Enhanced DNA vaccine potency by mannosylated lipoplex after intraperitoneal administration
Abstract
Background
Here we describe a novel DNA vaccine formulation that can enhance cytotoxic T lymphocyte (CTL) activity through efficient gene delivery to dendritic cells (DCs) by mannose receptor-mediated endocytosis.
Methods
Ovalbumin (OVA) was selected as a model antigen for vaccination; accordingly, OVA-encoding pDNA (pCMV-OVA) was constructed to evaluate DNA vaccination. Mannosylated cationic liposomes (Man-liposomes) were prepared using cholesten-5-yloxy-N-{4-[(1-imino-2-D-thiomannosylethyl)amino]butyl}formamide (Man-C4-Chol) with cationic lipid. The potency of the mannosylated liposome/pCMV-OVA complex (Man-lipoplex) was evaluated by measuring OVA mRNA in CD11c+ cells, CTL activity, and the OVA-specific anti-tumor effect after in vivo administration.
Results
An in vitro study using DC2.4 cells demonstrated that Man-liposomes could transfect pCMV-OVA more efficiently than cationic liposomes via mannose receptor-mediated endocytosis. In vivo studies revealed that the Man-lipoplex exhibited higher OVA mRNA expression in CD11c+ cells in the spleen and peritoneal cavity and provided a stronger OVA-specific CTL response than intraperitoneal (i.p.) administration of the conventional lipoplex and intramuscular (i.m.) administration of naked pCMV-OVA, the standard protocol for DNA vaccination. Pre-immunization with the Man-lipoplex provided much better OVA-specific anti-tumor effect than naked pCMV-OVA via the i.m. route.
Conclusions
These results suggested that in vivo active targeting of DNA vaccine to DCs with Man-lipoplex might prove useful for the rational design of DNA vaccine. Copyright © 2006 John Wiley & Sons, Ltd.
Introduction
DNA vaccine, plasmid DNA (pDNA)-encoding antigen from a pathogen, is of great interest in gene therapy as a means of immunotherapy against refractory diseases such as cancer and viral infections because the administration of naked pDNA-encoding antigen proteins induces not only an antibody response, but also a potent cytotoxic T lymphocyte (CTL) response in animal models 1-3. Recent immunological studies have demonstrated that gene transfection and subsequent activation of antigen-presenting cells (APCs), dendritic cells (DCs) and macrophages are important for efficient DNA vaccine therapy 4-6. Although some clinical trials involving melanoma, human imuunodeficiency virus, and HCV have been performed using topical administration of naked pDNA 7-9, the results are not good enough for clinical therapy. In order to overcome this problem, it is important to develop gene delivery carriers for in vivo APC-selective gene transfection.
In spite of the high transfection efficiency of viral vectors, they still need to be improved from the point of view of safety issues 10-12. The use of non-viral vectors is one of the possible approaches for in vivo gene delivery because they are free from some of the risks inherent in these systems. Furthermore, the characteristics of non-viral vectors can be more easily modified than those of viral vectors. To achieve targeted gene delivery, a number of receptor-mediated gene delivery systems have been developed 13-16 including our carriers 17-22. As far as in vivo selective gene delivery to APCs is concerned, mannose has been shown to be a promising ligand to target APCs because these cells have a large number of mannose receptors.
Recently, we have developed several types of macromolecular 18 and particulate 22, 23 gene carriers for macrophage-selective gene transfection in vivo. Among them, cationic liposomes containing cholesten-5-yloxy-N-{4-[(1-imino-2-D-thiomannosylethyl)amino]butyl} formamide (Man-C4-Chol) are some of the most interesting potential gene transfection carriers 22, 23 that can be efficiently recognized by mannose receptors on macrophages in liver. Man-C4-Chol exhibits bifunctional properties, i.e., an imino group for binding to pDNA via electrostatic interaction and a mannose residue for the cell-surface receptors in APCs 22. Therefore, a high density of mannose residues can be provided on the liposome surface without affecting the binding of the cationic liposomes to pDNA. More recently, we have demonstrated that intravenously administrated pCMV-OVA complexed with mannosylated liposomes (Man-lipoplex) enhances MHC class I antigen presentation, but no measurable CTL response was observed 24, suggesting that not only cell-selective gene transfection but also enhanced transfection efficiency in DCs is needed for gene therapy.
Intraperitoneal (i.p.) administration has some advantages as far as the transfection efficacy to DCs by Man-lipoplex is concerned; this is because of (i) high accessibility to APCs in the peritoneal cavity and lymph nodes, (ii) long retention of the lipoplex, (iii) the presence of few biocomponents that reduce transfection activity, and (iv) the high capacity of the lipoplex solution. Taking these factors into consideration, i.p. administrated Man-lipoplex would enhance gene expression in APCs resulting in efficient DNA vaccine therapy. However, few reports are available on the effect of i.p. administered Man-lipoplex on DNA vaccine therapy.
