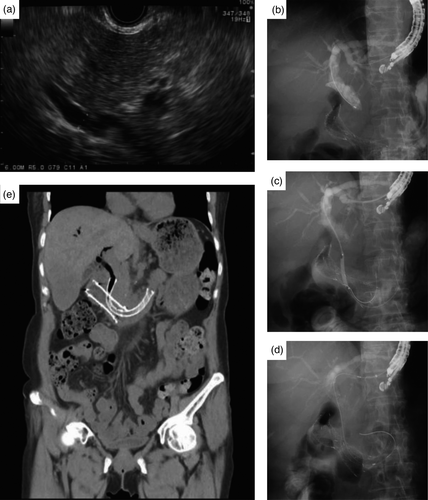
Endoscopic retrograde cholangiopancreatography and the subsequent EUS-BD were performed by one expert with experience of >5,000 cases of ERCP and at least 125 cases of EUS-BD before the study period.7 The detailed procedures of EASL are described in Figure 2 and Video 1. In brief, after puncturing the left intrahepatic duct with a 19-gauge EUS needle and crossing the distal bile duct stricture with placement of a guidewire in the duodenum, the guidewire was straightened in the bile duct and coiled in the distal duodenum for pushability and for an easier procedure. After the dilation of the fistula tract and distal biliary stricture with a 4-mm Hurricane balloon catheter (Boston Scientific), a long duodenal extension of a fully covered SEMS with at least 5 cm length of the stent was secured in the second and third portions of the duodenum (Figure 2A-E).
For the other EUS-guided drainage procedures in conventional EUS-BD group (n = 29; 15 in Kindai University, nine in University of Tokyo, and five in Gifu University), EUS-guided hepaticogastrostomy (EUS-HGS) with transmural stenting or EUS-guided antegrade uncovered metal stenting (EUS-AGUS) was performed according to the discretion of each endoscopist.
2.4 Definitions
Technical success was defined as satisfactory transpapillary deployment of the stent across the papilla with a long duodenal extension of a fully covered SEMS with at least 5 cm length of the stent (Figure 2E). Clinical success was defined as a decrease in bilirubin level to normal or to less than a quarter of the pretreatment value within the first month.8 Reintervention was defined as any type of endoscopic or percutaneous procedure for relieving stent obstruction. Stent obstruction requiring reintervention was diagnosed when a patient developed cholangitis or jaundice and/or when bile duct dilation was evident in imaging studies.9 Stent patency duration was defined as the period from the initial stent placement to the recurrence of stent obstruction requiring reintervention.9 Stent migration was defined as any displacement of the stent into the bile duct (proximal migration) or the duodenum (distal migration).9 Overall survival was calculated from the day of stent insertion to the last day of follow-up or death. Adverse events were classified according to the lexicon for endoscopic adverse events proposed by consensus guidelines.10
2.5 Statistical analysis
Descriptive statistics, including means, standard deviations, and percentages, were calculated. Categorical parameters are expressed as frequencies and proportions and compared using the chi-square test or Fisher's exact test. We estimated the cumulative stent patency and overall survival using the Kaplan-Meier method. All reported P-values are two-sided, and a P-value of <.05 was considered to indicate statistical significance. Data were analyzed using R program version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org). The result was additionally analyzed to compare with conventional EUS-BD.
To reduce the impact of treatment selection bias and potential confounding in an observational comparative study between EASL and conventional EUS-BD groups, the inverse probability of treatment weighting (IPTW) method based on propensity score analysis was used. With this technique, the weight for patients receiving stent 1 was the inverse of the propensity score, and the weight for patients not receiving stent 0 were the inverse of (1-propensity score). The propensity score was estimated with two treatments as the dependent variables in multiple logistic regression analysis that included all the variables in Table 3. Absolute standardized differences were used to diagnose the balance after propensity analysis. All absolute standardized differences after IPTW were <0.2. To assess the treatment effect, we performed IPTW-adjusted logistic or Cox model analysis with robust standard errors, as appropriate for the outcome. In addition, Fine and Gray competing-risk analysis was performed, in which death during the follow-up was considered a competing event for assessing reintervention.
