Lymphocytic Variant Hypereosinophilic Syndrome: Case Series From a Tertiary Referral Center in Canada
Funding: Luke Chen's research is funded by a philanthropic gift from the Hsu & Taylor Family through the VGH & UBC Hospital Foundation.
ABSTRACT
Background
Lymphocytic variant hypereosinophilic syndrome (L-HES) is a rare disorder characterized by persistent eosinophilia driven by aberrant T-cell populations. Diagnosis remains challenging due to the lack of standardized diagnostic criteria.
Methods
We retrospectively analyzed 18 patients diagnosed with L-HES between 2016 and 2023. Comprehensive flow cytometry was performed on peripheral blood samples.
Results
Nine patients demonstrated the classic sCD3−CD4+CD5+CD2+CD45RO+CD45RA− immunophenotype, ranging from 0.6% to 70% of total lymphocytes. Two patients showed variant sCD3−CD4+ phenotypes, five had expanded (> 10%) sCD3+CD4+CD7− T-cells, and two displayed aberrant CD8+ T-LGL populations. Clonality was established in all patients with nonclassic phenotypes by molecular TCR testing or based on uniform TRBC1. We assessed a serial gating strategy to quantify the classic L-HES phenotype and found this to be highly sensitive and specific with an estimated limit of detection of 0.06% of lymphocytes. Using this strategy, we identified decreased but detectable abnormal T-cells in all classic phenotype patients reassessed posttreatment, down to as low as 0.3% of lymphocytes. The identification of T-LGL phenotypes with eosinophilia is a novel finding.
Conclusion
Our study highlights the diverse immunophenotypic spectrum of L-HES, emphasizing the importance of comprehensive flow cytometry analysis for accurate diagnosis.
1 Introduction
Hypereosinophilic syndrome (HES) is characterized by persistent peripheral blood eosinophilia associated with end organ damage (>1.5 × 109 /L) [1-3]. Lymphocytic hypereosinophilic syndrome (L-HES) is a rare subtype of HES characterized by an aberrant T-cell population with gene rearrangements which lead to the overproduction of Th2 cytokines, most commonly Interleukin (IL)-4, IL-5, and IL-13 [4, 5]. In L-HES, the eosinophils are not clonal, but are reactive to eosinopoietic cytokines and chemokines produced by these clonal T-cells.
L-HES presents with a wide range of clinical manifestations, reflecting the involvement of multiple organ systems. These include cutaneous, soft tissue, rheumatological, gastrointestinal, and respiratory symptoms, all of which accompany end organ damage [6]. L-HES is difficult to distinguish from other causes of reactive eosinophilia including vasculitides, autoimmune disease, medications, parasitic infections, and IgG4-related disease [7, 8]. Accurate diagnosis is important for therapy, as patients with L-HES are less responsive to steroids, but more responsive to interferon alpha [9, 10]. JAK inhibitors and IL-5 inhibition are also emerging therapies [11, 12].
There are currently no standardized protocols or criteria for determining what constitutes an L-HES defining aberrancy. The general approach in most centers is to identify and quantify T-cell populations with abnormal marker expression patterns. sCD3−CD4+ T-cells are the most common/specific abnormal T-cell population described in the literature for L-HES [13-15]. A recent study by Carpentier et al. reported that sCD3−CD4+ T-cells in L-HES had other consistent immunophentopypic features, namely bright CD5, bright CD2, and homogeneous CD45RO and CD95 [16, 17]. Other aberrant T-cell immunophenotypes that have been reported in the literature for L-HES include sCD3+CD4+CD7− and sCD3+CD4−CD8− TCRαβ+; however, the sensitivities and specificities of these populations with respect to L-HES is not well established [4, 16, 18]. Notably, these two immunophenotypes are seen as minor T-cell populations in a variety of reactive states where they are often not clonal. It is therefore critical to demonstrate clonality, for example, based on molecular TCR studies or TRBC1 expression [19]. However, even then careful correlation with other clinical and laboratory findings is required [20, 21].
Here we report on a new cohort of 18 patients diagnosed with L-HES at our center. We found half of our patients have a sCD3−CD4+ T-cell population with a similar immunophenotype compared to what has recently been described in the literature. We show that this specific aberrant T-cell population can persist and be detected at low levels after treatment. Importantly we also report on patients without classic sCD3−CD4+ T-cells, but who have other features that support a diagnosis of L-HES. Comparisons between the two groups based on detailed clinical and laboratory characterization are provided, expanding the knowledge base of this rare and diagnostically challenging disease.