The objective of this paper was to clarify the DNA vaccine potency after i.p. administration of Man-lipoplex. In the present study, ovalbumin (OVA)-encoding pDNA (pCMV-OVA) was selected as a model DNA vaccine. Using in vitro and in vivo experiments, the transfection efficacy to APCs was evaluated by measuring the OVA mRNA using quantitative reverse-transcription polymerase chain reaction (RT-PCR). After immunizing with Man-lipoplex, OVA-specific CTL responses and its antitumor effects against inoculated E.G7-OVA cells (OVA expressing cells), and its parental cell line, EL4 cells (OVA non-expressing cells), were also evaluated. The results obtained were compared with those of conventional lipoplex and naked pCMV-OVA.
Materials and methods
Materials
Cholesteryl chloroformate, HEPES, concanavalin A, G418, and immunogloblin G were obtained from Sigma Chemicals Inc. (St. Louis, MO, USA). N-[1-(2,3-Dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA) and N-(4-aminobutyl)carbamic acid tert-butyl ester were obtained from Tokyo Chemical Industry Co. (Tokyo, Japan). Dioleoylphosphatidylethanolamine (DOPE) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). pVAX I, fetal bovine serum (FBS), and Opti-MEM I® were obtained from Invitrogen Co. (Carlsbad, CA, USA). Anti-CD11c monoclonal antibody (N418)-labeled magnetic beads were purchased from Miltenyi Biotec Inc. (Auburn, CA, USA). Nucleic acid purification kit magextractor®-RNA was purchased from Toyobo Co., Ltd. (Osaka, Japan). The first strand cDNA synthesis kit for RT-PCR, Lightcycler™ faststart DNA master hybridization probes, and a Lightcycler™-Primer/Probes set for mouse β-actin were purchased from Roche Diagnostics Co. (Indianapolis, IN, USA). Primers/probes for OVA were purchased from Nihon Gene Research Labs Inc. (Miyagi, Japan). All other chemicals were of the highest purity available.
Animals
Female ICR mice (4–5 weeks old) and C57BL/6 mice (6–8 weeks old) were purchased from the Shizuoka Agricultural Cooperative Association for Laboratory Animals (Shizuoka, Japan). All animal experiments were carried out in accordance with the Principles of Laboratory Animal Care as adopted and promulgated by the US National Institutes of Health and the guideline for animal experiments of Kyoto University.
Cell line
DC2.4 cells, a cell line of murine dendritic cells (DCs, haplotype H-2b) 25, were kindly provided by Dr. K. L. Rock (University of Massachusetts Medical School, Worcester, MA, USA). The expression of mannose receptors in this cell line has been confirmed elsewhere 26. Therefore, DC2.4 cells are a suitable model of DCs. EL4 cells (ATCC: TIB-39) and E.G7-OVA cells (ATCC: CRL-2113) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS and RPMI 1640 supplemented with 10% FBS, 4.5 g/l glucose, 10 mM HEPES, 1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol and 0.4 mg/ml G418.
Synthesis of Man-C4-Chol and Gal-C4-Chol
Man-C4-Chol and Gal-C4-Chol were synthesized as described previously 19, 22. Briefly, N-(4-aminobutyl)-(cholesten-5-yloxyl)formamide (C4-Chol) was synthesized from cholesteryl chloroformate and N-(4-aminobutyl)carbamic acid tert-butyl ester. The C4-Chol was reacted with 5 equivalents of 2-imino-2-methoxyethyl-1-thiomannoside or 2-imino-2-methoxyethyl-1-thiogalactoside 27 in pyridine containing 1.1 equivalents of triethylamine for 24 h. After evaporation of the reaction mixture in vacuo, the resultant material was suspended in water and dialyzed against water for 48 h, and then lyophilized.
Construction and preparation of pCMV-OVA
pCMV-OVA was constructed by subcloning the EcoRI chicken egg albumin (ovalbumin) cDNA fragment from pAc-neo-OVA 28, which was kindly provided by Dr. M. J. Bevan (University of Washington, Seattle, WA, USA), into the polylinker of pVAX I. pCMV-OVA was amplified in the E. coli strain, DH5α, then isolated, and purified using a Qiagen plasmid giga kit (Qiagen GmbH, Hilden, Germany). The endotoxin in pCMV-OVA solution was removed by the Triton X-114 method.
