Next Generation Sequencing Allows Identification of a Novel Mutation in the TfR2 Gene and Outperforms the Conventional Diagnostic Techniques
Rosa Lombardi and Paola Dongiovanni contributed equally to this study.
Funding: This study was supported by Italian Ministry of Health (Ricerca Corrente 2024 - Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico), Italian Ministry of Health (Ricerca Finalizzata Ministero della Salute GR-2019-12370172; RF-2021-12374481), PNRR-MCNT2-2023-12378295, and by 5×1000 2020 RC5100020B.
ABSTRACT
Introduction
Type 3 hereditary hemochromatosis (HH) is a rare genetic disease due to mutations in the transferrin receptor 2 (TFR2) gene.
Methods and Results
Here, we describe the case of an Italian patient presenting with hyperferritinemia and hepatic iron accumulation, not evidenced by magnetic resonance imaging, that was subsequently classified as HH Type 3 by the identification of the novel frameshift mutation c.523_524delC>T (p. Leu175Aspfs*41) in exon 4 of TFR2 gene through the whole exome sequencing (WES) approach.
Conclusion
WES would allow to diagnose rare HH-related diseases in patients with unexplained hepatic iron overload and/or aberrant circulating iron parameters.
Trial Registration
The authors have confirmed clinical trial registration is not needed for this submission.
1 Introduction
Hereditary hemochromatosis (HH) comprises a group of genetic disorders characterized by excessive iron absorption [1]. Overtime, iron accumulates in liver, pancreas, heart, joints, and pituitary gland, thus leading to life-threatening complications as liver cirrhosis, Type 2 diabetes (T2D), heart failure, arthropathy, skin pigmentation, and hypogonadism [2].
Most cases of HH are attributable to mutations in the HFE gene encoding a non-classical MHC Class I protein which negatively regulates iron absorption [3]. HFE, mainly expressed at the hepatocyte surface, competes with transferrin (Tf) to bind transferrin receptor-1 (TfR1) thus downregulating iron uptake. When serum iron levels increase, the iron-loaded Tf (holo-Tf) gains high affinity for TfR1, HFE is displaced from the HFE-TfR1 complex and becomes available to associate with transferrin receptor-2 (TfR2) and hemojuvelin (HJV). The HFE–TfR2–HJV complex triggers the bone morphogenetic protein (BMP)/SMAD signaling pathway to hepcidin gene (HAMP) expression [4].
The rs1800562 G>A (C282Y) mutation in the HFE gene (6p22.2, exon 4), leads to a protein misfolding thus preventing its expression at cell surface and dampening its negative regulation of iron absorption. C282Y homozygosity with an autosomal recessive transmission is associated with HH in 2–3/1000 subjects in the general population, with the higher prevalence in northern Europe where the diseases origin [1]. The other known rs1799945 C>G (H63D) mutation in the HFE gene is more frequent but has reduced pathogenicity except when inherited together the C282Y.
Actually, HH is a heterogeneous disorder and several mutations in other genes which participate in iron metabolism (non-HFE genes) have been associated with the disease. By far, HH definition has been split as follow: Type 1 (mutations in HFE gene), 2, 3, and 4. Mutations in HJV (BMP co-receptor) and HAMP genes (Types 2a and 2b HH, respectively) result in juvenile hemochromatosis, with the most severe phenotype [5]. Type 3 HH is a rare form of HH caused by genetic variations in the TFR2 gene [6] whereas Type 4 HH, also known as ferroportin (FPN) disease, is due to mutations in the solute carrier family 40-member 1 (SLC40A1) gene and differently from the other types, its transmission is autosomal dominant [7].
TFR2 gene is located on the long arm of chromosome 7 (7q22.1), encodes a Type II transmembrane glycoprotein which belongs to the TFR family sharing homology with TfR1 and codifies two main variants named TfR2α and TfR2β [8]. Tfr2α is mainly expressed in hepatocytes and erythroid cells. It is a protein of about 89 kDalton (kDa) and similarly to TfR1 has a short cytoplasmic tail, a transmembrane domain possibly involved in TfR2 homodimerization, and a large extracellular domain that bind di-ferric Tf (Fe2Tf).
TfR2β has an in-frame transcription start site in exon 4, its transcript lacks exons 1–3, resulting in a protein without the cytoplasmic and the transmembrane domain, and it is mostly expressed in spleen, heart, and brain. Since the TfR2β isoform lacks a signal peptide involved in the secretory pathway, it has been speculated that it might be a cytosolic 60 kDa protein similar to the TfR2α extracellular domain [8].
Type 3 HH is a rare recessive disorder with a similar phenotype to HFE-HH, showing increased serum ferritin, higher Tf saturation, and iron overload [9, 10]. Although most of the mutations involved in Type 3 HH cause the inactivation of both TfR2 isoforms, homozygosity for the M172K variant, which is located in exon 4, impairs TfR2β translation initiation codon, and patients affected showed cirrhosis, hypogonadism, cardiomyopathy, and arthritis [11].
Here, we describe the case of an Italian patient presenting with hyperferritinemia that was subsequently classified as HH Type 3 by the identification of a frameshift mutation in exon 4 of TFR2 gene.