2 Methods
At our tertiary referral center, we retrospectively analyzed 18 patients with persistent unexplained eosinophilia who were diagnosed with L-HES between 2016 and 2023. As a control, we also analyzed peripheral blood from patients without eosinophilia found to be negative for a lymphoproliferative disorder by flow cytometry run for indications other than eosinophilia (n = 19). The study was approved by the University of British Columbia Clinical Research Ethics Board (H23-03816) as a minimal risk study and did not require patient consent. All patient data were de-identified and unique patient numbers generated for data analysis. All patients were referred to hematology with clinical symptoms suspicious for L-HES, as well as evaluation to exclude reactive and other causes of eosinophilia. Clinical and laboratory data were reviewed.
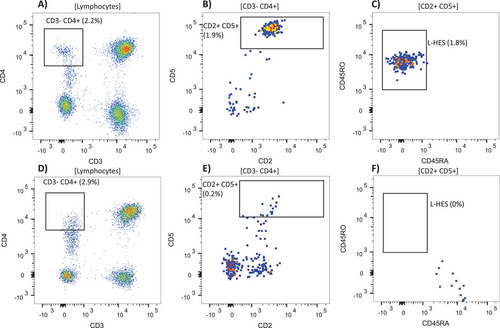
Flow cytometry was performed on peripheral blood as part of routine clinical evaluation. Lymphocyte populations were analyzed using a three-tube diagnostic panel, with one 13-color tube dedicated to T-cell and NK-cell-associated antibodies: CD2, sCD3, CD4, CD5, CD7, CD8, CD14, CD45, CD45RA, CD45RO, CD56, CD57, and CD279. Samples run after 2021 had an additional color for the marker TRBC1. The remaining two tubes focused on evaluation of B-cell populations; the data are not presented here as the objective of the current study is to quantitate abnormal T cell populations. Data were acquired on a BD FACS Canto II or BD LSRFortessa flow cytometer (Becton Dickinson; San Jose, California), and FCS data files were initially analyzed using BD FACSDiva, Kaluza, FlowJo v10, or a custom multidimensional clinical analysis software. Total lymphocytes were gated on using CD45 versus side scatter followed by a CD14 gate to exclude monocytes and sCD3−CD4+, sCD3+CD4+CD7−, and sCD3+CD4−CD8− subsets were initially characterized and quantified as part of routine clinical workup. A serial gating strategy to quantify the specific sCD3−CD4+CD5+CD2+CD45RO+CD45RA− L-HES phenotype was developed and retrospectively applied to L-HES patients at multiple time points in order to trend the clone size (minimum cluster size of 10 events to be considered positive, see Figure 1). The same gating strategy was applied to normal controls to assess limit of detection, which was also assessed for other selected phenotypes. The median fluorescence intensities (MFI) for selected markers were compared between the aberrant sCD3−CD4+ populations and normal sCD3+CD4+ T-cells from the same samples (22 samples from seven patients with raw data available for analysis).
The presence of a T-cell receptor (TCR) clonal rearrangement was determined using the BIOMED-2 guideline [22].
3 Results
Clinical and laboratory parameters for the 18 L-HES patients in our cohort are shown in Table 1. The cohort consisted of 11 males and 7 females, with ages at diagnosis ranging from 22 to 91 years. Presenting symptoms varied widely among patients, with the most common being skin-related issues such as pruritus, eczema, and rash. Notably, three patients were asymptomatic at the time of diagnosis. Initial peripheral blood eosinophil counts ranged from 1.7 to 88 × 109/L, with the majority of patients having counts below 5 × 109/L. Initial lymphocyte counts were generally within the normal range (0.4-4.6 × 109/L), with only two patients showing elevated levels. C-reactive protein (CRP) levels varied widely (from < 0.2 to 75.6 mg/L), with several patients exhibiting elevated levels indicative of ongoing inflammation. Serum protein electrophoresis (SPEP) results were predominantly normal, though some patients displayed polyclonal increases in the gamma region, and one patient showed a monoclonal band consistent with MGUS. Notably, immunoglobulin E (IgE) levels ranged from 26 to 45,900 IU/mL, with many patients demonstrating significantly elevated levels. Treatment strategies varied among patients, with most receiving prednisone, pegylated interferon, or a combination of both. Some patients required alternative or additional therapies such as hydroxyurea or imatinib, while a few remained untreated. Only one patient did not respond to treatment (L4).