3 RESULTS
3.1 Baseline characteristics
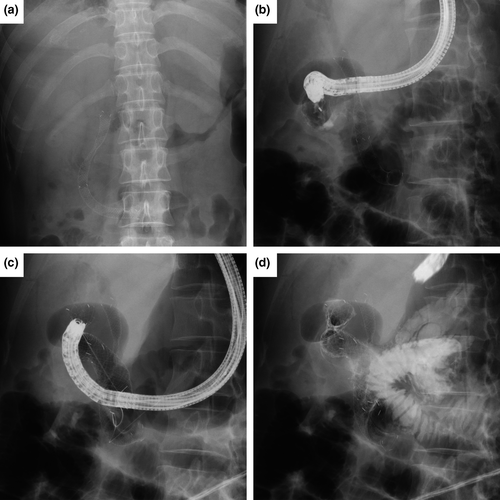
A total 25 patients were included in this study. The baseline characteristics are summarized in Table 1. The reasons for failed ERCP were DO in 18 patients (complete DO requiring duodenal stenting, n = 7) and SAA in seven patients (three total gastrectomy with Roux-en-Y gastrojejunostomy, four Billroth II and Roux-en-Y anastomosis). Among 18 patients with duodenal obstruction, seven were DO type I, 11 were DO type II. Regarding seven patients with duodenal stenting, the type of DO were type I (n = 3), and type II (n = 4). Uncovered stent was deployed in all patients (n = 7). EASL was performed after duodenal stent placement in four patients (median 18 days; one in type I, and three in type II DO [Figure 2E]), and vice versa in three patients (median 6 days; two in type I, and one in type II DO [Figure 3]).
| EASL (n = 25) | |
|---|---|
| Age, mean (y) | 60.2 ± 12.8 |
| Male sex, n (%) | 17 (68) |
| Diagnosis, n (%) | |
| Pancreatic cancer | 8 (32) |
| Cholangiocarcinoma | 3 (12) |
| Advanced gastric cancer | 11 (44) |
| Othersa | 3 (12) |
| Reason for failed ERCP, n (%) | |
| Duodenal obstruction | 18 (72) |
| Surgically altered anatomy | 7 (28) |
| Pretreatment chemistry, median (IQR) | |
| WBC (3/mm) | 6.5 (5-9.6) |
| Total bilirubin, mg/dL | 5.5 (0.9-9.4) |
| AST, IU/L | 122 (79-174) |
| ALT, IU/L | 134 (45-176) |
Note
- Data are presented as mean ± standard deviation, median (interquartile range), or number (%).
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; EASL, endoscopic ultrasound-guided antegrade covered stent placement with long duodenal extension; ERCP, endoscopic retrograde cholangiopancreatography IQR, interquartile range; WBC, white blood cell.
- a Others include duodenal cancer, pancreatic neuroendocrine tumor, and ovarian cancer in one patient each.
3.2 Clinical outcomes
3.2.1 Primary outcome
The technical success rate for EASL was 96% (n = 24/25). Technical failure occurred in one patient in the EASL group because the guidewire could not pass the stricture site of the common bile duct owing to complete obstruction by pancreatic cancer. The patient was managed with EUS-HGS with transmural metal stenting. All cases were suitable for EUS-HGS. However, for the evaluation of stent patency and reintervention rate of EASL, EUS-HGS with transmural stenting during the same session was not attempted. The median procedure time was 20.5 minutes (interquartile range 11.25).
3.2.2 Secondary outcomes
Clinical success was achieved in 21 patients (84%). In the other four patients, the reasons for clinical failure were advanced diseases in three patients and technical failure in one patient. With respect to adverse events, six occurred in the EASL group (two cases of cholangitis due to stent malfunction and four cases of postprocedural pancreatitis in non-pancreatic cancer patients). Reintervention for stent obstruction occurred in two cases (8.3%), which were managed with percutaneous transhepatic biliary drainage. The four cases of postprocedural pancreatitis were all mild and improved with conservative treatment. Two cases (one in a patient with ampullary cancer and one in a patient with pancreatic neuroendocrine tumor) of spontaneous distal migration (lost with stool production) related to shrinkage of the tumor after chemotherapy occurred during the follow-up; however, reintervention was not needed because the patients improved with chemotherapy. The median observational period was 2.7 month (95% confidence interval [CI]: 2.62-5.65). The median patency was 9.4 months (95% CI: 7.96-not available), and the overall survival was 2.73 months (95% CI 2.43-7.86) (Figure S1). The clinical outcomes in EASL are summarized in Table 2.
| Outcomes of EASL (n = 25) | |
|---|---|
| Technical success (%) | 24 (96) |
| Clinical success (%) | 21 (84) |
| Adverse event (%) | 6 (25) |
| Pancreatitis | 4 |
| Migration | 2 |
| Survival outcomes | |
| Reintervention (%) | 2 (8.3) |
| Median patency, mo | 9.40 (95% CI 7.96-NA) |
| Median overall survival, mo | 2.73 (95% CI 2.43-7.86) |
Note
- Data are presented as median (interquartile range) or number (%).
- Abbreviations: CI, confidence interval; EASL, endoscopic ultrasound-guided antegrade covered stent placement with long duodenal extension; NA, not available.
