QoL during KTd or KRd induction followed by K maintenance or observation in transplant noneligible patients with newly diagnosed multiple myeloma: Longitudinal and cross-sectional analysis of the randomized AGMT 02 study
Abstract
Understanding the impact of induction and maintenance therapy on patients’ quality of life (QoL) is important for treatment selection. This study aims to compare patient-reported QoL between patients treated with KTd or KRd induction therapy and K maintenance therapy or observation. QoL was assessed using the EORTC QOL-C 30 and QOL-MY20 questionnaires in the AGMT-02 study, in which 123 patients with newly diagnosed transplant ineligible multiple myeloma were randomized to nine cycles of either KTd or KRd induction therapy, followed by 12 cycles of K maintenance therapy, or observation. Longitudinal assessments showed statistically significant improvements in global health-related QoL, various disease symptoms and pain for both treatment regimens. KTd improved insomnia and fatigue, and KRd improved physical functioning. Cross-sectional comparisons indicated a “slight” superiority of KTd over KRd in several scales, with the exception of higher neuropathy scores with KTd. During maintenance, longitudinal comparisons showed no statistically significant changes. Cross-sectional comparisons revealed a “slight” improvement in cognitive functioning during carfilzomib therapy, but a worsening in most other QoL scales. Induction therapy led to improvements in most QoL items, while maintenance therapy with K maintenance was associated with “slight” or “moderate” impairments in several QoL scales compared with the observation group.
1 INTRODUCTION
The main goals of multiple myeloma therapy are to control the disease, extend survival [1], reduce disease-related symptoms and treatment-related adverse events, and thus improve quality of life (QoL) [2]. Several studies have shown that physicians often underestimate the impact of cancer-related symptoms such as nausea, fatigue or pain on patient's well-being [3]. Therefore, patient-reported outcomes (PROs) have become the gold standard for assessing the burden of the disease and the benefits and side effects of therapy [4]. The impact of a particular treatment on QoL in patients with multiple myeloma depends not only on the depth of response, but also to a considerable extent on the tolerability and toxicity of the individual drugs used for therapy, and on the patient's individual biological fitness and morbidities. In this study, we aimed to compare longitudinally and cross-sectionally QoL of patients randomized to nine cycles of either KTd or KRd and to compare longitudinally and cross-sectionally QoL of patients randomized to carfilzomib maintenance therapy or to observation after completion of induction therapy.
1.1 Patients
This open-label, randomized phase II trial included 123 transplant ineligible (TNE) patients with newly diagnosed multiple myeloma from 19 academic or major hospital centers in Austria and Germany [5]. Important inclusion criteria were ECOG performance status <2, GFR ≥30 mL/min, and LVEF ≥40%. Patient characteristics are shown in Table 1. Patients were randomized in a 1:1 ratio centrally via an electronic case reporting system to 9 cycles of KRd or KTd. One hundred twenty-three patients received therapy for at least one full cycle. Carfilzomib (K) was started at 20 mg/m2 at d 1+2 of cycle 1+2 and continued at 27 mg/m2 for the first 2 cycles (d 1+2, 8+9, 15+16 schedule). Thereafter, K was administered at 56 mg/m2 once weekly for a 28-day cycle. Patients received thalidomide (T) 100 mg/day (50 mg for patients > 75 years of age), d 1–28, or lenalidomide (R) 25 mg/d, d 1–21. Dexamethasone (d) 40 mg (20 mg for patients ≥ 75 years of age) once a week. After induction, patients with ≥SD were randomized 1:1 to maintenance therapy with carfilzomib (using the last tolerated dose) for 12 twice-monthly cycles, or to observation, stratified by prior induction therapy. The study protocol was approved by the Independent Ethics Committee or Institutional Review Board of the respective study centers. The study was conducted in accordance with the Declaration of Helsinki of the World Medical Association and is registered on clinicaltrials.gov (NCT02891811) and on the EU Clinical Trials Register (EudractCT 2016-000475-24).