| Study ID | Gender | Age at diagnosis (years) | Presenting symptom |
Eosinophil (giga/L) |
Flow | TCR PCR Clonality | Treatments received |
Lymph (giga/L) |
CRP (mg/L) |
SPEP | IgE (µg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | M | 38 | Mild pruritis | 2.3 | Classic | T-cell clonal | None | 1.6 | 0.7 | Normal | 449 |
| L2 | F | 36 | Eczema and cervical lymph-adenopathy | 2.2 | Classic | T-cell clonal | Prednisone followed by PEG INF | 2.3 | < 0.2 | Polyclonal increase in gamma region | 305 |
| L3 | F | 67 | Papular eczema | 3.3 | Classic | T-cell clonal | Prednisone | 1.4 | 1 | 0.3 g/l IgG lambda MGUS | 5300 |
| L4 | F | 48 | Ischemic stroke and fatigue | 1.8 | Classic | Negative | no response to prednisone or PEG INF | 1.1 | 0.5 | Normal | 620 |
| L5 | M | 54 | Urticaria | 88 | Classic | T-cell query clonal | Prednisone followed by PEG INF; Hydroxyurea | 0.95 | 5.8 | Normal | 197 |
| L6 | M | 56 | No symptoms | 25 | Classic | T-cell clonal | Prednisone followed by PEG INF | 2.1 | 6.4 | Normal | 81 |
| L7 | M | 22 | Episodic angioedema | 28.8 | Classic | T cell clonal | PEG INF | 1.3 | < 0.3 | Normal | 26 |
| L8 | F | 62 | Prurigo nodularis | 10 | Classic | T-cell clonal | PEG INF | 4.6 | 8.7 | Normal | 5532 |
| L9 | F | 30 | Pneumonitis | 1.7 | Classic | T-cell clonal | Prednisone | 1.9 | < 0.3 | Normal | 45,900 |
| L10 | M | 60 | Urtacaria rash | 2 | Variant classic | T cell query clonal | Prednisone followed by PEG INF; Imatinib | 1.1 | 0.6 | Normal | 5153 |
| L11 | M | 90 | Puritic rash | 3.8 | Variant classic | T cell clonal | Prednisone | 1.1 | 22 | Polyclonal increase in gamma region | 40,030 |
| L12 | M | 59 | Severe chronic eczema | 2.00 | Non classic | T-cell clonal | Prednisone | 0.9 | 3.9 | Normal | 6978 |
| L13 | M | 91 | Extensive erythroderma, progressive renal insufficiency | 2.2 | Non classic | T-cell clonal | PEG INF | 0.5 | 15.6 | Normal | 978 |
| L14 | F | 57 | Eosinophilic myocarditis | 10.2 | Non classic | T cell clonal | Prednisone followed by PEG INF | 2.2 | 24.6 | Borderline polyclonal increase in gamma region | 128 |
| L15 | F | 81 | No symptoms | 2.4 | Non classic | T-cell clonal | None | 0.4 | 75.6 | Irregular gamma region with inflammatory changes | 1367 |
| L16 | M | 70 | Pulmonary eosinophilia | 2.6 | Non classic | T-cell query clonal | Prednisone | 1 | 0.6 | Normal | 952 |
| L17 | M | 73 | No symptoms | 1.9 | T-LGL | T-cell clonal | Prednisone followed by PEG INF | 3.3 | 1.5 | Normal | 61 |
| L18 | M | 52 | Drenching night sweats | 16.9 | T-LGL | Inconclusive | Prednisone | 2.8 | 7.8 | Polyclonal increase in gamma region | 54 |
- Note: Bolded values are above the reference range; italicized values are below the reference range.
- Abbreviation: PEG INF: pegalyated interferon.
There were nine patients with an aberrant sCD3−CD4+CD5+ (typically bright) CD2+CD45RO+CD45RA− population, which we refer to as the classic phenotype, ranging from 0.6% to 70% of total lymphocytes at diagnosis (Figure 2A–C and Table 2). In addition, there were two patients with variant sCD3−CD4+ immunophenotypes showing dim to negative CD5 and dim or variable CD45RO expression (both ≥ 5% of lymphocytes), five patients with > 10% sCD3+CD4+CD7− T-cells, and two patients with aberrant CD8+ T-LGL populations with restricted TRBC1 (Table 2). There was corroborating evidence of T-cell clonality by PCR in all but two patients. One had the classic sCD3−CD4+ phenotype (L4) and one with a T-LGL clone (L18). L18 had inconclusive clonality by PCR but showed homogenous TRBC1 expression by flow cytometry. The size of the T-cell clone did not show significant correlation with the eosinophil count at initial diagnosis (Pearson r = −0.17; p = 0.5).
