Generation of cell hybrids via a fusogenic cell line
Abstract
Background
Hybrids obtained by fusion between tumour cells (TC) and dendritic cells (DC) have been proposed as anti-tumour vaccines because of their potential to combine the expression of tumour-associated antigens with efficient antigen presentation. The classical methods used for fusion, polyethylene glycol (PEG) and electrofusion, are cytotoxic and generate cell debris that can be taken up by DC rendering the identification of true hybrids difficult.
Methods
We have established a stable cell line expressing a viral fusogenic membrane glycoprotein (FMG) that is not itself susceptible to fusion. This cell line has been used to generate hybrids and to evaluate the relevance of tools used for hybrid detection.
Results
This FMG-expressing cell line promotes fusion between autologous or allogeneic TC and DC in any combination, generating ‘tri-parental hybrids’. At least 20% of TC are found to be integrated into hybrids.
Conclusions
It is speculated that this tri-parental hybrid approach offers new possibilities to further modulate the anti-tumour effect of the DC/TC hybrids since it allows the expression of relevant immunostimulatory molecules by appropriate engineering of the fusogenic cell line. Copyright © 2006 John Wiley & Sons, Ltd.
Introduction
Since the discovery of the first tumour-associated antigens (TAA) against which T cell immunity can be directed, many others have been identified in various tumour types 1-3. Tumours do however arise because of their ability to escape from immune surveillance due, among other things, to downregulation of signals required for proper presentation of antigens and induction of tolerance 4. Different strategies to achieve TAA presentation by dendritic cells (DC), the most potent antigen-presenting cells, have been explored 5. These cells have the property to internalise and cross-present antigens through phagocytosis of apoptotic, or even whole tumour cells 6. DC can be pulsed with tumour peptides or whole tumour cell lysates or transduced with TAA-encoding genes 7. Presentation of TAA by MHC class II or class I leads to priming or cross-priming of CD4+ and CD8+ T cells 8.
One promising approach to achieve TAA presentation in an immunogenic context is the generation of hybrids between tumour cells (TC) and DC 9-11. These hybrids combine the expression of a great number of TAA with presentation by MHC class I in the presence of the required co-stimulatory signals 12. Different approaches to TC/DC fusion have been used, the most frequent being polyethylene glycol (PEG) and electrofusion. They have been validated in vitro, where fusion products were shown to induce cytotoxic T-lymphocyte (CTL) activation 13-20, and in animal tumour models in vivo in which they induced an efficient curative and protective immune response 10, 21-32. The safety of hybrid cell vaccination has been shown in clinical trials with some encouraging anti-tumour effects 33-35. However, data are as yet insufficient to assess a clear therapeutic benefit 36-39. For some of these assays hybrids were selected for on HAT medium 10, 22, 23, 29, 40 or enriched by magnetic bead selection 17, 21, 24, 41, 42 or by fluorescence-activated cell sorting (FACS) 18, 27, 28. Purified hybrids were shown to be more efficient immunogens than the bulk of ‘fused’ cells 27, 28. Only in three instances were purified hybrids analysed for the presence of markers of both fusion partners 10, 17, 28. One hybrid between a mastocytoma cell line and mouse DC could be kept in long-term culture but the expression of the different markers was not followed over time 10. On the other hand, hybrids between mouse melanoma B16 and immature, bone-marrow-derived DC had only a very short life span 28, whereas human cell hybrids (Mel5/PBL-derived DC) could be kept in culture for longer periods, but tended to lose the expression of APC markers 17.
Another approach to cell fusion uses the expression of fusogenic viral glycoproteins (FMG). FMG have been previously shown to mediate fusion between neighbouring cells leading to the formation of highly immunogenic syncytia of tumour cells 43 or murine TC/DC hybrids 28. We have explored the possibility to fuse human TC and DC via the FMG of gibbon ape leukaemia virus (GALV), a C-type retrovirus. GALV-FMG targets a distinct surface receptor Pit-1 (formerly Glvr-1) that belongs to the type III sodium-dependent phosphate transporter/retrovirus receptor gene family 44, 45. The Pit-1 receptor is widely expressed in mammalian tissues 46, but is functional for GALV infection in a restricted number of species, including humans. Mouse cells and some hamster cells, like CHO-K1, are resistant to GALV infection 47.
