Acute myeloid leukemia in SRP54-mutated congenital neutropenia
Abstract
SRP54 mutations have recently been implicated in congenital neutropenia (CN) and the in-frame deletion, p.Thr117del, is the most common pathogenic mutation reported. The largest study of SRP54-mutated CN to-date followed 23 patients for a median of 15 years. No patients developed a hematologic malignancy in that study. Given the known risk of leukemia in other CNs it is crucial to know whether patients with SRP54-mutated CN have an increased risk of leukemia. We report the first case of leukemia in a patient with SRP54-mutated CN. A 15-year-old male with SRP54-mutated CN (p.Thr117del) was diagnosed with acute myeloid leukemia with myelodysplasia-related changes on a screening bone marrow evaluation. Next generation sequencing of the leukemia cells identified CSF3R and RUNX1 mutations. These mutations commonly co-exist in CN-associated malignancies and suggest leukemogenesis in SRP54-mutated CN may occur in a similar manner to other CNs. He was successfully treated with CPX-351 followed by hematopoietic cell transplant (HCT) and remains in remission at a follow-up time of 9 months. Although conclusions from this single report must be limited, this has potentially significant implications for both screening and treatment practices for these patients, including the role and timing of HCT.
1 INTRODUCTION
The SRP54 gene encodes signal recognition particle (SRP) 54 GTPase protein and mutations in SRP54 have been linked to congenital neutropenia (CN) and a Schwachman-Diamond-like syndrome [1]. Bellanne ́-Chantelot et al. [1] described 23 subjects with SRP54 mutations who had neutropenia as well as neurodevelopmental delay and exocrine pancreatic deficiency. SRP54 is one of at least 25 known genes implicated in CN, many of which have an increased risk of hematologic malignancies [2]. SRP54-mutated CN can result in lethal infections and patients rely on exogenous granulocyte colony stimulating factor (G-CSF) to limit infections. At least six patients with SRP54 mutations have undergone hematopoietic cell transplant (HCT) [1, 3-5]. None of these patients was transplanted for a hematologic malignancy and there have been no published reports of hematologic malignancies in patients with SRP54 mutations. We describe our experience with the first reported case of leukemia in a patient with SRP54-mutated CN.
2 METHODS
All clinical data were compiled prospectively.
3 RESULTS/DISCUSSION
A 15-year-old male with CN secondary to a heterozygous SRP54 mutation (p.Thr117del) underwent an annual surveillance bone marrow aspirate and was found to have 38% myeloblasts (Figure 1). The patient had no prior history of myelodysplasia or blast populations on surveillance bone marrow studies. He was diagnosed with CN at 2 months of age when he was found to have an absolute neutrophil count (ANC) of 0 × 103 cells/μl and a known maternal history of CN. The genetics of his CN were undefined until a repeat analysis was performed at 15 years of age and showed a pathogenic SRP54 mutation. He began treatment with G-CSF at 2 months of age and responded well (Figure 1A). He was largely managed with a dose of 3 μg/kg/day. He did not have any severe or life-threatening infections and never required intravenous antibiotic therapy but did have recurrent mild infections treated with oral antibiotics (e.g., acute otitis media). He did not have malabsorption or growth failure and his weight ranged from the 34th to 91st percentiles throughout childhood. No dysmorphic features were present. He did require an individualized education program (IEP) at school for a mild neurodevelopmental delay, but no objective measures of cognitive delay were available.