| Parameter | KTd (n = 63) | KRd (n = 60) |
|---|---|---|
| Median age, years (range) | 75.0 (58–84) | 75.0 (55–84) |
| Gender | ||
| Male/Female | 50.8%/49.2% | 60.0%/40.0% |
| ISS Stage | ||
| I/II/III | 23.8%/41.3%/34.9% | 23.3%/38.3%/38.3% |
| R-ISS Stage | ||
| I/II/III | 14.9%/72.3%/12.8% | 22.4%/67.3%/10.2% |
| Not available | N = 15 | N = 12 |
| ECOG-Status | ||
| 0/1 | 47.6%/52.4% | 56.7%/43.3% |
| Isotype | ||
| IgA/IgG/IgD | 25.8%/48.4%/1.6% | 14.8%/63.9%/1.6% |
| Light chain only | 24.2% | 19.7% |
| Cytogenetics | ||
| t(4;14) | 6 of 51 (11.8%) | 2 of 52 (3.9%) |
| del 17p | 6 of 51 (11.8%) | 5 of 51 (9.8%) |
| ampl 1q21 | 19 of 50 (38.0%) | 19 of 51 (37.3%) |
| None | 25 of 45 (55.6%) | 27 of 48 (56.3%) |
| Missing data | 9 without cytogenetics | 6 without cytogenetics |
| Median follow-up (months) | 39.3 | 34.8 |
2 METHODS
Health-related quality of life was assessed using the EORTC questionnaires EORTC QOL-C30 [6] and the myeloma subscale QOL-MY20 [7]. The questionnaires were completed in paper form prior to the start of study treatment, and subsequently on day 1 of each treatment cycle, at the start of maintenance treatment, and monthly thereafter until the end of month 12, or at the time of treatment discontinuation or disease progression. This study focused on twelve pre-selected and clinically relevant QoL domains. Nine functional and symptom scales of the QLQ-C30 questionnaire (health-related global QoL, physical, role, social, cognitive, and emotional functioning, as well as pain, fatigue, and insomnia) and three symptom scales of the QLQ-MY20 instrument (neuropathy, disease symptoms, and side effects of treatment). The QLQ-MY20 and QLQ-C30 domains were scored in accordance with their published guidelines [12, 8]. Scores were converted into scales ranging from 0 to 100. Baseline data of the individual domains/items were normalized to allow meaningful comparisons between KTd and KRd outcomes, as well as between K maintenance and observation groups. For the functional scales, higher scores indicate a better QoL, whereas for the symptom scales, lower scores indicate a better state of health.
As a guideline for interpreting meaningful important improvements during the course of therapy, an arbitrary difference of 5–10 points in a subscale was associated with “little improvement” or “little deterioration” [9], while a change of >10–20 points in a subscale was considered a “moderate change”, and >20 as “very large” change. A “moderate change” corresponds to the previously defined minimal important difference (MID) required to suggest clinical relevance [10]. Evidence-based guidelines were used to interpret the longitudinal change from baseline within a group and the cross-sectional scores [11]. The normality of the distribution was tested using the Shapiro–Wilk test. Pearson's χ2 test was used to compare qualitative variables, while the Wilcoxon signed-rank test was used to compare the quantitative variables before and after treatment, as well as to compare the scores between the end of induction and the start of maintenance therapy. A value of p < 0.05 was considered statistically significant. Compliance rates at each scheduled assessment were calculated as the number of compliant patients divided by the number of patients with clinical data at that assessment. Due to the high compliance rate, no imputation was performed for the few missing values. The data cut-off was June 31, 2022.