| Study ID | Flow phenotype | Abnormal population immunophenotype | Clone size (% of lymphocytes) | TCR PCR Clonality |
|---|---|---|---|---|
| L1 | Classic | sCD3-CD4+CD5+CD2+CD7-CD45RO+ | 1.8% | T-cell clonal |
| L2 | Classic | sCD3-CD4+CD5+CD2+CD7-CD45RO+ | 58.0% | T-cell clonal |
| L3 | Classic | sCD3-CD4+CD5+CD2+CD7-CD45RO+ | 34.0% | T-cell clonal |
| L4 | Classic | sCD3-CD4+CD5+CD2+CD7-CD45RO+ | 10.0% | non clonal |
| L5 | Classic | sCD3-CD4+CD5+CD2+CD7-CD45RO+ | 7.2% | T-cell query clonal |
| L6 | Classic | sCD3-CD4+CD5+CD2+CD7-CD45RO+ | 15.4% | T-cell clonal |
| L7 | Classic | sCD3-CD4+CD5+CD2+CD7-CD45RO+ | 0.6% | T-cell clonal |
| L8 | Classic | sCD3-CD4+CD5+CD2+CD7-CD45RO+ | 70.0% | T-cell clonal |
| L9 | Classic | sCD3-CD4+CD5+CD2+CD7-CD45RO+ | 1.2% | T-cell clonal |
| L10 | Variant classic |
sCD3-CD4+CD5dimCD2-CD7- TRBC1- heterogeneous CD45RA/CD45RO expression |
9.4% | T cell query clonal |
| L11 | Variant classic | sCD3-CD4+CD5-CD2+CD7-CD45RO+ | 5.0% | T cell clonal |
| L12 | Nonclassic | sCD3+CD4+CD7- | 11.4% | T-cell clonal |
| L13 | Nonclassic | sCD3+CD4+CD7- | 25.7% | T-cell clonal |
| L14 | Nonclassic | sCD3+CD4+CD7- | 14.4% | T cell clonal |
| L15 | Nonclassic | sCD3+CD4+CD7- | 13.5% | T-cell clonal |
| L16 | Nonclassic | sCD3+CD4+CD7- | 10.8% | T-cell query clonal |
| L17 | T-LGL | CD8+CD5-CD56+CD57+ uniform TRBC1+ | 22.0% | T-cell clonal |
| L18 | T-LGL | CD8+CD5-CD7-CD56+CD57+ uniform TRBC1+ | 4.8% | inconclusive |
Comparing the nine patients with the classic L-HES phenotype to those with the nine patients with other T-cell phenotypes. The difference in age between the two groups is statistically significant (p < 0.05). Patients with the other phenotype tended to be older than those with the classic phenotype: the mean age for patients with the classic phenotype is 47.9 years versus 70.3 years for patients with other phenotypes. Comparing the initial peripheral blood eosinophil count using the Mann–Whitney U test, there was no significant difference in eosinophil counts between the two groups. There was no difference in treatment response between the classic and other phenotype groups nor differences in the presence of autoimmune comorbidities between the two groups.