Preparation of cationic liposomes
Liposomes were prepared using the method reported previously 19, 22. Briefly, DOTMA, Chol, and Man-C4-Chol or Gal-C4-Chol were mixed in chloroform at a molar ratio of 2 : 1:1 : 0, 2 : 1:0 : 1, and 2 : 2:0 : 0 to prepare Man-liposomes, Gal-liposomes, and cationic liposomes, respectively. Then, the mixture was dried, vacuum desiccated, and resuspended in sterile 20 mM HEPES buffer (pH 7.8) or 5% dextrose solution in a sterile test tube for in vitro and in vivo experiments, respectively. After hydration, the dispersion was sonicated for 5 min in a bath sonicator to produce liposomes and then sterilized by passing through a 0.45 µm filter (Nihon-Millipore Ltd., Tokyo, Japan).
Preparation of lipoplex for in vitro study
Lipoplex was prepared using the method reported previously 19, 22. Briefly, equal volumes of pCMV-OVA and stock liposome solution were diluted with Opti-MEM I® in 15 ml Falcon tubes. Then, pCMV-OVA solution was added rapidly to the surface of the liposome solution at a charge ratio (−/ +) of 1.0 : 2.3 using a micropipette (Pipetman®, Gilson, Villier-le Bel, France) and the mixture was agitated rapidly by pumping it up and down twice in the pipette tip.
Preparation of lipoplex for in vivo study
All cationic liposome/pCMV-OVA complexes for in vivo experiments were prepared under the optimal conditions for cell-selective gene transfection as reported previously 29-31. Briefly, equal volumes of pCMV-OVA and stock liposome solution were diluted with 5% dextrose in 15 ml tubes. Then, pCMV-OVA solution was added rapidly to the surface of the liposome solution using a micropipette and the mixture was agitated rapidly by pumping it up and down twice in the pipette tip. The mean particle sizes were measured by dynamic light scattering spectrophotometry (LS-900; Otsuka Electronics Co., Ltd., Osaka, Japan). The zeta-potential of the lipoplexes was measured by the laser-Doppler electrophoresis method with a Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK).
Uptake characteristics by DC2.4 cells
Uptake study was performed by the method reported previously 19, 22. Briefly, the DC2.4 cells were plated on a 24-well plate at a density of 0.65 × 105 cells/cm2 and cultivated in 500 µl RPMI supplemented with 10% FBS. Twenty-four hours later, the culture medium was replaced with an equivalent volume of Hanks medium (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) containing 1 kBq/ml [32P]-pCMV-OVA, 0.5 mg/ml cold pCMV-OVA and cationic liposomes at a charge ratio (−/+) of 1.0 : 2.3. After incubation for given time periods, the solution was quickly removed by aspiration, the cells were washed five times with ice-cold HBSS buffer and then solubilized in 0.3 M NaOH solution with 10% Triton X-100 (0.3 ml). The radioactivity was measured by liquid scintillation counting (LSC-500; Beckman, Inc., Tokyo, Japan) and the protein content was determined by a modification of the Lowry method. The effect of the presence of 0.125 mg/ml mannan was determined in the same system.
Transfection activity by DC2.4 cells
DC2.4 cells were seeded in 10.5 cm2 dishes at a density of 0.65 × 105 cells/cm2 in RPMI 1640 medium supplemented with 10% FBS. After 24 h in culture, the culture medium was replaced with Opti-MEM I® containing 0.5 µg/ml pCMV-OVA and cationic liposomes. Six hours later, the incubation medium was replaced again with RPMI 1640 supplemented with 10% FBS and incubated for an additional 6 h. Then, the cells were scraped and suspended in 200 µl pH 7.4 phosphate-buffered saline (PBS). Total RNA was isolated from DC2.4 cells with MagExtractor MFX-2000 (Toyobo Co., Ltd., Osaka, Japan) and MagExtractor-RNA following the manufacturer's instructions. Reverse transcription of mRNA was carried out using a first strand cDNA synthesis kit as follows: total RNA was added to the oligo dT primer (0.8 µg/µl) solution, and incubated at 42 °C for 60 min with a program temperature control system PC-808 (Astec Co., Ltd., Fukuoka, Japan). Real-time PCR was performed using the Lightcycler™ quick system 350S (Roche Diagnostics Co., Indianapolis, IN, USA) with hybridization probes. Primer and hybridization probes for OVA cDNA were constructed as follows: primer, 5′-GCGTCTCTGAATTTAGGG-3′ (forward) and 5′-TACCCCTGATACTACAGTGC-3′ (reverse); hybridization probes, 5′-CTTCTGTATCAAGCACATCGCAACCAACG-3′-fluorescein isothiocyanate (FITC) and Lightcycler™-Red640 (LCRed)-5′-CGTTCTCTTCTTTGGCAGATGTGTTTCCCC-3′. The PCR reaction for detection of the OVA gene was carried out in a final volume of 20 µl containing: (i) 2 µl DNA Master hybridization probes 10× (DNA Master hybridization probes kit); (ii) 1.6 µl 25 mM MgCl2; (iii) 1.5 µl forward and reverse primers (final concentration 0.75 µM); (iv) 1 µl2 µM FITC-labeled hybridization probes and 2 µl2 µM LCRed-labeled probes (final concentrations 0.2 and 0.4 µM, respectively); (v) 5.4 µlH2O; (vi) 5 ml cDNA or pCMV-OVA solution. For the mouse β-actin cDNA measurements, samples were prepared in accordance with the instruction manuals. After an initial denaturation step at 95 °C for 10 min, temperature cycling was initiated. Each cycle consisted of denaturation at 95 °C for 10 s, hybridization at 60 °C for 15 s, and elongation at 72 °C for 10 s. The fluorescent signal was acquired at the end of the hybridization step (F2/F1 channels). The total number of cycles performed was 40. The mRNA copy numbers were calculated for each sample from the standard curve using the instrument software (‘Arithmetic Fit Point analysis’ for the Lightcycler). Results were expressed as relative copy numbers calculated relative to β-actin mRNA (copy number of OVA mRNA/copy number of β-actin mRNA).
Quantification of OVA mRNA in CD11c+ cells after i.p. administration by quantitative PCR
pCMV-OVA (100 µg) or lipoplex was injected via the i.p. route. Spleens and peritoneal cells were harvested 6 h after i.p. administration and single cell suspensions of spleen cells were prepared in ice-cold RPMI 1640 medium. Ice-cold RPMI 1640 medium (5 ml) was injected and then peritoneal cells were collected as a cell suspension in RPMI medium. Following this, red blood cells were removed by incubation with Tris-NH4Cl solution for 10 min at room temperature. Positive selection of CD11c+ cells was carried out by magnetic cell sorting with auto MACS (Miltenyi Biotec Inc., Auburn, CA, USA) following the manufacturer's instructions. Briefly, the cell suspension was incubated with PBS containing 1 mg/ml IgG to block the Fcγ receptors of macrophages. Then, CD11c+ cells were labeled by incubating with anti-CD11c monoclonal antibody (N418)-labeled magnetic beads. After washing three times, CD11c+ cells were collected by auto MACS. Total RNA was isolated from the recovered CD11c+ cells with a MagExtractor MFX-2000 (Toyobo Co., Ltd., Osaka, Japan) and MagExtractor-RNA following the manufacturer's instructions. Reverse transcription and quantitative PCR of OVA and β-actin mRNA were performed as described in the section ‘Transfection activity by DC2.4 cells’.
Induction of OVA-specific CTL
C57BL/6 mice were immunized with naked pCMV-OVA (50 or 100 µg) or lipoplexes by i.p., i.m., or intradermal (i.d.) administration three times at intervals of 2 weeks. Two weeks after the last immunization, the spleens of each group were harvested and a single cell suspension was prepared in ice-cold RPMI 1640 medium. Then, the spleen cells were resuspended in RPMI 1640 medium supplemented with 10% FBS and 2-mercaptoethanol. The recovered spleen cells were plated in a 25-cm flask at 5 × 106 cells/ml along with MMC and E.G7-OVA cells and treated for 1 h (100 µg/ml, 1 h). Four days after cultivation, non-adherent cells were harvested, washed, and plated with relevant or irrelevant target cells at effector/target (E/T) ratios of 100 : 1, 50 : 1, 25 : 1, and 12.5 : 1. The target cells were E.G7-OVA cells or their parental cell line, EL4 cells. The target cell (E.G7-OVA or EL4 cells) suspensions in RPMI medium (2.5 × 107 cells/ml) were incubated with 51Cr (7.4 MBq/ml) for 1 h. Following incubation, the cells were washed five times and then resuspended at 2 × 105 cells/ml. The target cells (E.G7-OVA or EL4 cells; 2 × 104 cells) were added to each well of a 96-well microtiter plate, along with 2 × 106, 1 × 106, 5 × 105, or 2.5 × 105 spleen cells and the plates were mixed and incubated for 4 h at 37 °C and 5% CO2 in an incubator. After further centrifugation, 100 µl supernatant was collected from each well and the radioactivity released was measured in a gamma counter. The percentage 51Cr release was calculated as follows: specific lysis (%) = [(experimental 51Cr release — spontaneous 51Cr release)/(maximum 51Cr release — spontaneous 51Cr release)] × 100). The percentage OVA-specific 51Cr release was calculated as (% of 51Cr release from E.G7-OVA)—(% of 51Cr release from EL4).
