Erythrocytapheresis as a strategy to manage anemia and iron overload in nondeletional hemoglobin H disease
Abstract
Hemoglobin H (HbH) disease is associated with anemia, ineffective erythropoiesis, and iron overload. We report a case of a patient with HbH/Hb Constant Spring disease, who was maintained on chronic transfusions as an adult due to symptomatic anemia. Over time, he developed iron overload and was started on chelation therapy but did not have an adequate response to chelation. We then added erythrocytapheresis to chelation therapy and were able to successfully decrease his iron burden while managing his anemia. Therapeutic erythrocytapheresis may be an effective treatment strategy for iron overload in HbH disease that is refractory to chelation.
1 INTRODUCTION
Hemoglobin H (HbH) disease is a form of alpha thalassemia caused by deletional or nondeletional mutations in three of the four alpha globin genes, which results in an imbalance in globin chains and subsequent hemolysis and extramedullary hematopoiesis [1]. HbH/Hemoglobin Constant Spring (HbH/HbCS) is the most common nondeletional alpha thalassemia in Asia and is increasing in prevalence in North America as a result of immigration [1]. This variant is characterized by moderate to severe hemolytic anemia, ineffective erythropoiesis and iron overload. Patients with HbH/HbCS have symptoms at a younger age, higher severity of hemolysis, and are more likely to require transfusions [2].
Iron overload is a commonly encountered complication in HbH/HbCS disease, particularly among adults, and can occur for a variety of reasons. While chronic or intermittent red blood cell (RBC) transfusions can cause iron overload in this population, iron overload can occur even in the absence of transfusions, possibly due to increased absorption of iron or due to iron release into the bloodstream due to hemolysis and RBC turnover [2, 3]. Iron overload can be particularly challenging to manage in adults with thalassemia, many of whom require transfusion throughout their lives. Iron chelation therapy has been studied in transfusion-dependent patients with iron overload [4] and also used in patients with thalassemia not receiving routine transfusions. However, adherence to therapy can be difficult due to gastrointestinal discomfort, liver and kidney toxicity, and high cost associated with chelation agents [5].
We report the case of an individual with HbH/HbCS disease requiring chronic transfusions for worsening symptomatic anemia with severe iron overload refractory to both oral and parenteral chelation strategies, successfully managed with erythrocytapheresis.
2 CASE PRESENTATION
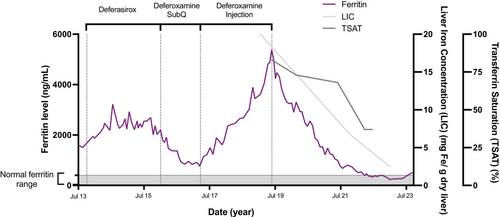
A 36-year-old male of Laotian origin was diagnosed with HbH/HbCS disease at the age of 2. He received red cell transfusions approximately once annually since around 6 months of age and intermittently throughout early childhood and adolescence. His family history was notable for a sibling with HbH/HbCS disease who died at age 39 from iron overload-related complications. At age 23, the patient was also diagnosed with human immunodeficiency virus (HIV) infection (not acquired from transfusions), and he developed pancytopenia, hepatosplenomegaly, and became symptomatic from anemia with profound fatigue. Bone marrow biopsy revealed profound erythroid hyperplasia and iron deposition. He started treatment with antiretroviral therapy and started simple transfusion therapy for symptomatic anemia. His transfusion regimen included 2–3 units of packed red blood cell (PRBC) every 3 weeks, and his pretransfusion hemoglobin (Hb) was maintained in the 9–10.5 g/dL range (reference 13.7–17.5 g/dL). Over time, he developed iron overload and his serum ferritin level started to rise (Figure 1) and transferrin saturation was elevated at 96%. We consider other causes for increased ferritin levels, including acute and chronic inflammation and steatohepatitis, but his presentation appeared to be consistent with iron overload given his transfusion burden. He was started on chelation therapy with deferasirox and gradually titrated to a maximum dose of 40 mg/kg per day. He reported difficulty tolerating the medication due to gastrointestinal side effects including nausea, abdominal cramping and diarrhea. Despite his reported compliance, his ferritin levels gradually increased from 1507 to 2201 ng/mL (reference 30–400 ng/mL). He was then started on subcutaneous deferoxamine, which he initially tolerated well and was effective in decreasing his ferritin levels down to 761 ng/mL, but he developed cellulitis of the abdominal wall after misplacement of the needle. Following this, he elected to discontinue subcutaneous deferoxamine and received post-transfusion deferoxamine for iron chelation. This strategy was not effective, and his ferritin continued to rise after deferoxamine was discontinued, from 761 ng/mL in March 2017 to 3894 ng/mL November 2018. At this time, he started film-coated deferasirox, and his dose was increased to 21 mg/kg, which he tolerated well and without side effects. However, ferritin levels persistently increased to 5381 ng/mL in June 2019. Magnetic resonance imaging (MRI) showed diffuse reticuloendothelial iron deposition in liver, spleen, and bone marrow, with estimated liver iron concentration (LIC) measuring over 20 mg Fe/g dry liver (number was too high to be quantified; Figure 1). Given significant iron burden, we considered adding deferiprone to his chelation plan but withheld it given risk of worsening of baseline neutropenia due to HIV and patient concerns regarding difficulty in adhering to a three times daily dosing regimen. We discussed a brief trial of erythrocytapheresis as an alternative strategy to maintain Hb level and decrease iron burden with our apheresis team. He started on his first erythrocytapheresis