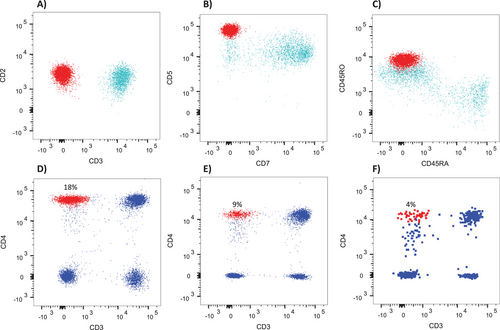
Based on 22 samples measured at various time points from patients with the classic sCD3−CD4+ phenotype, the CD5 MFI was on average 3.4× brighter compared to sCD3+CD4+ T-cells from the same samples (95% CI = 3.0 to 3.7), CD2 was on average 1.3× brighter (95% CI 1.3 to 1.4), while CD4 was similar between the sCD3− and sCD3+ subsets (mean 1.1, 95% CI 1.0 to 1.1). In addition, CD7 was completely or partially negative in all patients with the classic phenotype. In all samples collected after initial diagnosis (14 samples from 6 patients), a persistent population with the same aberrant phenotype was detectable, often at low levels (< 1% in six samples with 0.3% being the lowest level detected) using a standardized gating strategy (Figure 1). Five of these patients had repeat flow cytometry at a time point after receiving treatment, which showed persistence of aberrant T-cells with the same phenotype present at diagnosis at decreased or stable levels in all cases (Figure 2D–F), coinciding with decreases in the eosinophil count (Table 3). As an example, patient L5 showed a decrease in classic sCD3−CD4+ T-cells from 7.2% to 0.3%, with a concurrent decrease in eosinophils from 88 × 109 to 0.1 × 109/L. To further validate the significance of such a low level of aberrant T-cells with the classic phenotype, we looked at 19 normal control blood samples run with the same panel/serial gating strategy. This gave an average of 0.014% of lymphocytes residing within the final gate (SD = 0.014%, theoretical limit of detection = 0.06%), with none of the control samples showing a cluster of ≥ 10 events with the classic L-HES phenotype. In addition, no clusters of ≥ 10 events with the classic phenotype were found in patients identified as having nonclassic phenotypes (10 samples from 8 patients with data available for re-analysis). By contrast, the average percent in the control samples of total sCD3−CD4+ lymphocytes was 1.1% (SD = 0.9%) and sCD3+CD4+CD7+ lymphocytes was 3.5% (SD = 1.9%).
| Study ID | Treatment | Pretreatment CD3-CD4+ % of total lymph | Posttreatment CD3-CD4+ % of total lymph | Pretreatment eosinphil count | Posttreatment eosinophil count | T-cell clonality |
|---|---|---|---|---|---|---|
| L2 | PrednisonePEG INF | 58 | 1.8 | 2.2 | 0 | Clonal |
| L5 | Prednisone PEG INF, hydroxyurea | 7.2 | 0.3 | 88 | 0.1 | Query clonal |
| L6 | Prednisone PEG INF | 18 | 4.1 | 25 | 1.2 | Clonal |
| L7 | PEG INF | 0.6 | 0.6 | 28.8 | 0.3 | Clonal |
| L8 | PrednisonePEG INF | 70 | 12 | 10 | 0.3 | Clonal |
- Abbreviation: PEG INF: pegalyated interferon.
4 Discussion
Our study highlights the critical role of flow cytometry in the diagnosis of lymphocytic variant hypereosinophilic syndrome (L-HES) yet shows these patients may display a range of immunophenotypes. The classic immunophenotype of sCD3−CD4+CD5+(bright)CD2+CD45RO+CD45RA− emerged as the most common and specific marker for L-HES, consistent with previous findings in the literature [15–18, 23, 24]. We found similar aberrancies as described previously, including bright CD5 and CD2, alongside loss of CD7 [4, 16, 18]. This distinct phenotype was not observed in any of our control samples, highlighting its specificity for L-HES.
In our cohort, half of the patients displayed this classical immunophenotype. We detected populations as small as 0.6% of total lymphocytes at initial diagnosis, which still proved to be clinically relevant. Interestingly, these abnormal populations persisted, albeit for most patients at decreased levels, even after treatment. Given the high specificity of this classic phenotype, we propose that even small populations of cells with this immunophenotype should be considered significant. This underscores the importance of acquiring sufficient events and using appropriate sequential gating strategies to detect these often small abnormal populations with a minimal marker combination including CD45, sCD3, CD4, CD2, CD5, CD45RO, and CD45RA (CD95 has been reported as another marker expressed in classic L-HES but was not included in our panel). For laboratories without dedicated L-HES panels, a serial gating strategy such as the one presented here could be incorporated into existing T-cell panels to ensure these populations are not overlooked. A standalone panel design could also be considered, which may not require as many fluorochromes as a comprehensive lymphoma diagnosis panel. One patient in our cohort with the classic phenotype had negative clonality studies (L4). Interestingly, patient L4 was the only patient in our cohort to not respond to treatment.
While the classic phenotype demonstrated high specificity, our study also identified nonclassic abnormal populations, with sCD3+CD4+CD7− T-cells being the most prevalent. However, these cells are not exclusive to pathological conditions; they can be found in healthy individuals and their frequency tends to increase with age. Moreover, elevated levels of these cells have been observed in a variety of noneosinophilic disorders, particularly those involving chronic inflammation or autoimmune processes. Interestingly, there was no significant difference between autoimmune comorbidities when comparing the classic phenotype group to the nonclassic group, however, there was a notable difference in age with the latter group being significantly older.