Evaluation of protection against transplanted tumor cells in mice
C57BL/6 mice were immunized three times by i.p. or i.m. administration of naked pCMV-OVA (100 µg) or lipoplex at 2-week intervals. Then 2 weeks after the last immunization, E.G7-OVA (1 × 106) or EL4 (1 × 106) cells were inoculated subcutaneously (s.c.) into the back of the mice. The survival of the mice was monitored up to 100 days after inoculation of the E.G7-OVA or EL4 cells.
Results
Particle sizes and zeta-potentials of Man-lipoplexes
To investigate the physicochemical properties of lipoplexes, the particle size and zeta-potential of each lipoplex were evaluated. Both lipoplexes showed a clear-cut distribution pattern and the mean particle sizes of the Man-lipoplexes and conventional lipoplex were 114 ± 7.8 and 116 ± 11.5 nm (n = 3), respectively. Zeta-potential analysis showed that the zeta-potential of the Man-lipoplex and the conventional lipoplex at a charge ratio (−/+) of 1.0 : 2.3 was 62.1 ± 1.85 and 64.1 ± 1.74 mV (n = 3), respectively. These results show that there was almost no difference in physicochemical properties between the two complexes. The galactosylated lipoplex (Gal-lipoplex) also showed a similar size distribution and zeta-potential (data not shown).
Uptake characteristics of Man-lipoplex by DC2.4 cells in vitro
To evaluate the potency of the Man-lipoplex in terms of targeted delivery to DCs, the uptake of the lipoplexes and subsequent transfection to cells were evaluated. In this study, we used DC2.4 cells, a cell line derived from DCs, as a model DC expressing mannose receptors 26. The [32P] Man-lipoplex was taken up by DC2.4 cells more efficiently than the conventional [32P] lipoplex (Figure 1a) and this was significantly reduced in the presence of an excess of mannan (Figure 1b). In contrast, the uptake with conventional lipoplex was not significantly inhibited by an excess of mannan (Figure 1b).
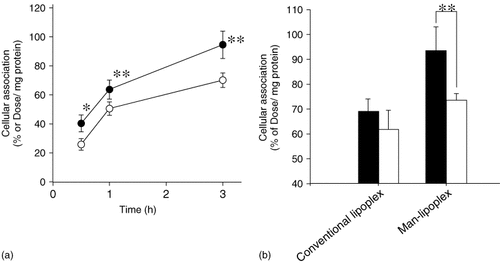
Cellular association of the Man-lipoplex in DC2.4 cells. (a) Cellular association time-course of 32P-labeled Man-lipoplex (●) and lipoplex (○) in DC2.4 cells at 37 °C. pCMV-OVA (0.5 µg/ml) was complexed with cationic liposomes at a charge ratio (−/ +) of 1.0 : 1.6. Each value represents the mean ± standard deviation (S.D.) (n = 3). (b) Cellular association of 32P-labeled lipoplex or Man-lipoplex in the absence (▪) or presence (□) of 0.125 mg/ml mannan in the culture medium. Each value represents the mean + S.D. (n = 4). Statistical analysis was performed by Student's t-test (*P < 0.05, **P < 0.01)
Transfection characteristics of Man-lipoplex with respect to DC2.4 cells in vitro
We next investigated the transfection activity of the Man-lipoplex with respect to DC2.4 cells. As shown in Figure 2a, the highest gene expression was observed in the Man-lipoplex. In the presence of an excess of mannan, the gene expression of the Man-lipoplex was significantly inhibited (Figure 2b). In contrast, the gene expression with conventional lipoplex and naked pDNA was not significantly inhibited in the presence of an excess of mannan (Figure 2b).

Transfection activity of the Man-lipoplex in DC2.4 cells. (a) Transfection activity of naked pCMV-OVA or lipoplexes in cultured DC2.4 cells. The concentration of pDNA was fixed at 0.5 µg/ml in all experiments. Each value represents the mean + S.D. (n = 3). (b) Transfection activity of naked pCMV-OVA or lipoplexes in the absence (▪) or presence (□) of 0.125 mg/ml mannan. The concentration of pCMV-OVA was fixed at 0.5 µg/ml in all experiments. Each value represents the mean + S.D. values (n = 4). Statistical analysis was performed by analysis of variance (*P < 0.05)
Effect of Man-lipoplex administration routes on CTL response
To investigate the effect of the administration route of the Man-lipoplex, the induction of an OVA-specific cytotoxic response with a 51Cr release assay using E.G7-OVA cells (OVA-expressing cells), and its parental cell line, EL4 cells (OVA-non-expressing cells), was examined. We found that the CTL activity induced by i.p. administration of the Man-lipoplex was higher than that induced by i.m. or i.d. administration of the Man-lipoplex (Figure 3a). Furthermore, increasing the amount of pCMV-OVA of the Man-lipoplex markedly enhanced the CTL response induced by i.p. administration (Figure 3b). Furthermore, the CTL activity of the Man-lipoplex following i.p. administration was significantly higher than that induced by i.m. administration of naked pCMV-OVA (Figure 3c).