3 RESULTS
3.1 Patient population
Patient characteristics are shown in Table 1. QoL data at baseline were available from 122 of the 123 patients who had received at least one study dose of KTd or KRd, with one data set missing from the KRd group. QoL data were recorded from 80 of 84 patients who started maintenance therapy, with 38 patients randomized to K maintenance therapy and 42 patients to observation. Details of the study flow are shown in Figure S1. The median follow-up was 38.5 months.
3.2 Compliance
Compliance rates were consistently high throughout the study period. During induction therapy more than 97% of patients who were still on treatment completed the questionnaires as required at each predefined time point. During the maintenance phase, 80 of 84 patients who were eligible for maintenance therapy participated in the QoL study, and 95% or more of patients still on treatment or observation completed the questionnaires.
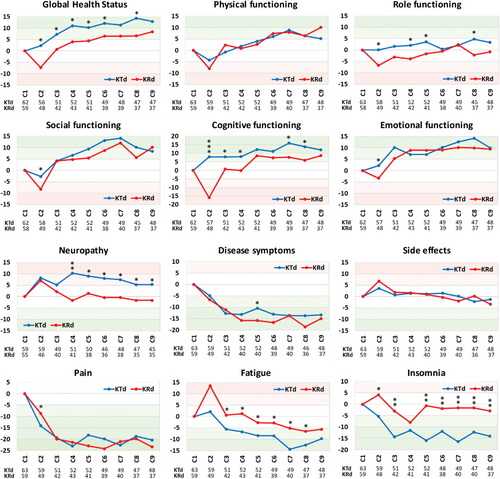
3.3 Longitudinal follow-up of functional and symptom scales, and single items from the beginning until the end of induction therapy in both treatment groups
The scores at the start of induction therapy (baseline scores) of all scales were normalized for better comparison of the two treatment groups. In Figure 1 the longitudinal average scores of all 12 functional and symptom scales are depicted. The comparison of the baseline scores with those observed at the end of induction therapy showed statistically significant improvements in global health-related QoL, disease symptoms, and in pain for both treatment regimens (KTd, p = 0.001, p = 0.027, and p = 0.01, respectively, and KRd p = 0.01, p < 0.001, and p = 0.001, respectively, [Table 2]). KTd induction therapy led to significant improvements in fatigue and insomnia (p = 0.022 and p = 0.002, respectively), while with KRd a statistically significant improvement in physical function was noted (p = 0.035). An exception to this general observation was neuropathy, which worsened during KTd (−5.29), but the difference between the first and last assessment was not statistically significant (p = 0.387). Another notable observation was the marked decline (compared with baseline) in most functional scores after the first cycle of KRd, indicating a fast deterioration in well-being, particularly cognitive function (−17). This impairment improved at least to the baseline levels, but generally exceeded those, with continuation of therapy.
| Functional Scales | Little worsening >−10 to −5 | No change >−5 to 5 | Little improvement >5 to 10 | Moderate improvement >10 to 20 | Very much improvement >20 | p-value | |
|---|---|---|---|---|---|---|---|
| KTd | KRd | ||||||
| Global Health Score | KRd (8.36) | KTd (12.91) | 0.001 | 0.010 | |||
| Physical functioning | KTd (5,13) | KRd (10.10) | 0.312 | 0.035 | |||
| Role functioning | KTd (3,34) KRd (−0.83) | 0.063 | 0.238 | ||||
| Social functioning | KTd (8.34) | KRd (10.08) | 0.213 | 0.486 | |||
| Cognitive functioning | KRd (8.59) | KTd (12.02) | 0.704 | 0.061 | |||
| Emotional functioning | KTd (9.89) KRd (9.44) | 0.061 | 0.213 | ||||
| Symptom Scales | Little worsening <10 to 5 | No change <5 to −5 | Little improvement <−5 to −10 | Moderate improvement <−10 to −20 | Very much improvement <−20 | p-value | |
|---|---|---|---|---|---|---|---|
| KTd | KRd | ||||||
| Neuropathy | KTd (5,29) | KRd (−1.69) | 0.387 | 0.519 | |||
| Disease symptoms | KTd (−13.39) KRd (−15.06) | 0.027 | <0.001 | ||||
| Side effects | KTd (−1.31) KRd (−3.39) | 0.607 | 0.555 | ||||
| Pain | KTd (−20.35) KRd (−23.42) | 0.010 | 0.001 | ||||
| Fatigue | KTd (−9.75) KRd (−5.63) | 0.022 | 0.289 | ||||
| Insomnia | KRd (−2.92) | KTd (−14.02) | 0.002 | 0.943 | |||
- Bold values indicates statistical significant.