For our experiments, a C-terminally truncated form of GALV-FMG was used, which lacks the 16 amino acid R-peptide of the wild-type protein 48. The R-peptide normally serves to restrict fusion of the viral envelope until it is cleaved during viral infection. This modification renders the protein constitutively highly fusogenic to Pit-1-expressing cells.
We first induced cell fusion by transducing one of the partners, usually the tumour cell, with a plasmid or a viral vector to express the GALV-FMG before the addition of the second partner. Although syncytia could be observed by light microscopy, no hybrids were identified as judged by flow cytometry after labelling with specific markers for each cell type. We have developed a method to circumvent the need for transduction by establishing a stable FMG-expressing cell line that is easily grown to large amounts and that induces fusion between any cells providing that they express the Pit-1 receptor.
Materials and methods
Culture media and cell lines
All culture media (Life Technologies) were supplemented with 10% fetal calf serum (FCS) (PAA), 1% sodium pyruvate and antibiotics (penicillin 50 U/ml and streptomycin 50 µg/ml or gentamycin 50 mg/ml) to give complete medium.
Chinese hamster ovary (CHO-K1) cells and cell lines derived thereof were grown in complete Ham's F-12 medium. The cell line CHO-FMG was established by stable transfection with pCR3.1GALV 43 as described in the Results section.
The human 518.A2 melanoma cell line 49 and derived cells were grown in complete Dulbecco's modified Eagle's medium (DMEM). 518-eGFP cells express enhanced green fluorescent protein (eGFP); they are stably transfected with plasmid pTet-on eGFP neo that was derived from pTet-on eGFP 50. These cells were kept in culture under inducing conditions, i.e. in the presence of doxycycline, 2 µg/ml, with medium changed three times a week. Approximately 90% of cells expressed the transgene as determined by FACS analysis
The 518-EGFR cell line was obtained by stable transfection of 518.A2 cells with expression plasmid pEFIN-EGFR (kind gift from Euroscreen, Belgium) carrying the cDNA for the epidermal growth factor receptor (EGFR) and the neomycin resistance gene (neoR). Cells were selected in G418-containing medium and cloned by limiting dilution.
The HeLa-eGFP cell line, expressing eGFP, was obtained by stable transfection with pTReGFP 51 and was grown in complete DMEM.
Human dendritic cells (DC) were obtained from healthy donors using the following protocol. Peripheral blood mononucleated cells (PBMC) were isolated from buffy coats by gradient centrifugation on Lymphoprep (Nycomed). CD14+ monocytes were positively selected by magnetic cell sorting (MACS, Miltenyi Biotec) using CD14 microbeads (Miltenyi Biotec) and grown in complete RPMI 1640 HEPES containing also 1% L-glutamine, 50 µM mercaptoethanol, 800 U/ml GM-CSF (Leucomax) and 1000 U/ml IL-4 (R&D Systems). Cells were restimulated with cytokines on days 3 and 5. DC were phenotyped by flow cytometry after labelling with antibodies directed against HLA class I markers, HLA-DR, CD1a, CD80, CD86, CD3 and CD14 (Becton Dickinson, San Jose, CA, USA). DC maturation was performed by the addition of poly I : C (Sigma) (10 µg/ml) for 1 day.
Plasmids and vectors
The pCR3.1GALV vector expresses the GALV-FMG under the regulation of the cytomegalovirus (CMV) promoter 43.