Effect of the route of administration and dose on immunization with naked pCMV-OVA or Man-lipoplexes. Mice were injected with 50 µg pCMV-OVA as naked pCMV-OVA or Man-lipoplexes biweekly three times before the experiment. (a) CTL activity primed by i.p. (▪), i.d. (□) and i.m. (●) administration of the Man-lipoplexes or CTL activity in the no-treatment group (○). OVA-specific cell lysis at various effector/target (E/T) ratios was calculated from the % 51Cr release from EL4 cells and from E.G7-OVA cells. Each value represents the mean of 4–5 experiments. (b) CTL response induced by the Man-lipoplexes (▪) and naked pCMV-OVA (□) given i.p. at a dose of 50 or 100 µg/mouse. OVA-specific 51Cr release at an E/T ratio of 50 : 1 was calculated from the following equation: OVA-specific 51Cr release = %51Cr release from E.G7-OVA cells − %51Cr release from EL4 cells. Each value represents the mean + S.D. (control group: n = 3, other groups: n = 5). Statistical analysis was performed by analysis of variance (**P < 0.01). (c) CTL response induced by the Man-lipoplexes given i.p. and naked pCMV-OVA given i.m. at a dose of 50 or 100 µg/mouse. OVA-specific 51Cr release at an E/T ratio of 50 : 1 was calculated from the following equation: OVA-specific 51Cr release = %51Cr release from E.G7-OVA cells − %51Cr release from EL4 cells. Each value represents the mean + S.D. (control group: n = 3, other groups: n = 5). Statistical analysis was performed by analysis of variance (**P < 0.01)
Effect of lipoplex mannosylation on CTL response
As shown in Figure 4a, the Man-lipoplex induced a much higher CTL response than the conventional lipoplex. However, the Gal-lipoplex induced a much lower CTL response than the Man-lipoplex (Figure 4b), suggesting that the Man-lipoplex induces a strong CTL response via a mannose receptor-mediated mechanism.

Effect of mannosylation of cationic liposomes on the induction of a CTL response. Mice were injected three times with naked pCMV-OVA (100 µg) or lipoplexes (pCMV-OVA; 100 µg) biweekly. (a) CTL response induced by the Man-lipoplexes (▪) and the lipoplexes (●) or that of the no-treatment group (▵). OVA-specific 51Cr release was calculated from the following equation: OVA-specific 51Cr release = %51Cr release from E.G7-OVA cells − %51Cr release from EL4 cells. Each value represents the mean ± S.D. (n = 4–5). (b) CTL response induced by the Man-lipoplex (▪) or the Gal-lipoplex (□) or CTL response in the no-treatment group (▵). OVA-specific 51Cr release was calculated from the following equation: OVA-specific 51Cr release = %51Cr release from E.G7-OVA cells − %51Cr release from EL4 cells. Each value represents the mean ± S.D. (n = 5). Statistical analysis was performed by analysis of variance. Significant difference between the no-treatment group (*P < 0.05, **P < 0.01) or pCMV-OVA given i.m. (††P < 0.01)
Gene expression characteristics of Man-lipoplex on CD11c+ cells in the spleen and peritoneal cavity after i.p. administration
To clarify the transfection activity of the Man-lipoplex with regard to DCs after i.p. administration, the OVA mRNA in the CD11c+ cells in the spleen and peritoneal cavity was determined 6 h after i.p. administration of naked pCMV-OVA (100 µg) or Man-lipoplex and conventional lipoplex using quantitative RT-PCR. The relative copy number of OVA mRNA in the Man-lipoplex injected group was the highest of all in both peritoneal CD11c+ cells (Figure 5a) and splenic CD11c+ cells (Figure 5b).

