Changes in indicators of cerebral metabolic stress following treatment with voxelotor in children and adolescents with sickle cell anemia
Jeremie Estepp is currently employed by Agios Pharmaceuticals, Cambridge, MA. Robert Ogg is currently employed by HII, McLean, VA.
Abstract
Voxelotor is a small molecule that reduces the polymerization of sickle hemoglobin by increasing its affinity for oxygen. In patients with sickle cell anemia, it has been postulated that increasing hemoglobin-oxygen affinity could limit oxygen offloading from hemoglobin, causing an increase in cerebral metabolic stress. To investigate this hypothetical concern, we used multimodal brain imaging to define the effects of voxelotor on cerebral blood flow and oxygen extraction. We followed four patients for 2–5 months during and/or after voxelotor therapy. This study showed no observable increase in cerebral blood flow or oxygen extraction fraction during treatment.
1 INTRODUCTION
Sickle cell anemia (SCA) is an inherited hematological disorder caused by a glutamic acid to valine substitution in beta-globin which results in sickling of red cells. Repetitive ischemic injury and inflammation caused by anemia and hemolysis result in progressive end-organ damage, including the brain. Patients with SCA are at risk of stroke and silent cerebral infarcts (SCI) [1], which are associated with significant cognitive decline [2].
Brain infarcts occur in SCA due to increased cerebral metabolic stress [3]. Both cerebral blood flow (CBF) and oxygen extraction fraction (OEF) are elevated in SCA due to reduced arterial oxygen content (CaO2) [3]. CBF and CaO2 encompass cerebral oxygen delivery, and OEF quantifies the transfer of available oxygen supply into the brain tissue. Ischemia occurs when the cerebral metabolic rate of oxygen utilization does not meet metabolic demands.
Current treatment options for SCA remain limited, with hydroxyurea being the primary disease-modifying therapy. Chronic red blood cell transfusions are effective for primary stroke prevention in the setting of elevated transcranial Doppler velocities and for secondary stroke prevention [4]. Hydroxyurea may also reduce stroke risk [5]. Chronic transfusions reduce global CBF and OEF [6], and hydroxyurea reduces whole-brain OEF [7]. Allogeneic hematopoietic stem cell transplantation and gene therapy have emerged as potentially curative options for SCA and are known to reduce CBF and OEF to levels consistent with same-age peers without SCA [8]. However, stem cell transplantation and gene therapy are accessible only to a small number of patients with severe disease complications.
In 2019, Oxbryta (voxelotor), was approved for the treatment of SCA patients ≥ 12 years of age and use was expanded to patients 4 to < 12 years of age in 2022. Voxelotor is a first-in-class, small molecule that reduces the polymerization of sickle hemoglobin by increasing its affinity for oxygen [9]. This results in decreased hemolysis and an increase in hemoglobin. However, it has been postulated that increasing hemoglobin-oxygen affinity could limit oxygen offloading from hemoglobin, thus impairing tissue oxygenation [10]. There have been safety concerns that decreased oxygen delivery to the brain, may increase stroke risk [11]. To date, no adverse neurologic events have been reported from clinical trials [12]. A recent study using optical spectroscopies suggested that OEF and CBF decreased with voxelotor [13]. To further examine the potential effects of voxelotor on cerebral metabolic stress, we used multimodal brain imaging to define the effects of voxelotor therapy on grey matter CBF and OEF in children and adolescents with SCA.
2 METHODS
Data were collected during a clinical study to evaluate the use of voxelotor in children and adolescents (NCT02850406, HOPE Kids). St. Jude enrolled 16 participants in the HOPE Kids trial, and four of those patients received multimodal neuroimaging as part of a separate study. Study activities were IRB-approved, and all participants provided informed consent. Four children with SCA underwent multimodal brain imaging on a 3T Siemens magnetic resonance imaging (MRI) scanner utilizing a multi-channel head coil. Conventional anatomical MRI included 3D sagittal T1, T2, and 2D axial fluid attenuation inversion recovery (FLAIR) T2 images of the whole brain. Susceptibility-weighted imaging (SWI) and quantitative susceptibility mapping were obtained from the magnitude and phase images of a 3D SWI sequence and were used to estimate OEF. Arterial spin labeling MRI was used to obtain perfusion-weighted images and to calculate resting state CBF.