AAV2-CMV.GALV.FMG (AAV2-GALV) and AAV5-CMV.GALV.FMG (AAV5-GALV) vectors were constructed and produced at the Vector Core of the University Hospital in Nantes. They also express the GALV-FMG under the control of the CMV promoter. Titres were 3.7 × 109 (AAV2) and 4 × 1010 (AAV5) total particles/ml. 518.A2 cells were most efficiently transduced with AAV serotype 2 (not shown). CHO-K1 cells were infected with AAV serotype 5 52.
Antibodies
Anti-CHO monoclonal antibodies (mAb) are directed against a non-identified determinant of CHO cells. Anti-FMG antibodies were obtained after genetic immunisation, as described 53. The generation of hybridomas and mAb purification have been described 53.
Fusion protocol
CHO-FMG cells were mixed with either tumour cells or DC in different ratios in a 24-well dish. Then, 2 × 105 cells were plated per well unless indicated otherwise and fusion was allowed overnight. The non-fusogenic CHO-K1 parental cell line was substituted for CHO-FMG in negative control co-culture samples. Multinucleated fusion products were detected by light microscopy. For some samples the visualisation of multinucleated cells was facilitated by Diff-Quik staining (Dade Behring), according to the manufacturer's directions.
Transduced cells were fused according to the same protocol, except that one of the fusion partners was either transfected or infected for 5 h, detached by trypsinisation and mixed with the second partner. Transfection was done with FuGene (Roche). For infection, cells were incubated with vector in a small volume (1/10) for 1 h before medium was added and infection was allowed to proceed for another 4 h.
Flow cytometry
Fusion products were analysed for the existence of hybrids by immunofluorescent flow cytometry (FACS). Cells were either labelled before fusion with PKH-26-GL (Sigma), according to the manufacturer's instructions, or after fusion with specific antibodies.
For marking with antibodies, cells were recovered from culture dishes with phosphate-buffered saline (PBS) containing 5 mM EDTA. Subsequent staining and washing steps were performed in PBEN buffer (PBS-EDTA 1 mM, bovine serum albumin (BSA) 0.1% and NaN3 0.1%) in a volume of 100 µl. Analysis of CHO/HeLa cell fusions was done with a primary anti-CHO mAb and a phycoerythrin (PE)-coupled secondary goat anti-mouse-Ig Ab (Biosource, Camarillo, CA, USA). Analysis of CHO/DC fusions was performed with a PE-conjugated mouse anti-HLA-DR Ab (Becton Dickinson) and a biotin-conjugated anti-CHO antibody followed by avidin-fluorescein isothiocyanate (FITC) conjugate (BD Pharmingen). EGFR expression was detected with FITC- or PE-coupled anti-EGFR antibody (Santa Cruz). Triple fusion products (DC/CHO/518EGFR) were labelled with FITC-conjugated anti-EGFR and PE-conjugated anti-HLA-DR.
Flow cytometry was performed on a FACScan (Becton Dickinson) or Cytomics FC500 (Beckman Coulter). Data obtained by FACScan were analysed using the WinMDI 2.6 software.
Confocal microscopy
Fusion was performed on sterile coverslips placed in 6-well dishes. Each well contained a total of 106 cells. After overnight incubation cells were rinsed with PBS and stained as for FACS with the following modifications. The washing and staining buffer was PBS, 0.1 mM CaCl2, 0.1% BSA and 0.1% NaN3. CHO/HeLa fusions were stained with Alexa 594 labelled goat anti-mouse Ig as secondary antibody (Molecular Probes, Leiden, The Netherlands). Cells were subsequently washed twice with washing buffer and once with PBS before fixation with PBS, 4% paraformaldehyde for 2 h. Coverslips were mounted on microscope slides using Vectastain hardset (Vector Laboratories). Imaging was performed on a Leica TCS confocal microscope.
Results
Cell fusion via viral fusogenic glycoproteins is an interesting alternative to more widely used methods like PEG or electrofusion, that are associated with a high mortality of cells 54. Classically, hybrid formation between the two different cell types is monitored by flow cytometry, the two partners being identified either by membrane staining before fusion or by labelling with specific antibodies after fusion.