The greatest clinically relevant improvement was observed with both regimens in pain (KTd −20.35, KRd −23.2), followed by an improvement in disease symptoms (KTd-13.39, KRd −15.06). KTd led to a “moderate” improvement in insomnia (−14.2), global health status (+12.91), and cognitive functioning (+12.02), while KRd resulted in a “moderate” improvement of physical (+10.10) and social functioning (+10.08). The data for the “slight” improvement are shown in Table 2.
3.4 Cross-sectional comparison of functional and symptom scales between KTd and KRd from the beginning and longitudinal follow-up until the end of induction therapy
The most consistent differences in functional scales between both regimens during the course of induction therapy favoring KTd were observed for global health status, role, and cognitive functioning (difference > 5 score points), indicating slightly heightened well-being (Table 2). The marked impairment in cognitive function at cycle 2 during KRd treatment was mainly seen in the group of very elderly patients (≥75 years) and the small group of unfit patients. For the symptom cores, a similar superiority of KTd over KRd was observed for fatigue and insomnia. The difference in scores amounted >5 points for fatigue at cycle 2 and >10 score points for insomnia in six of nine comparisons. In contrast, KTd therapy led to higher neuropathy scores (>10 at cycle 4, and thereafter >5 score points differences) compared with KRd, which apart from cycle one with a slight worsening, showed stable results during the induction phase.
3.5 QoL at the end of induction therapy and the start of maintenance treatment
After nine cycles of induction therapy, 80 of 84 eligible patients were randomized to receive 12 cycles of carfilzomib maintenance therapy or observation. Forty-two patients were assigned to active treatment and 38 to observation. The median time between the end of induction and the start of the maintenance phase was 38 days (5–126 days). A comparison of the scores between the two time points “end of induction” and “start of maintenance” showed no major difference for 8 of the 12 scales. However, a clinically relevant difference with a worsening of scores was observed in patients randomized to carfilzomib maintenance therapy compared with the observation group for the role (−11.20), social (−7.70), and cognitive (−6.18) functioning, and fatigue (+7.51) compared with patients randomized to observation (Table 3). The statistical comparison of scores at the two time points showed a statistically significant difference for fatigue (p = 0.036) indicating negative perceptions of patients randomized to carfilzomib maintenance therapy.
| Scales | Treatment group (2nd rand.) | End of induction (C9) | Start of maintenance (M1) | Δ of score points between C9 and M1 | p-value | Δ of score points between K vs O at M1 |
|---|---|---|---|---|---|---|
| Role functioning | K-monotherapy | 61.04 (30.73) | 63.60 (28.83) | 2.56 | 0.587 | −11.20 |
| Observation | 60.98 (32.09) | 74.80 (30.82) | 13.82 | 0.128 | ||
| Social functioning | K-monotherapy | 75.20 (28.90) | 78.07 (24.81) | 2.87 | 0.427 | −7.70 |
| Observation | 77.71 (26.74) | 85.77 (24.30) | 8.06 | 0.386 | ||
| Cognitive functioning | K-monotherapy | 79.54 (23.41) | 76.75 (25.52) | −2.79 | 0.662 | −6.18 |
| Observation | 79.76 (24.13) | 82.94 (23.43) | 3.18 | 0.697 | ||
| Fatigue | K-monotherapy | 40.36 (23.21) | 33.04 (18.64) | −7.32 | 0.036 | 7.51 |
| Observation | 39.44 (23.25) | 25.53 (26.42) | −13.91 | 0.077 |
- Bold values indicates statistical significant.