in June 2019 with use of Optia Spectra Apheresis System. Bilateral arm-vein needles were used for access, and exchange using 4 units of antigen-matched PRBCs was performed. Anticoagulation was ACD-A at 13:1 (whole blood:ACD-A) and calcium gluconate (2 g in 120 mL normal saline) given via the return line at 100 mL/h. Preapheresis Hb was maintained at 10 g/dL on average, target postapheresis hematocrit (Hct, reference 40%–50%) was at least 33% or preprocedure Hct, whichever was higher. After three treatments of exchange every 4 weeks, we attempted deplete/exchange mode, reducing the Hct initially by three percentage points below preprocedure Hct prior to beginning the exchange portion of the procedure with the goal of more efficient iron removal, which he tolerated well. He continued deferasirox (film-coated) tablets throughout this time. Ferritin levels started to decrease after five treatments, and over time his depletion phase was increased to a maximum of five percentage points below starting Hct, and ferritin continued to decrease to less than 500 mg/mL at the 35th treatment and his chelation was discontinued. Iron saturation was 96% at the start of chelation therapy and 83% at the start of erythrocytapheresis; it decreased to 57% at the time of chelation discontinuation. Serial lipid panels and erythrocyte sedimentation rates remained within normal limits. Repeat liver MRI of the abdomen obtained in 2022 and 2023 showed a reduction in LIC to an estimated 2.5 mg Fe/g dry liver (Figure 1). The patient has experienced no complications from the apheresis procedures; all procedures were performed via peripheral vein access, and he continues a regimen of every 4-week RBC exchange with 4 units of PRBCs with intermittent deplete–exchange (vs. exchange only) to maintain ferritin below 500 ng/mL.
3 DISCUSSION
Iron overload is a well-recognized life-threatening complication of long-term transfusion therapy in thalassemia patients. Excess iron accumulation can affect multiple organs including the liver, heart, and endocrine system [6]. Iron chelation therapy is used to remove excess iron and to reverse-related complications [7, 8], however, it can be difficult to tolerate due to side effects, with studies showing variable compliance, making management of iron overload challenging [5, 9].
Erythrocytapheresis has been shown to be safe and efficient in managing anemia and stabilizing serum ferritin level in sickle cell disease (SCD) patients [10], and there have been attempts to introduce it into other hemoglobinopathies and hemolytic anemias including thalassemia as a strategy to manage iron overload [11]. However, studies to support erythrocytapheresis in thalassemia patients are anecdotal and show mixed results. A study from Wall and Bolster showed no benefits of erythrocytapheresis with or without chelation therapy in seven adult beta thalassemia major patients did not show any benefit in iron reduction and patients were observed to have increased extramedullary hematopoiesis, syncope, and antibody formation [11]. On the other hand, a recent study from Van Hattem et al. showed successful reduction of serum ferritin using erythrocytapheresis combined with chelation therapy in three pediatric patients with beta thalassemia major [12]. Erythrocytapheresis has been used as a successful strategy in hereditary hemochromatosis to decrease iron overload [13].
The role of erythrocytapheresis in alpha thalassemia syndromes, specifically, HbH disease is not known. In our case, we were able to manage both the anemia and iron overload successfully with this modality. A possible reason for the success of this approach could be that the preapheresis Hb level was able to be maintained at 9–10.5 g/dL. High initial Hb level would theoretically make it easier to achieve a negative net RBC load, thus enabling net negative iron balance. In addition, over time, we were able to bring the target Hct (postprocedure Hct) below pretreatment Hct levels, and this also likely helped achieve a negative iron balance.
Additional advantages of using erythrocytapheresis over standard PRBC transfusions include shorter treatment duration when compared to straight PRBC transfusions and more streamlined transfusion protocols using the same number of cumulative PRBC units annually. Potential disadvantages to this approach include the need for additional trained staff, resources, and equipment.
In conclusion, we were able to successfully manage both anemia and iron overload in a patient with nondeletional HbH disease using therapeutic erythrocytapheresis. This may be an effective treatment strategy for this thalassemia population where there is a significant iron overload burden.
AUTHOR CONTRIBUTIONS
Ke Zhang reviewed patient data and wrote first draft of the manuscript, John Bliamptis reviewed patient data, reviewed and wrote the manuscript, Janice Park provided oversight for erythrocytapheresis and reviewed the manuscript draft, Amber P. Sanchez conceptualized apheresis plan, provided oversight for erythrocytapheresis, and reviewed the manuscript draft, Patricia Kopko provided transfusion medicine support and reviewed the manuscript, Srila Gopal conceptualized the apheresis plan, managed thalassemia and iron chelation, reviewed the manuscript, and obtained informed consent from the patient for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING INFORMATION
There was no specific funding or grants associated with this work.
ETHICS STATEMENT
The authors have confirmed ethical approval statement is not needed for this submission.
PATIENT CONSENT STATEMENT
The patient provided verbal and written consent to have their case published.
CLINICAL TRIAL REGISTRATION
The authors have confirmed clinical trial registration is not needed for this submission.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.