Double-stained cells in co-culture samples
Data obtained by flow cytometry, for the setting-up of a fusion protocol, showed that simple co-culture of the human 518.A2 melanoma cell line with DC generated a significant background of double-labelled cells. Indeed, both 518.A2 cells stained before co-culture with the membrane stain PKH and 518-EGFR cells labelled with anti-EGFR antibodies after co-culture showed a significant amount of double-stained cells in the absence of any cell fusion agent (Figure 1a). Arguing that this background could be due to phagocytosis of cell debris by DC, we set up the protocol using two types of tumour cells (TC), 518.A2 or 518-EGFR on one hand and 518-eGFP on the other. 518.A2 and 518-EGFR cells were identified by membrane staining with PKH or anti-EGFR antibodies, respectively. The other partner, 518-eGFP, was detected through the cytoplasmic green fluorescence. Co-culture of these cells for 24 h again led to a background of double-stained cells which was very variable from one experiment to the other, ranging from 1% to 9% (average 5%), and which was independent of the staining method used (PKH or anti-EGFR) (Figure 1b).
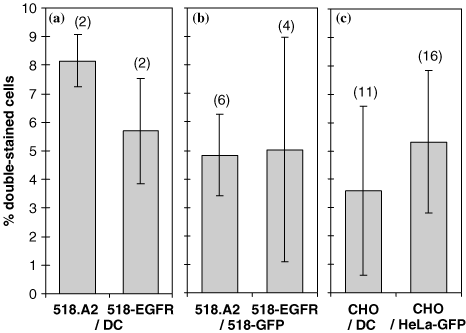
Background of double-stained cells in the absence of fusogenic treatment. Mixtures of cells were analysed by FACS for the presence of markers of each cell type. 518.A2 cells were labelled with PKH before co-culture, 518-EGFR, CHO and DC were stained with the appropriate antibodies after co-culture. HeLa-eGFP cells were detected through their green cytoplasmic fluorescence. Cells were mixed at a ratio of 1 : 1 and incubated overnight. The figures in parentheses indicate the number of experiments
Fusion of GALV-FMG-transduced tumour cells
One of the fusion partners, 518-EGFR, was transiently transfected with plasmid pCR3.1GALV or infected with an AAV2-GALV vector that codes for the gibbon ape leukaemia virus fusogenic membrane protein, GALV-FMG, before being mixed with HeLa-eGFP cells. This did not significantly increase the number of double-stained cells as determined by FACS analysis although syncytia could be detected by light microscopy after 24 h (results not shown). Light microscopy does however not distinguish fusion that occurs among the FMG-expressing population from hybrid formation between different cell types. The former might be favoured by the proximity of cells during division and might decrease the formation of hybrids by withdrawing fusion partners from the pool of FMG-expressing cells. We therefore chose to transduce cells that would not fuse among themselves, such as CHO-K1. These cells are not infected by GALV due to a low level of Pit-1 receptor expression 55.
Transduction of CHO-K1 cells with GALV-FMG
CHO-K1 cells were transiently transfected with the pCR3.1GALV plasmid or infected with AAV5-GALV at a multiplicity of infection (MOI) of 10 infectious particles per cell. After overnight incubation with 518-EGFR cells at a ratio of 1 : 1, the number of double-labelled cells (anti-CHO-PE, anti-EGFR-FITC) was equivalent with non-transduced and transduced CHO (Figure 2a). Similar results had been obtained with pCR3.1GALV-transfected CHO cells and HeLa-eGFP (7.6 ± 3.5% and 6.9 ± 3.4% in co-culture and fusion samples, respectively, for 5 experiments) (results not shown). Light microscopy analysis of Diff-Quik-stained cells showed however some multinucleated cells indicating that hybrids had been formed (Figure 2b). Our results suggest that potential hybrids might go undetected in the background of ‘false positive’ cells. The pool of FMG-expressing cells may be too small or the level of FMG expression too low after transient transduction to allow for efficient hybrid formation. The number of FMG-expressing cells was variable between experiments as determined with anti-FMG antibodies, ranging from 14% to 41% of infected and 17% to 70% of transfected CHO cells after 24 h. Transduction with the control plasmid pCR3.1eGFP yielded similar results. Also, in both AAV-GALV-infected CHO and 518-EGFR, the level of FMG expression varied greatly and was low for most cells (Figure 2c, not shown for 518-EGFR).