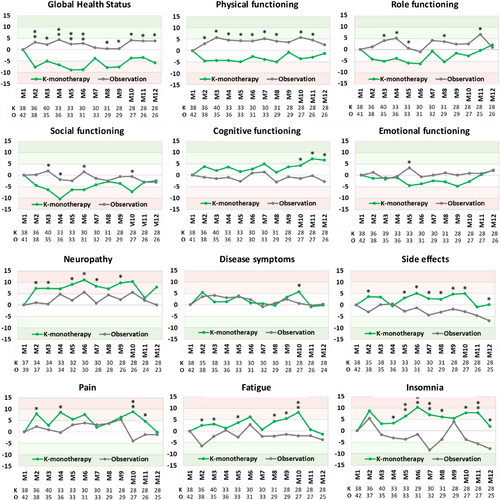
3.6 Longitudinal follow-up of functional and symptom scales, and single items from the beginning until the end of carfilzomib maintenance therapy or observation and cross-sectional comparison
Differences in the mean EORTC QLQ-C30 scores between baseline and each cycle of maintenance with K-monotherapy or observation are shown in Figure 2. The comparison between the normalized scores at the beginning and the end of the maintenance phase showed no significant differences (Table 4). However, a “slight” improvement in cognitive functioning was noted during carfilzomib maintenance therapy (+6.58) and in the observation group for side effects of therapy (−6.82) and for insomnia (−7.84). It is noteworthy the neuropathy worsened “slightly” at the end of carfilzomib maintenance therapy (7.85).
| Functional Scales | Little worsening >−10 to −5 | No change >−5 to 5 | Little improvement >5 to 10 | Moderate improvement >10 to 20 | Very much improvement >20 | p-value M1 vs. M12 | |
|---|---|---|---|---|---|---|---|
| K | O | ||||||
| Global Health Score | K (−5.80) | O (3.85) | 0.105 | 0.730 | |||
| Physical functioning | K (−1.12) O (2.63) |
0.425 | 0.714 | ||||
| Role functioning | K (1.88) O (0.84) |
0.545 | 0.677 | ||||
| Social functioning | K (−2.47) O (−3.08) |
0.238 | 0.688 | ||||
| Cognitive functioning | O (−2.81) | K (6.58) | 0.139 | 0.954 | |||
| Emotional functioning | K (2.26) O (2.01) |
0.792 | 0.856 | ||||
| Symptom Scales | Little worsening <10 to 5 | No change <5 to −5 | Little improvement <−5 to −10 | Moderate improvement <−10 to −20 | Very much improvement <−20 | p-value M1 vs. M12 | |
|---|---|---|---|---|---|---|---|
| KTd | KRd | ||||||
| Neuropathy | K (7.85) | O (0.04) | 0.245 | 0.386 | |||
| Disease symptoms | K (−0.22) O (0.30) |
0.439 | 0.661 | ||||
| Side effects | K (0.19) | O (−6.82) | 0.981 | 0.586 | |||
| Pain | K (−0.13) O (−1.19) |
0.681 | 0.262 | ||||
| Fatigue | K (−1.29) O (−3.73) |
0.853 | 1.000 | ||||
| Insomnia | K (1.88) | O (−7.84) | 0.950 | 0.287 | |||
The cross-sectional comparisons showed a “slight” improvement (>5 score points) in cognitive functioning during the last three cycles of carfilzomib maintenance therapy, but deterioration of scale points on most or almost all 12 assessments during the maintenance phase. This was particularly true for global health status (>10 on 5 of 12 assessments) while a difference >5 points in favor of the observation group was observed in physical, role, and social functioning. Compared with the observation group, maintenance therapy with carfilzomib therapy was associated with more neuropathy, side effects of therapy, fatigue, pain (>5), and more insomnia (>10 in 4 out of 12 assessments).