2 Case Presentation
A 61-year-old Italian man was evaluated in June 2024 at the Metabolic and Liver Disease outpatient service at Fondazione IRCCS Cà Granda, Ospedale Policlinico Milano (Milan, Italy) for hyperferritinemia associated with thalassemic trait, Gilbert syndrome, and dyslipidemia. He reported a familial history positive for cardiovascular disease and T2D. In addition, he had two brothers with thalassemic trait, one of which with a history of liver transplantation for HCV and potus related cirrhosis (Brother I). The thalassemic trait and Gilbert syndrome were also observed in his 31-year old son but not in his daughter (Table 1).
| Proband | Brother I | Brother II | Daughter | Son | |
|---|---|---|---|---|---|
| Gender | Male | Male | Male | Female | Male |
| Age, years | 68 | 75 | 79 | 29 | 33 |
| BMI, kg/m2 | 18.7 | 24.1 | 21.5 | 25 | 22.3 |
| Presence of IFG/T2D | No | Yes | No | No | No |
| Dyslipidemia | Yes | No | Yes | Yes | No |
| Iron, µg/dL | 192 | 67 | 153 | 195 | 115 |
| Ferritin, µg/L | 1114 | 380 | 166 | 21 | 373 |
| Transferrin, mg/dL | 188 | 237 | 218 | 334 | 227 |
| Transferrin saturation (%) | 73 | 19 | 49 | 26.3 | 36 |
| Hb, g/dL | 11.5 | 12.4 | 14 | 12.7 | 13.1 |
| MCV, fL | 63 | 66 | 77 | 86 | 62 |
| TFR2 c.523_524delC>T | c.[523_524del, =] | c.[523_524del, =] the | c.[=, =] | c.[=, =] | c.[523_524del, =] |
| HFE c.187C>G (H63D) | CG | CC | CC | CG | CG |
| HFE c.845G>A (C282Y) | GG | GG | GG | GG | GG |
| HBB c.118C>T (codon 39) | CT | CT | CT | CC | CT |
- Note: [=]: wild type allele.
- Abbreviations: BMI, body mass index; HBB, hemoglobin subunit beta; IFG, impaired fasting glucose; T2D, Type 2 diabetes.
Proband's diet was balanced without excessive consumption of red meet, saturated/trans fats, chocolate, and candies. He denied alcohol consumption and smoking. He was a normal weight subject although with a scant physical activity.
At presentation, the physical examination revealed blood pressure of 105/60 mmHg, weight of 56 Kg, BMI of 18.7 Kg/m2. Blood tests indicated increased ferritin levels (988 µg/L) and Tf saturation (65%), along with total and unconjugated bilirubin levels of 2.5 and 1.88 mg/dL, respectively, in the absence of hemolysis (normal LDH 155 μ/L, haptoglobin 77 mg/dL, and reticulocyte count 1.78%) and normal transaminases. Hormonal tests (LH, FSH estrogens, prolactin, testosterone, DHEA-S, and TSH) were within normal ranges. The patient referred pains in the back and hand, tests were negative for rheumatoid factor, ENA, ANA, PCR, and ESR whereas bilateral hands x-ray showed arthropathy.
Abdominal ultrasound (US) showed no pathologic findings, whereas a transient elastography by Fibroscan reported a liver stiffness measurement slightly increased (6.8 kPa).
Magnetic resonance imaging (MRI) scan with gradient recalled echo (GRE) T2*-weighted imaging revealed only mild iron deposition in the liver (T2* 5.22 m/s, normal values > 6.3 m/s) without accumulation in the heart. Since there was a discrepancy between ferritin levels and MRI results, we further investigated iron accumulation in the liver by performing a biopsy which depicted a widespread iron deposition within hepatocytes (2+) and Kupffer (3+) cells, consistent with a clinical HH diagnosis (Figure 1A).

HH was highly suspected also by persistently high serum levels of ferritin (maximum 1114 µg/L) and Tf saturation (maximum 73%) since 2017.
At first genetic screening, DNA was isolated from peripheral blood mononuclear cells (PBMCs) to assess the mutations in the HFE gene and the proband resulted heterozygous for the H63D variant (Table 1).
Since the latter could not explain the increased iron parameters, we performed whole-exome sequencing (WES) using the Illumina NextSeq2000. DNA was fragmented and target sequences were enriched by PCR methods (SureSelect Technology). All single-nucleotide variants were filtered via multiple databases and variants frequency was evaluated by dbSNP, using European descent as the reference population.
The proband had a frameshift mutation c.523_524delC>T (p.Leu175Aspfs*41; rs1803510959) in heterozygosity, in the exon 4 of TFR2 gene (Figure 1B). The target sequence of the suspected pathogenic mutation (delAG) was confirmed by Sanger sequencing (ABI 3130, Thermo-Fisher Scientific). It has been reported as a sequence variant which causes a disruption of the translational reading frame, since the number of deleted nucleotides is not a multiple of three (https://www.ensembl.org) and it is classified as pathogenic for Type 3 HH according to ACMG criteria and the in silico prediction programs (CADD 32, PaPi 0.988 and ClinVar) [6, 12, 13].