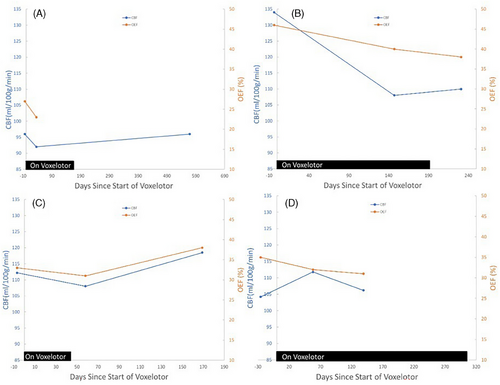
All MRI data was collected between May 2017 and June 2019. Post eligibility screening, consenting, and enrollment into the HOPE Kids clinical trial (NCT02850406), each participant received a baseline MRI evaluation, within 4 weeks before treatment with voxelotor, and two follow-up MRI evaluations. The second MRI evaluation occurred 40–151 days after treatment began (see Figure 1). Participant 3 discontinued voxelotor 7 days prior to the 2nd MRI and 118 days prior to the 3rd MRI. Participants 1 and 2 underwent a third MRI evaluation post-discontinuation of voxelotor, whereas participant 4 remained on voxelotor through all three MRI evaluations.
Laboratory parameters, including total hemoglobin and daytime oxygen saturation, were collected on the day of each MRI evaluation.
3 RESULTS
Table 1 displays demographic characteristics, lab values, CBF, and OEF of the four participants at baseline and following treatment with voxelotor. At baseline, participants’ ages ranged from 10.3 to 17.1 years. All participants were Black/African American, two participants were female, and two were male. Three participants had the HbSS genotype, and one had HbSB0. Three of the four patients received a stable hydroxyurea dose at baseline and during all MRI/MRA evaluations (Participants 1, 2, and 3). One patient (Participant 4) was not treated with hydroxyurea during the study. No patient had a history of stroke or experienced a stroke during the study. Transcranial Doppler velocities were within the normal range (< 170 cm/s) at baseline and during treatment for all patients. Baseline CBF as measured by MRI (range = 96–134 mL/100 g/min) was elevated compared to healthy same-age peers [14].
| ID | Genotype | Age at baseline (years) | Sex | Hydroxyurea treatment | HgBa at MRI* | O2Sat (%) at MRI* | CBFb,* | OEF (%)* |
|---|---|---|---|---|---|---|---|---|
| 1 | HbSB0 | 14.65 | M | Y | 10.5, 11.4, 9.7 | 0.98, 1, 0.99 | 96, 92, 96 | 0.27, 0.23, # |
| 2 | HbSS | 12.82 | F | Y | 7.4, 8, 8.1 | 0.98, 0.96, 0.96 | 134, 108, 110 | 0.46, 0.40, 0.38 |
| 3 | HbSS | 17.13 | F | Y | 9, 7.4, 8 | 0.96, 1, 0.96 | 112, 108, 118 | 0.33, 0.31, 0.38 |
| 4 | HbSS | 10.29 | M | N | 9.6, 10.1, 10.1 | 0.99, 0.99, 0.98 | 104, 112, 106 | 0.35, 0.32, 0.31 |
- Abbreviations: CBF, cerebral blood flow; HgB, total hemoglobin; O2Sat, daytime oxygen saturation; OEF, oxygen extraction fraction; TCD, transcranial Doppler.
- a Gram per deciliter.
- b mL/100 g/min.
- * Bold values were collected while treated with voxelotor (participants 1, 2, and 4) or within 1 week after treatment was completed (Participant 3).
- # Oxygen extraction fraction could not be calculated for this patient due to missing data.
- The three values for HgB, O2Sat (%), CBF, and OEF represent baseline, and two additional points either during (bold) or after treatment ended (not bold).
Figure 1 and Table 1 display changes in total hemoglobin, CBF, and OEF following treatment with voxelotor. Three of four patients displayed an increase in hemoglobin during treatment. Three of four patients displayed a decrease in CBF, and all four displayed a decrease in OEF during treatment. The only patient that did not display an initial decrease in CBF (Participant 4), subsequently showed a return to near baseline at his final MRI. After stopping treatment with voxelotor, all three patients displayed an increase in CBF. OEF increased for Participant 3 and slightly decreased for Participant 2 post-treatment.