4 DISCUSSION
Induction therapy with nine cycles of KTd or KRd led to a statistically significant improvement in the scores for global health, disease symptoms, and pain (Table 2). In addition, KTd led to a significant improvement in fatigue and insomnia, while KRd led to a similar improvement in physical functioning. Carfilzomib-based therapy induced a numerical improvement in all functional scales except role functioning. KTd resulted in higher scores for global health status, role, and cognitive functioning compared with KRd. Pain severity (−20) and disease symptoms (−15) decreased substantially within the first three cycles with similar improvements with KTd and KRd therapy. The observed improvements in fatigue were more pronounced with KTd therapy, and insomnia improved only during treatment with KTd. The latter observation is not surprising as thalidomide was originally introduced as a nonbarbiturate sleep-inducing drug [12] and a beneficial effect of thalidomide on insomnia has been reported in previous studies comparing melphalan and prednisolone alone (MP) and with thalidomide (MPT) [13, 14]. Improvements were also noted in fatigue with KTd being “slightly” superior to KRd. Importantly, disease symptoms improved significantly after two cycles with both treatment regimens, while the side effects of therapy showed a tendency for worsening after cycle one with KRd, but improved marginally thereafter, and remained fairly constant during the remainder of the induction phase. Another important finding was the difference in the neuropathy scores between the two regimens. The first treatment cycle was associated with a “slight” worsening of the neuropathy score with both regimens, which improved to baseline values at cycle 4 in the KRd group and remained rather stable thereafter. In the KTd group, the neuropathy score deteriorated at most at cycle 4 (−10). Thereafter, the severity of thalidomide-associated neuropathy symptoms tended to decrease with continued treatment, possibly due to a reduction in the thalidomide dose. In the only other direct comparison between thalidomide and lenalidomide in combination with melphalan and prednisone in the ECOG E1A06 study, a statistically significant higher neuropathy score was reported in the thalidomide-containing MPT combination [14]. In contrast to our comparison, no differences between MPT and MPR in functional scores assessed with the Functional Assessment of Cancer Therapy (FACT) instrument were observed.
Our study revealed a previously little-known phenomenon, namely a marked decrease in QoL scores for several items at the end of cycle one in patients in the KRd group. This phenomenon was particularly pronounced for cognitive functioning, and fatigue where the difference from baseline was >15 score points (“moderate”). The marked impairment in cognitive functioning in the KRd group at cycle 2 was mainly observed in patients 75 years of age or older in the small subgroup of unfit patients. There are only a few publications that report similar consequences of lenalidomide therapy. In a small study of three patients, a homogeneous neuropsychological pattern was observed after taking lenalidomide characterized by deficits in verbal and visual-spatial long-term memory, and a decrease in attentional and executive functions [15]. Myeloma experts from France presented two patients with memory loss as a result of lenalidomide intake in two patients [16]. The authors commented that these symptoms were consistent with the well-known chemo-brain and speculated that previous mild cognitive impairment, age, and the presence of cerebrovascular lesions might predispose people to these adverse events, which according to our findings, appear to be transient. One possible explanation for this previously unrecognized effect may lie in the longer intervals (often every three cycles, or even longer) between QoL assessments, chosen for most clinical studies [17-19]. This practice is often driven by the additional burden of monthly assessments for patients and more by the additional workload for study teams.