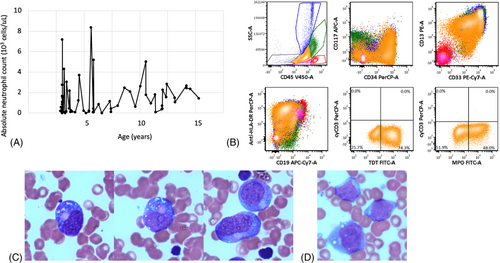
Immunophenotyping of the patient's myeloblasts is described in Figure 1. Fluorescence in situ hybridization (FISH) identified a deletion of 5q31.2 (EGR1) in 68% of cells and an AML-specific FISH panel for RUNX1, KMT2A, and CBFB was negative. Next generation sequencing performed by FoundationOne HEME identified mutations in CSF3R (Q776) and RUNX1 (p113L). No other abnormalities were identified on cytogenetics or FoundationOne sequencing. The patient was diagnosed with acute myeloid leukemia (AML) with myelodysplasia-related changes based on the 2016 World Health Organization myeloid neoplasm classification [6]. His G-CSF was stopped and he began therapy with CPX-351 (liposomal daunorubicin-cytarabine). Deletion of 5q is a poor prognostic finding in AML and this patient's therapy was chosen based on superior outcomes using CPX-351 compared to conventional chemotherapy in patients with AML and myelodysplastic syndrome (MDS)-like changes [7]. He received one cycle of CPX-351 as per the Children's Oncology Group study, AAML1831 consisting of 60 mg/m2 doses on day 1, day 3, and day 5. Overall, he tolerated this regimen well but developed significant gingivitis and periodontitis complicated by a hematoma. He received 7 days of intravenous vancomycin and tranexamic acid-soaked gauze and these complications completely resolved upon count recovery. A repeat bone marrow analysis was performed 35 days after the start of CPX-351 and showed a hypocellular marrow for age (45%–55% cellularity) but no morphologic evidence of leukemia. FISH did not identify the previously seen 5q31.2 deletion and minimal residual disease analysis by Hematologics (Seattle, WA, USA) was also negative for leukemia. The ANC at the time of these studies was 0.81 × 103 cells/μl. He proceeded to HCT after cycle 1 of CPX-351.
The patient received a peripheral blood stem cell (PBSC) graft from a 10/10 matched unrelated male donor after conditioning with busulfan (3.2 mg/kg/dose, four doses) and cyclophosphamide (50 mg/kg/dose, four doses). Graft versus host disease (GvHD) prophylaxis included cyclosporine, mycophenolate mofetil, and abatacept. Platelet and neutrophil engraftment occurred on day +12. Engraftment studies on day +15 showed >98% donor chimerism. Bone marrow studies on day +100 also showed full donor chimerism and no evidence of leukemia or myelodysplasia. He developed steroid-responsive late onset acute GvHD of the skin and liver and chronic oral GvHD 7 months after HCT, but otherwise has not had any significant complications at a follow-up time of 9 months. Table 1 compares this patient's HCT details to other published HCTs in patients with SRP54 mutations.
| Patient 1 | Tamura et al. [3] | Carapito et al. [5] | Bellanne ́-Chantelot et al. [1] | Carden et al. [4] | |
|---|---|---|---|---|---|
| SRP54 mutation(s) | p.Thr117del |
p.Gly225Asp p.Gly274Asp |
Patient 1: pGly226Glu Patient 2: pThr115Ala |
Patient 1: p.Thr117del Patient 2: p.Gly226Glu |
p.Thr117del |
| Other diagnoses | AML with myelodysplastic changes | None |
Patient 1: none Patient 2: none |
Patient 1: none Patient 2: none |
Chromosome 22q11.2 deletion syndrome |
| Age at HCT, sex | 15 years, male | 8 months, female |
Patient 1: 4 years, male Patient 2: 1 year, female |
Patient 1: 1.5 years, male Patient 2: 0.5 years, male |
2 years, male |
| Conditioning regimen | Busulfan, cyclophosphamide | Fludarabine, cyclophosphamide, etoposide, melphalan | Patient 1: not reported |
Patient 1: not reported Patient 2: not reported |
Alemtuzumab, fludarabine, melphalan |
| Donor, match | Unrelated, 10/10 | Unrelated, mismatched |
Patient 1: unrelated, genoidentical Patient 2: unrelated |
Patient 1: not reported Patient 2: not reported |
Sibling, 8/8 |
| Graft | PBSC | Cord |
Patient 1: not reported Patient 2: cord |
Patient 1: not reported Patient 2: not reported |
Cord |
Cell dose Nucleated (cells/kg) CD34+ (cells/kg) |
10.9 x 108 8.9 x 106 |
2 x 108 6.3 x 105 |
Patient 1: not reported Patient 2: not reported |