Notably, this novel mutation was located three nucleotides downstream from the previously described variant M172K [11]. In order to investigate whether the frameshift mutation could dampen the transcription of isoform β as the M172K variant [10] and the (BMP)/SMAD signaling pathway, we compared the expression of TfR2 (both isoforms), HAMP genes, and of phospho-SMAD1/5/8 in liver biopsies obtained from one patient without hemochromatosis (no-HH), one patient with Type 1 HH (homozygous for the C282Y mutation, 282YY), one patient with Type 3 HH (homozygous for the Q672X mutation in the TfR2 gene (672XX) and the proband (p.Leu175Aspfs*41), through real-time PCR (qRT-PCR), and western blot (WB) (Table 2). At qRT-PCR we observed that the proband showed the lowest TfR2α mRNA levels, whereas the expression of isoform β was unchanged compared to the other conditions (Figure 1C,D). As expected, HAMP mRNA levels were reduced in both patients with Types 1 and 3 HH as well as in the proband compared to no-HH, thus supporting an iron metabolism dysregulation (Figure 1E). Accordingly, WB analysis revealed an inactivation of the SMAD1/5/8 signaling in the proband and the 282YY patients compared to no-HH (Figure 1F).
| No-HH | 282YY | 672XX | |
|---|---|---|---|
| Gender | Male | Male | Male |
| Age, years | 52 | 67 | 49 |
| BMI, kg/m2 | 29 | 24 | 28.6 |
| Presence of IFG/T2D | No | No | No |
| Dyslipidemia | Yes | Yes | Yes |
| Iron, µg/dL | 91 | 121 | 261 |
| Ferritin, µg/L | 648 | 909 | 2040 |
| Transferrin, mg/dL | 40 | 194 | 214 |
| Transferrin saturation (%) | 26 | 43 | 85 |
| Hb, g/dL | 15.7 | 15.1 | 14.8 |
| MCV, fL | 87 | 91 | 89 |
- Note: 282YY and 672XX refer to HFE and TFR2 homozygosity for the mutated allele, respectively.
- Abbreviations: BMI, body mass index; IFG, Impaired Fasting Glucose; T2D, Type 2 Diabetes.
Then, we performed WES also in the proband's relatives (Brother I, Brother II, son, and daughter) for good clinical practice (Figure 2). The rs1803510959 mutation was identified in heterozygous in Brother I and in the son thus allowing the diagnosis of Type 3 HH (Table 1). The proband was then started with venesection to obtain iron depletion. Conversely, since both mutated relatives presented with normal iron profiles, we decided only to monitor them over time without any therapeutic approach.
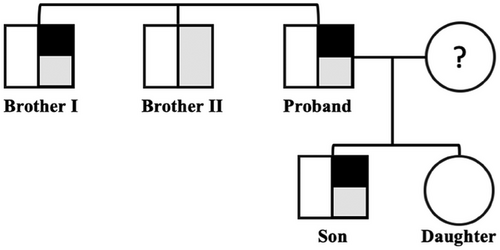
In summary, WES analysis allowed us to discover a novel mutation in the TFR2 gene associated with the rare Type 3 HH phenotype. Our results highlight the importance of exploiting advanced sequencing techniques to diagnose rare HH-related diseases in patients with unexplained iron accumulation in the liver and/or aberrant circulating iron parameters. In this case, non-invasive approaches (MRI) were ineffective in detecting hepatic iron overload whereas genetic screening has permitted a definitive diagnosis, thus guiding in the clinical management (i.e. bloodletting) as provided by the treatment guidelines of other type of HH [14].
Moreover, this study revealed the coexistence between the beta thalassemic trait and a TFR2 mutation. To date it is still debated whether the former may have a detrimental or a beneficial impact on iron status [15-18]. Here, we found the beta thalassemic trait in all the proband's family except for the daughter. However, we cannot claim the existence of a co-inheritance between the TFR2 mutation and HBB trait as Brother II was negative for the TFR2 deletion. The lack of hepatic biopsy in relatives limits possible speculation about the damaging or protective effect of the thalassemic trait on iron overload thus paving the way for further investigations.
Author Contributions
M.L., data analysis and interpretation, data generation, and manuscript drafting. E.P. and M.M., data analysis and interpretation. F.C., C.B., G.P., patients' enrolment and data generation. M.M., data generation. A.L.F., patients' enrolment, manuscript revision, and funding acquisition. R.L., patients' enrolment, manuscript drafting, data analysis and interpretation, and funding acquisition. P.D., study design, manuscript drafting, data analysis and interpretation, and funding acquisition. All authors read and approved the final manuscript.
Acknowledgments
The Department of Pathophysiology and Transplantation, University of Milan, is funded by the Italian Ministry of Education and Research (MUR): Dipartimento di Eccellenza Program 2023–2027.
Ethics Statement
The study was approved by the Ethical Committee at Fondazione IRCCS Cà Granda, Ospedale Maggiore Policlinico Milano and conformed to the 1975 Declaration of Helsinki (Ethics Commettee no. 1473 3.11/2020-153).
Consent
All participants provided a written informed consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