Another interesting finding was the substantial improvement in several dimensions of QoL in the approximately 1-month interval between discontinuation of induction therapy and randomization to maintenance therapy in patients assigned to the observation group. Improvement was particularly pronounced in role, social, and cognitive functioning, and in fatigue underscoring the relief from negative expectations in those selected to discontinue chemotherapy, while those randomized to maintenance chemotherapy anticipated the burden of chemotherapy with its negative consequences for wellbeing. Such a finding has not yet been observed in myeloma patients, but is not unexpected [20] and seems reminiscent of the psychological phenomenon of “hedonic adaptation” [21, 22]. Patients undergoing chemotherapy may initially experience a sense of distress. Over time, they adapt and the discomfort becomes the new normal, often associated with a sense of acceptable well-being. However, if the distress is suddenly removed or the therapy is discontinued, there may be a sudden increase in well-being, which seems to explain the remarkable improvement in patients randomized to observation.
The QoL data observed during maintenance therapy showed no statistically significant difference between the start and the end of the maintenance phase in any of the scales assessed in either the carfilzomib maintenance or the observation group. The cross-sectional comparisons showed discordant findings with “little” improvement in cognitive function during the last three cycles of carfilzomib maintenance therapy, but a negative impact of carfilzomib maintenance on QoL in most functional and symptom scales. The difference exceeded 10 score points for insomnia and health-related QoL and more than 5 score points for neuropathy. These differences in QoL are not trivial but were not recognized by the clinical care teams, consistent with many reports indicating that physicians often underestimate the symptoms of their patient's disease and the consequences of therapy [23, 24]. This was also true for neuropathy [25], which in the cross-sectional comparisons was “slightly” more severe during carfilzomib maintenance therapy than in the untreated observation group. However, none of the treated patients discontinued the bi-weekly carfilzomib maintenance therapy, indicating that carfilzomib maintenance can be given for long periods.
While the discrepant impact of carfilzomib on QoL during the induction and maintenance phases may seem counterintuitive, the reduction in disease burden after initiation of therapy appears to explain the rapid improvement in health-related QoL. In the maintenance phase, in well-controlled disease, even monotherapy with carfilzomib has an impact on certain dimensions of quality of life compared with untreated controls.
QoL was not assessed in the FORTE [26] and ATLAS [27] trials, which examined the effects of carfilzomib-based maintenance therapy in younger, transplant eligible, patients, but neither study reported neuropathy as a complication of KR or KRd maintenance therapy. Both studies reported prolonged PFS with carfilzomib-based maintenance therapy, while in this study carfilzomib maintenance therapy did not improve PFS, as previously reported [5]. The CARFI trial randomized patients to either carfilzomib-dexamethasone or observation after salvage transplantation and found no clinically relevant impairment in the QoL sum score in patients on maintenance, but improvement in several QoL domains was only observed in the observation group [28].
In conclusion, induction therapy with KTd and KRd resulted in a significant improvement in global health, disease symptoms, and pain. Physical functioning improved significantly under KRd and fatigue and insomnia under KTd treatment. The cross-comparison of the two regimens showed a clinically meaningful benefit of KTd for several scales except for neuropathy, which worsened under KTd.
The study revealed a previously little-known phenomenon, which demonstrates a significant decrease in several QoL scales after the first cycle of KRd, with a particularly pronounced deterioration in cognitive functioning and fatigue in elderly patients. The short treatment-free interval (median, 38 days) between the end of induction therapy and randomization to carfilzomib maintenance or control resulted in a marked discrepancy in scores for role, social, and cognitive functioning, and fatigue between patients randomized to carfilzomib or control suggesting that the perception of continuing chemotherapy led to a worsening of these QoL scales. Maintenance therapy with carfilzomib or observation did not result in statistically significant changes during the 12-month study period. The cross-sectional comparison showed a clinically relevant impairment of all functional scales (during the 12 months of therapy or at specific time points) and led to a clinically relevant impairment of all symptom scales with the exception of disease symptoms with carfilzomib maintenance therapy. These results emphasize the importance of carefully monitoring QoL throughout the entire treatment period to gain a comprehensive understanding of the effects of specific therapies.