Patient 1: not reported Patient 2: not reported |
2 x 108 8.8 x 105 |
| GvHD prophylaxis | Cyclosporine, mycophenolate, abatacept | Tacrolimus, methotrexate |
Patient 1: not reported Patient 2: not reported |
Patient 1: not reported Patient 2: not reported |
Cyclosporine, mycophenolate |
| Neutrophil engraftment | Day +12 | Day +19 |
Patient 1: not reported Patient 2: not reported |
Patient 1: not reported Patient 2: not reported |
Day +14 |
| Complications |
Late acute GvHD Chronic GvHD |
None |
Patient 1: none Patient 2: VOD, death |
Patient 1: not reported Patient 2: not reported |
CMV viremia |
| Follow-up time from HCT | 9 months | 14 months |
Patient 1: 2 years Patient 2: death (2 months) |
Patient 1: 9.5 years Patient 2: 1 year |
6 months |
- Abbreviations: AML, acute myeloid leukemia; CMV, cytomegalovirus; GvHD, graft versus host disease; PBSC, peripheral blood stem cells; VOD, veno-occlusive disease.
HCT is commonly used for the treatment of CNs. Indications include G-CSF refractoriness or the development of MDS and/or acute leukemia. SRP54-mutated CN was first described in 2017 [5] and a study of 23 patients with these mutations did not have single case of leukemia or MDS (median follow-up time of 15 years) [1]. The in-frame deletion seen in our patient (p.Thr117del) was the most common mutation in that study [1]. Deep sequencing of 17 patients in that cohort did not identify CSF3R, RUNX1, or TP53 mutations, which have been implicated in leukemogenesis in CNs. Based on these observations, Oyarbide and Corey [8] questioned whether longer follow up would unveil a risk for leukemic transformation, potentially with different cooperating mutations. Our patient developed AML at 15 years of age and tumor sequencing identified CSF3R and RUNX1 mutations. CSF3R mutations occur in up to 80% of CN patients who develop myeloid malignancies and commonly coexist with RUNX1 mutations [9]. Skokowa et al. [9] studied 31 patients with CN and MDS/leukemia and found that 80.5% of patients with RUNX1 mutations had concurrent CSF3R mutations, suggesting that these mutations together contribute to leukemic transformation in CNs. The presence of these leukemogenic mutations in our patient suggests overlap exists between the pathways of malignant transformation in SRP54-mutated CN and more common causes of CN (e.g., ELANE and HAX1 mutations). This is an important observation since SRP54 mutations have not previously been linked to an increased risk of leukemia.
Although conclusions from this single report of AML in SRP54-mutated CN must be limited, this finding has significant implications for the management of patients with SRP54-mutated CN, particularly regarding screening practices and the role of HCT. A long-term follow-up study of patients with SRP54 mutations is needed to further characterize the incidence and biology of leukemia in this patient population.
ACKNOWLEDGMENTS
We would like to thank our patient and his family for consenting to this research and for their commitment to improving outcomes for patients with blood disorders.
AUTHOR CONTRIBUTIONS
Anthony Sabulski wrote the manuscript, collected data, and designed the table. David D. Grier provided pathology expertise and designed the figure. Kasiani C. Myers edited the manuscript and provided bone marrow failure syndrome expertise. Stella M. Davies edited the manuscript, collected data, and provided bone marrow failure syndrome expertise. Jeremy D. Rubinstein designed the study, provided leukemia expertise, and edited the manuscript.
CONFLICT OF INTEREST
The authors have no conflicts or financial interests to disclose.
ETHICS STATEMENT
This study describes the clinical treatment and outcomes of a patient who received care at our center. All ethical standards were complied with during this study. There is no identifying data included in the manuscript. No animal work was done in this study.
FUNDING INFORMATION
No funding was received for this work.
PATIENT CONSENT STATEMENT
The patient consented to the HCT tissue repository at our institution.