In vivo mRNA gene expression in CD11c+ cells in the peritoneal cavity (a) and spleen (b) after i.p. administration to mice. Lipoplex or Man-lipoplex was prepared at a charge ratio (−/ +) of 1.0 : 2.3 in 5% dextrose. Six hours after injection, OVA mRNA and β-actin mRNA eluted from CD11c+ cells were measured by quantitative two-step RT-PCR. Each value represents the mean + S.D. (n = 3–4). Statistical analysis was performed by analysis of variance (*P < 0.05)
Anti-tumor responses of Man-lipoplex after immunization
To assess the protective anti-tumor effect, EL4 and E.G7-OVA cells were transplanted into pre-immunized mice. Pre-immunization with the Man-lipoplex prolonged the survival time after transplantation of E.G7-OVA cells compared with pDNA or conventional lipoplex (Figure 6a). However, all formulations failed to prolong the survival rate after transplantation of EL4 cells (Figure 6b).

The anti-E.G7-OVA cell (a) or EL4 cell (b) tumor effect following pre-immunization by i.p. administration of various formulations or i.m. administration of naked pCMV-OVA solution (100 µg). Mice were injected with naked pCMV-OVA (○), the Man-lipoplex (▪), or the lipoplex (●) given i.p., naked pCMV-OVA given i.m. (▴), or no treatment (▵). E.G7-OVA (a) or EL4 (b) cells were transplanted into mice 2 weeks after the last immunization and the survival rate was determined (n = 7 for all groups in (b) and for Man-lipoplex in (a), n = 10 for all groups but the Man-lipoplex in (a))
Discussion
DNA vaccine represents an exciting novel approach in vaccine development. The vaccine construct is created by insertion of a DNA encoding the desired antigen into a pDNA. The extent to which the pDNA is able to transfect cells is dependent on the application route and delivery carrier used. The encoded protein is then expressed in the transfected cells in vivo and, consequently, an immune response is elicited to the expressed antigen. However, to date, there have been few reports on in vivo gene therapy based on targeted non-viral gene delivery. In our series of experiments, we have been developing APC-selective in vivo gene carrier systems for use in gene therapy 22-24, 32. In the present study, we describe the Man-lipoplex given i.p. as a novel approach to enhance therapeutic potency of DNA vaccine therapy in vivo.
Since some clinical trials have involved the local administration of naked pDNA 7-9, we evaluated the OVA-specific CTL response following the local administration of naked pDNA. As shown in Figure 3c, i.p. administration of Man-lipoplex induced a higher OVA-specific CTL response than local administration of naked pDNA. To demonstrate the antigen-specific anti-tumor effects induced by vaccination, E.G7-OVA cells (OVA expressing cells), and its parent cell line, pre-immunized mice were inoculated with EL4 cells (OVA non-expressing cells). Corresponding to the CTL response, the anti-tumor effects of the Man-lipoplex were observed only in E.G7-OVA cells and the effects of the Man-lipoplex were much greater than those following local administration of naked pDNA (Figure 6). These results suggest that the Man-lipoplex is an effective gene carrier for DNA vaccination used as cancer therapy.
To demonstrate mannose receptor-mediated gene transfection of the Man-lipoplex, its transfection characteristics in DCs were evaluated in both in vitro and in vivo experiments. As shown in Figures 1 and 2, the Man-lipoplex showed significantly higher uptake and transfection activity than the conventional lipoplex and this was reduced in the presence of mannan, a mannose receptor ligand. These in vitro results suggest that the Man-lipoplex is taken up by mannose receptor-mediated endocytosis by a dendritic cell line, DC2.4 cells. This observation is in good agreement with our previous report showing that the Man-lipoplex is taken up by mannose receptor-mediated endocytosis by primary cultured mouse peritoneal macrophages 22, 32. To evaluate the importance of mannose receptor-mediated gene transfection to DCs, we also evaluated the involvement of the mannose receptor-mediated mechanism in the CTL response. The Gal-lipoplex was selected because it differed only from the Man-lipoplex by a sugar moiety. In a previous study, we have already confirmed that the Gal-lipoplex was taken up by asialoglycoprotein receptor-mediated endocytosis after intraportal administration 33, 34. As shown in Figure 4b, the CTL response of the Gal-lipoplex was significantly lower than that of the Man-lipoplex. These in vitro and in vivo results suggest that the Man-lipoplex is efficiently taken up by DCs via mannose receptor-mediated endocytosis.
As control cationic liposomes, cationic liposomes composed of 3β-[N,N′,N′-dimethylaminoethane]carbamoyl] cholesterol hydrochloride (DC-Chol liposomes) represent a feasible formulation for clinical trials involving gene therapy via the i.p. route 35 and it has also been reported that DC-Chol liposomes enhance DNA vaccine potency following i.p. administration in mice 36. However, our preliminary experiment using pCMV-Luc demonstrated that DOTMA/Chol liposomes exhibited a much higher transfection activity than DC-Chol liposomes following i.p. administration (data not shown); therefore, DOTMA/Chol liposomes were selected as control liposomes for DNA vaccine therapy.