AUTHOR CONTRIBUTIONS
Heinz Ludwig and Richard Greil conceptualized and designed the study. Heinz Ludwig and Ilvy Schweitzer were responsible for data analysis and drafted the manuscript. All authors were responsible for locally conducting the study, and data collection; edited, and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors acknowledge the valuable contribution of the study team of the AGMT, particularly Dr. Daniela Wolkersdorfer, Mag. Manuela Fersterer, Mag. Alexandra Keuschnig, and Mag. Dr. Judith Schuster. The authors also acknowledge the support of Julie Krainer, BSc, MSc for preparing this revision. The study was supported in part by the Austrian Forum Against Cancer and by a research grant of AMGEN.
CONFLICT OF INTEREST STATEMENT
The study was supported by AMGEN which provided support for the conduct and analysis of the trial, and by the Austrian Forum against Cancer which supported in part the scientific assistance of HL. HL declares receiving honoraria for lectures or advisory boards from Janssen, BMS, Takeda, Pfizer, Sanofi, and Stem line and research support from AMGEN and Sanofi. TM received honoraria from AbbVie and BMS. MS received honoraria from Janssen, AbbVie, BMS, and Pfizer. BH received honoraria from BMS, Amgen, AbbVie, and Janssen-Cilag. KP received research funding and honoraria for consultancy from Amgen, BMS, Janssen, and Roche. WW is an employee of syndena GmbH and received research funding from Amgen, Takeda, BMS-Celgene, Janssen-Cilag, Novartis, Roche, Sanofi, and oncotyrol and received honoraria for participation in steering and safety committees from Amgen, BMS-Celgene, and Morphosys and received honoraria for consultancy from Amgen, Takeda, BMS-Celgene, EUSA Pharma, Gilead, AbbVie, Janssen-Cilag, GSK, Incyte, Kite, Novartis, Morphosys, Merck, Pfizer, Roche, Sandoz, and Sanofi, and received honoraria from Fujimoto and Myelom- und Lymphomselbsthilfe. MTK received research funding from Janssen and honoraria from GSK, Sanofi, Pfizer, Janssen, Amgen, BMS-Celgene, and Takeda. AP received honoraria for participation in advisory boards from Novartis, Kite-Gilead, Amgen, Celgene, Janssen, Roche, Sandoz, AstraZeneca, AbbVie, Takeda, Sanofi, Pfizer, Saegen, Daiichi Sankyo, and received travel support from Kite-Gilead, Janssen, Roche, AstraZeneca, Pfizer, and Daiichi Sankyo. CS received research funding from AstraZeneca, Janssen-Cilag, and Roche, and received honoraria for consultancy from AbbVie, AstraZeneca, BMS-Celgene, Janssen-Cilag, Roche, and Takeda. SMS received honoraria for consultancy from Jazz Pharmaceuticals, Novartis, Amgen, BMS, and Gilead. HA received research funding from Janssen and received honoraria from Janssen, Amgen, BMS, and Takeda. SK received honoraria from Amgen, BMS-Celgene, and Sanofi, and received travel grants from BMS and Sobi. RG received research funding, travel support, and honoraria for consultancy and participation in advisory boards from AbbVie, Takeda, Daiichi Sankyo, Gilead, MSD Merck, BMS-Celgene, Novartis, AstraZeneca, Janssen-Cilag, and Hoffmann-La Roche. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
The study protocol was approved by the Independent Ethics Committee or Institutional Review Board of the respective study centers.
PATIENT CONSENT STATEMENT
Patients were informed in detail about the purpose and conduct of the study by the principal investigator or subinvestigators. All patients received a consent form containing the information on the aim of the study, the treatment arms, possible benefits and risks and had at least 24 h to study the document, and signed the form in the presence of the study staff.
CLINICAL TRIAL REGISTRATION
The AGMT 02 study is registered with clinicaltrials.gov (NCT02891811) and with EudractCT, 2016-000475-24.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from AGMT, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the AGMT and Heinz Ludwig.