3.2.3 Comparative analyses using IPTW with propensity scores
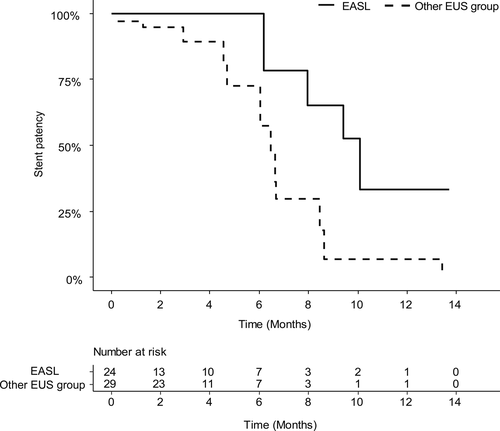
Baseline characteristics between EASL and conventional EUS-BD groups were shown in Table S1. The clinical success rate was 96.6% in the conventional EUS-BD and 84% in the EASL group. The clinical success did not differ between the two groups after IPTW adjustment (odds ratio [OR] 0.186, confidence interval [95% CI] 0.018-1.885; P =.155). With respect to adverse events, five occurred in the conventional EUS-BD group (three cholangitis, one hemobilia, one bile peritonitis) and six in the EASL group (two cholangitis, four postprocedural pancreatitis in non-pancreatic cancer patients). After IPTW, there was no statistical difference (OR 1.157, 95% CI 0.264-5.070; P =.846). The reintervention rate was lower in the EASL group than in the conventional EUS-BD; however, the difference showed marginal significance (hazard ratio 0.242, 95% CI 0.057-1.035; P =.056) (Table 3). After IPTW adjustment, the median stent patency in the EASL and conventional EUS-BD groups was 10.1 and 6.5 months, respectively (P =.018; Figure 4).
| Binary outcomes | Group | Crude | IPTW | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Other EUS-guided drainage (n = 29) | EASL (n = 25) | OR | 95% CI | P-value | OR | 95% CI | P-value | |||
| Technical success (%) | 29 (100) | 24 (96) | - | |||||||
| Clinical success (%) | 28 (96.6) | 21 (84) | 0.188 | 0.009 | 1.382 | .147 | 0.186 | 0.018 | 1.885 | .155 |
| Adverse event (%) | 5 (17.2) | 6 (25) | 1.600 | 0.418 | 6.360 | .490 | 1.157 | 0.264 | 5.070 | .846 |
| Survival outcomes | HR | 95% CI | P-value | HR | 95% CI | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reintervention (%) | 11 (37.9) | 2 (8.3) | 0.16 | 0.034 | 0.711 | .017 | 0.155 | 0.031 | 0.772 | .023 |
| Reinterventiona (%) | 11 (37.9) | 2 (8.3) | 0.21 | 0.050 | 0.904 | .036 | 0.242 | 0.057 | 1.035 | .056 |
| Stent patency | 12 (41.4) | 4 (16.7) | 0.266 | 0.084 | 0.839 | .024 | 0.236 | 0.071 | 0.777 | .018 |
| Overall survival (%) | 23 (79.3) | 22 (88.0) | 1.29 | 0.717 | 2.321 | .395 | 1.35 | 0.773 | 2.339 | .294 |
Note
- Abbreviations: CI, confidential interval; EASL, endoscopic ultrasound-guided antegrade covered stent placement with long duodenal extension; HR, hazard ratio; IPTW, inverse probability treatment weighting; OR, odds ratio.
- a Fine and Gray competing-risk analysis with death as the competing event.
4 DISCUSSION
EUS-HGS with transmural stenting has a potential risk of serious adverse events, such as proximal migration of the stent, whereas EUS-AGUS has a risk of tumor ingrowth.11 Furthermore, obstruction by sludge or stones often occurs despite EUS-HGS with transmural stenting to bypass the stricture.12 Therefore, in conventional EUS-BD in patients with DO or SAA after a failed ERCP, reintervention related to stent dysfunction may frequently be required.
In this pilot study, EASL showed a 96% technical success rate and an 84% clinical success rate, which were comparable to the rates of other EUS-guided biliary drainages.13 The EASL group showed a low intervention rate (2/24) during the follow-up period, which may be lower than that of other modalities.14 The median stent patency of the EASL group was 9.4 months, which seems to be comparable to a recent study on EUS-HGS with partially covered metal stenting, which showed a median stent patency of 6.3 months in 110 patients (75 with DO and 16 with SAA) with malignant biliary obstruction.12 The low reintervention rate and comparable stent patency of EASL may have resulted from a decrease in reflux cholangitis (Figure 3D) owing to the long duodenal extension and the prevention of tumor ingrowth by a covered metal stent. However, this interpretation may be premature because of the relatively small number of patients included in our pilot study.