Distinguishing between a normal variant and a truly abnormal population of sCD3+CD4+CD7− T-cells often requires additional context and supporting evidence. In our cohort, we had corroborating evidence that expanded sCD3+CD4+CD7− T-cells were significant based on positive molecular clonality studies and the lack of detectable T-cells with the classic sCD3−CD4+ phenotype using a sensitive flow cytometry assay. Based on our findings and the inherent variability of this cell population, we propose a threshold of ∼10% of total lymphocytes for considering a sCD3+CD4+CD7− population as potentially significant in the L-HES context, although this should likely be validated individual institutions since there could be variability due to patient population differences and technical factors. Incorporating new markers such as TRBC1/TRBC2 to directly assess the clonality of sCD3+CD4+CD7− T-cell populations identified by flow cytometry could potentially improve specificity, but this requires further study, as the cases we had in our cohort preceded having TRBC1 in our flow cytometry panel. At this time, we strongly emphasize that this phenotype should never be interpreted in isolation: clinicians should always consider it in conjunction with the patient's clinical presentation, other laboratory findings, and molecular evidence of T-cell clonality.
A novel finding in our study was the identification of patients with aberrant T-large granular lymphocyte (T-LGL) populations. Specifically, we observed T-LGL phenotypes in two patients who presented with clinical features of L-HES. This observation warrants careful consideration in the context of L-HES diagnosis and management. T-LGL leukemia is typically characterized by the clonal expansion of cytotoxic T cells with a sCD3+CD8+CD57+ phenotype, although CD4+ variants have been reported. The co-occurrence of T-LGL phenotypes with hypereosinophilia presents a diagnostic challenge, as it blurs the lines between distinct hematological entities. Among our two patients, L17's bone marrow showed mild eosinophilia and normal trilineage hematopoiesis. He received first line oral cortical steroids. Clinically, when he was weaned from his steroids, he had rebound eosinophilia and suspected end organ damage in the form of cardiomyopathy. He was subsequently restarted on steroids, followed by second line interferon therapy and responded well. L18's bone marrow showed marked eosinophilia with focal fibrosis with no evidence of KIT mutation. L18 only received first line oral cortical steroid therapy and responded well. Both patients had normal karyotypes and negative FISH for PDGFRA, PDGFRB, FGFR1, and JAK2. Neither patient had intrasinusoidal infiltrates and their clones persisted over time without lymphadenopathy. Future studies should investigate the functional role of T-LGL in hypereosinophilia, particularly their potential cytokine-driven contribution, as seen in sCD3−CD4+ L-HES.
Our findings both support and extend previous research on L-HES. Similar to the studies by Lefevre et al. and Carpentier et al., we observed the classic sCD3−CD4+CD5+CD2+CD45RO+CD45RA− immunophenotype in a significant proportion of L-HES patients [15–17, 23]. We demonstrated that this phenotype can be reliably detected at very low levels in L-HES patients due to its high specificity. Uniquely, our study examined flow cytometry results for patients with L-HES posttreatment, revealing the persistence of aberrant T-cell populations at decreased or stable levels. This aspect, not addressed in previous studies, provides valuable insights into the durability of these cell populations. Future work could examine whether continued trending of these small residual populations could have clinical value as a form of measurable residual disease testing. Our case series also contained other phenotypes reported in literature for L-HES, including increased sCD3+CD4+CD7− T-cells in patients without an identifiable classic L-HES population. Notably, our identification of T-LGL phenotypes in some L-HES patients represents a novel finding not reported in either previous study. Overall, our study provides a comprehensive perspective on L-HES, highlighting the importance of thorough flow cytometry analysis both at diagnosis and during follow-up. Our findings suggest that the immunophenotypic landscape of L-HES may be more diverse and persistent than previously recognized. We propose that incorporation of targeted gating strategies or dedicated panels for diagnosis of L-HES could improve detection rates and disease monitoring in clinical practice.
Acknowledgments
We gratefully acknowledge Dr. Florence Roufosse for her helpful comments and insights for this manuscript. Luke Chen's research is supported by a philanthropic gift from the Hsu & Taylor family through the VGH & UBC Hospital Foundation.
Conflicts of Interest
L.Y.C. Chen has received consulting fees from Recordati Rare Diseases and from Glaxo-Smith-Kline. None of the other authors have any declarations.
Open Research
Data Availability Statement
Data are available upon reasonable request to the corresponding author.