To further evaluate the effectiveness of lipoplex mannosylation, gene expression in APCs, OVA-specific CTL activity and the antitumor effect were compared with those obtained using conventional lipoplex. Since the lipid composition of the liposomes (DOTMA/Chol vs. DOTMA/Chol/Man-C4-Chol) and the physicochemical properties (zeta-potential and particle size (see Results section)) of the lipoplex and Man-lipoplex are almost the same, the effect of mannosylation of lipoplex could be investigated by such comparisons. After i.p. administration of lipoplex or Man-lipoplex, the gene expression in CD11c+ cells in the spleen and peritoneal cavity of Man-lipoplex was significantly higher than that of the conventional lipoplex (Figure 5a and 5b). Furthermore, the OVA-specific CTL response (Figure 4) and anti-tumor effect (Figure 6a) of the Man-lipoplex were significantly higher than that of the conventional lipoplex. These results convinced us that lipoplex mannosylation could enhance DNA vaccine potency.
As far as the effect of the administration route on the CTL activity by Man-lipoplex was concerned, the CTL activity following i.p. administration was higher than that following s.c. and i.m. administration (Figure 3a). We previously reported that the transfection efficiency of the lipoplex was lower than that of naked pDNA because the lipoplex is only localized at the injection site due to its cationic and macromolecular nature 37. Thus, the lower CTL activity following s.c. and i.m. administration of the Man-lipoplex may be partly explained by our previous observation. In contrast, i.p. administrated Man-lipoplex is considered to reach the APCs in the peritoneal cavity and lymph nodes. Even the (large sized) cancer cells could distribute to the lymph nodes from the peritoneal cavity when undergoing metastasis 38, 39. Thus, these observations strongly suggest that i.p. administration is an effective administration route for gene transfection to APCs by the (Man-)lipoplex.
In the present study, we have demonstrated the effectiveness of i.p. administration of the Man-liposome formulation. This type of infusion of not only drugs such as cisplatin 42 and paclitaxel 43 but also cationic liposome/pDNA 44 has already been performed in clinical trials for ovarian cancer therapy. Regarding the i.p. administration method, implantable infusion pumps have been developed for a number of diseases 45 and there has been remarkable progress in endoscopic and laparoscopic surgical techniques 46. These progresses in surgical techniques and devices might make i.p. administration of the Man-liposome formulation a conventional and feasible approach for the clinical application of DNA vaccine therapy.
In this study, we demonstrated that the Man-lipoplex enhanced gene expression via a mannose receptor-mediated mechanism. Since our previous study demonstrated that the Man-lipoplex were rapidly sorted from endosomes to lysosomes after uptake via mannose receptors 47, only a small part of pDNA seems to be released from endosome/lysosome to the cytosol and enters the nucleus for gene expression. Taking these findings into consideration, further modulation of intracellular sorting with some functional device should lead to more efficient gene expression in APCs. So far, functional materials such as influenza virus hemagglutinin subunit HA-2 (mHA2) 17, fusigenic peptide, and polyhistidine 48 have been grafted to the vectors to improve the intracellular sorting of cationic carrier/pDNA complexes. Modulation by grafting such functional molecules might be effective in the further development of the Man-lipoplex.
A large number of diseases can be potentially prevented or cured by DNA vaccination 40. Recent developments in genomics technology have identified new target antigens, not only for infectious disease pathogens, but also for a large number of tumor-associated antigens 41 and this has increased the possibility of using DNA vaccines for a variety of infectious diseases and cancer therapies. Since the pDNA that encodes a variety of antigens has almost the same physicochemical properties as a polyanion, our Man-liposomes are expected to be applicable to a range of DNA vaccine therapies to enhance the CTL response.
In conclusion, we demonstrate that the Man-lipoplex produces an extremely high antigen-specific CTL response. In addition, intraperitoneal administration is an effective route for the APCs-selective gene transfection by the Man-lipoplex. Although further optimization is required, this information will also be valuable for the future use, design, and development of a Man-lipoplex to enhance the potential of DNA vaccine therapy.
Acknowledgements
We are grateful to Dr. M. J. Bevan and Dr. K. L. Rock for providing us with pAc-neo-OVA and DC2.4 cells, respectively. This work was supported in part by Grants-in-Aid for Scientific Research from Ministry of Education, Culture, Sports, Science, and Technology of Japan, by Health and Labour Sciences Research Grants for Research on Advanced Medical Technology from the Ministry of Health, Labour and Welfare of Japan, by the Kao Foundation for Arts and Sciences, and by the Shimizu Foundation for the Promotion of Immunology Research.