Gwon et al introduced percutaneous antegrade placement of a double-stent system with an outer self-expanding uncovered stent and an inner expanded polytetrafluoroethylene-covered stent for MDBO.6 As the length was 21 cm, the distal end was located in the third or fourth duodenal portion or in the jejunum, similar to the concept of our study although the stent was much longer in their study. However, 10 patients (23.8%) experienced stent occlusion by food and biliary sludge, which needed reintervention. As it was a single study, the interpretation of the study results was limited; however, the occlusion of the covered metal stent could be attributed to e-polytetrafluoroethylene, which is reported to be vulnerable to the formation of biofilm, or to the excessively long extension of the stent, which can increase antegrade flow resistance.15 As we used an 11-13-cm-long stent in our pilot study, it can be presumed that the antegrade flow resistance would be lower than that with the stent used by Gwon et al.
In terms of overall adverse events, four patients had a mild degree of postprocedural pancreatitis, which rarely occurs in EUS-HGS. In this study, 68% of the patients in the EASL group had non-pancreatic cancer, which is a risk factor for pancreatitis after metal stent placement.16 None of the four patients with post-ERCP pancreatitis in our study had pancreatic cancer. Therefore, EASL may be considered for patients with pancreatic cancer after a failed ERCP. As another merit of EASL, a duodenal stent for accompanying type II DO could be safely inserted as the distal end of the biliary covered stent is placed in the third portion of the duodenum (Figure 2E and Figure 3). As usual, the biliary stent patency via the pre-positioned duodenal stent can be affected by the duodenal stent patency. In our pilot study, none of patients experienced recurrent biliary obstruction in patients (0%, 0/3) with EASL with pre-positioned duodenal stent with stent-in-stent fashion (duodenal stent first and EASL later in type II DO, Figure 2E). Recurrent biliary obstruction for EASL without duodenal stent was observed in two patients (11.8%, 2/17). However, it is hard to tell whether biliary stent patency or recurrent biliary obstruction in EASL would not be affected by pre-positioned duodenal metal stent patency with this small number and limited observation time. Covered or uncovered duodenal metal stent could be used subsequently after EASL by side-by-side stent placement (EASL first and duodenal stent placement later in type II DO, Figure 3). In our pilot study, however, only one patient with type II DO had side-by-side EASL and uncovered duodenal metal stent placement. For the evaluation of side-by-side EASL and covered or uncovered duodenal metal stent patency and the ideal way of deploying both biliary and duodenal metal stent in EASL, therefore, future larger studies are required.
This study had several limitations. First, this was a single-arm study with a small number of included patients for EASL. Therefore, we performed additional comparative analysis with IPTW of propensity scores to overcome this matter, but the heterogeneity of the comparative group with a small patient number makes it difficult to conclude that there is a benefit to our method; therefore, the present study may be a basis for further well-designed studies in the future. Second, a recent study about EUS-HGS combined with transmural and antegrade covered stenting was proposed for prolonged stent patency and reduced reinterventions.11 However, the cost of the metal stent used for this procedure should not be neglected. Further comparative studies with a cost-effectiveness analysis between EASL and EUS-HGS combined with transmural novel plastic and antegrade stenting would be of interest.17 Third, the inherent limitations of a retrospective analysis remain. Fourth, as the life expectancy of the patients was short, there was insufficient time to observe stent dysfunctions related to the stent.
In summary, EASL has acceptable technical and clinical success rates, patency, and adverse events but a higher rate of postprocedural pancreatitis in patients with non-pancreatic cancers. The low number of reinterventions may be an attractive pilot study result warranting further studies on EASL. Future randomized trials comparing the EASL and conventional EUS-BD in patients with unresectable pancreatic cancer with DO or SAA after a failed ERCP are needed to validate our findings.
ACKNOWLEDGMENTS
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, Republic of Korea, the Ministry of Food and Drug Safety) (project number: KMDF_PR_20200901_0266, 999100693)
CONFLICT OF INTEREST
All authors disclose no financial relationships relevant to this article.
AUTHOR CONTRIBUTIONS
Conception and design: Do Hyun Park. Acquisition of the data: Do Hyun Park, Mamoru Takenaka, Kosuke Minaga, Shinya Uemura, Takuji Iwashita, Tomotaka Saito, Yousuke Nakai. Analysis and interpretation of the data: Seon Ok Kim, Hoonsub So, Do Hyun Park. Drafting of the article: Hoonsub So, Dongwook Oh, Do Hyun Park. Critical revision of the article for important intellectual content: Mamoru Takenaka, Kosuke Minaga, Shinya Uemura, Takuji Iwashita, Tomotaka Saito, Yousuke Nakai. Final approval of the article: Do Hyun Park.
Open Research
DATA AVAILABILITY STATEMENT
The datasets of this study are available from the corresponding author upon reasonable request.