4 DISCUSSION
Voxelotor is a first-in-class, small molecule that increases the affinity of hemoglobin for oxygen and thereby inhibits sickle hemoglobin polymerization. Given its mechanism of action, it has been postulated that voxelotor may impair tissue oxygenation and lead to an increase in CBF [10]. Our data from all four patients followed for 2–5 months during voxelotor treatment did not display worsening metrics of cerebral metabolic stress (CBF and OEF) while treated with voxelotor. Intriguingly, cerebral tissue oxygenation potentially worsened (as measured by increased CBF) in three patients after discontinuation of voxelotor, which was not associated with worsening anemia. The mechanisms underlying this potential finding are not clear; however, increases in hemoglobin with other disease-modifying therapies are associated with decreases in CBF and OEF [6-8]. The in vivo effect of increased oxygen affinity, decreased hemolysis, and increased hemoglobin with voxelotor may be linked to the reduction in CBF and OEF but other unaccounted physiologic changes with therapy may also be contributing factors [12].
Recently, studies presented longitudinal data assessing CBF or other markers of neurovascular function following voxelotor treatment. One study demonstrated stable CBF and reduced OEF at 3-month follow-up using MRI (n = 15) [15]. The second study found significant decreases in OEF and CBF 4 weeks after treatment with voxelotor using diffuse optical spectroscopy. These changes persisted in weeks 8 and 12 (n = 8) [13]. Building off this prior work, we followed patients for up to 5 months after treatment initiation and captured changes in cerebral metabolic stress after discontinuation of voxelotor in three of four patients. Our findings are consistent with these reports, as we observed a decrease in OEF for all patients and a decrease in CBF for three of four patients after voxelotor was initiated.
The small sample and inconsistent timing of data collection limit conclusions that can be drawn from the current case series. However, our findings are not consistent with the hypothesis that voxelotor significantly impairs oxygen transfer to the brain. Rather, consistent with prior work, CBF and OEF appear to decrease slightly following voxelotor treatment and may increase after stopping treatment. Cognitive endpoints are needed to determine the functional consequences of these cerebrovascular changes. Larger studies and further investigation are required to better characterize the effects on stroke risk in SCA.
ACKNOWLEDGMENTS
The authors would like to thank Mitchell Weiss, MD, PhD for his review and suggestions to improve the manuscript. This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC). AMH received funding from K23HL166697 during the study. Global Blood Therapeutics, which was acquired by Pfizer Inc., provided funding for this study under the GBT440-007 trial – HOPE Kids, NCT02850406.
CONFLICT OF INTEREST STATEMENT
Andrew M. Heitzer—Consultant Fees: Global Blood Therapeutics.
Clark Brown—Former employee: Legacy Global Blood Therapeutics / Pfizer; Equity ownership: Pfizer; Consultant fees: Global Blood Therapeutics; Research funding: Forma Therapeutics, Global Blood Therapeutics, Imara, Novartis, Pfizer.
Mark Davis—Employee: Pfizer; Equity ownership: Pfizer; Former employee: Global Blood Therapeutics.
Sandy Dixon—Employee: Pfizer; Equity ownership: Pfizer; Former employee: Global Blood Therapeutics.
Jeremie Estepp—Research Funding: Global Blood Therapeutics, Forma Therapeutics, Pfizer, Eli Lilly and Co, NHLBI, ASH; Consultant Fees: Daiichi Sankyo, Esperion, and Global Blood Therapeutics. Following completion of the activities contained in this manuscript, Jeremie Estepp changed employers to Agios Pharmaceuticals, which had no role in the design of the study, analysis or interpretation of the results, or drafting of the manuscript.
Cliff Takemoto—Site-PI, Global Blood Therapeutics; Site-PI, Forma Therapeutics; Data Safety Committee, Novartis.
Jane Hankins–Royalties from UpToDate.
ETHICS STATEMENT
The authors have confirmed ethical approval statement is not needed for this submission.
PATIENT CONSENT STATEMENT
The authors have confirmed patient consent statement is not needed for this submission.
CLINICAL TRIAL REGISTRATION
The authors have confirmed clinical trial registration is not needed for this submission.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