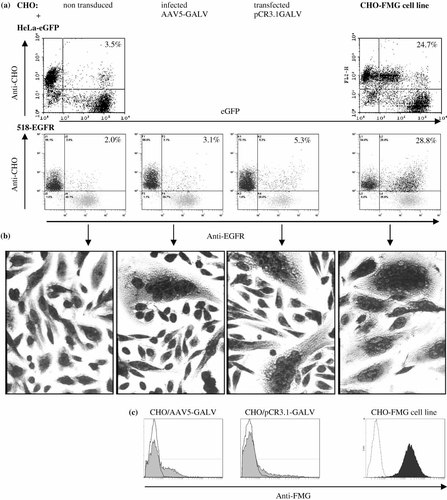
Fusion efficiency of transduced cells compared to the CHO-FMG cell line. CHO cells were transfected with pCR3.1GALV or infected with AAV5-GALV at an MOI of 10 infectious particles per cell. After overnight co-culture with 518-EGFR at a 1 : 1 ratio, cells were labelled with anti-CHO followed by PE-coupled anti-mouse and anti-EGFR-FITC antibodies for flow cytometry (a, 518-EGFR) or stained with Diff-Quik for microscopy (b). HeLa-eGFP and 518-EGFR cells were also fused overnight with the CHO-FMG cell line (a, b, right panels). (c) To evaluate the level of FMG expression in transduced CHO cells and in the CHO-FMG cell line they were stained 24 h after transduction with anti-FMG and PE-conjugated anti-mouse antibodies for flow cytometry. Results are representative of four (AAV5-GALV), three (pCR3.1GALV) and > ten (CHO-FMG) experiments
Establishment of a stable cell line, expressing FMG
To increase the number of FMG-expressing cells, a stable cell line was derived from CHO-K1 by transfection with pCR3.1GALV and selection on G418. Only a small proportion of cells (<6%) in the initial clones were found to express GALV-FMG when analysed by flow cytometry using a murine anti-FMG antiserum. Therefore, FMG-expressing cells were purified by magnetic cell sorting (MACS) with the same anti-FMG antiserum and resulting FMG-positive cells were cloned by limiting dilution. The selected CHO-FMG clone, CHO-FMG 10M6, was more than 95% positive for GALV-FMG expression and the level of expression was about 10-fold higher than for transduced cells (Figure 2c). It will be referred to as ‘CHO-FMG’. FMG expression has been stable over the tested time period of 8 months providing cells are grown in selective medium containing G418. No syncytia formation was observed by light microscopy in the CHO-FMG cultures (data not shown). On the other hand, CHO-FMG cells fused efficiently with Hela-eGFP (Figure 2a) and 518-EGFR cells (Figures 2a and 2b). Indeed almost all cells were involved in syncytia as shown by light microscopy of Diff-Quik-stained cells (Figure 2b). Hybrids involving eGFP-expressing cells displayed at least one log lower eGFP activity than aggregates (Figure 2a, results not shown for 518-eGFP). This allowed us to discriminate double-stained cells generated by co-culture from those resulting from cell fusion (true hybrids) as they are detected in different parts of the dot plots. The staining of fused cells was checked by confocal microscopy (data not shown). In overnight cultures of CHO-FMG and HeLa-eGFP cells syncytia could clearly be seen due to their green cytoplasmic stain. Although their membrane stain could usually not be seen, such syncytia are expected to arise only if at least one CHO-FMG cell is fused with HeLa-eGFP cells. No such cells were found in co-cultures of CHO and HeLa-eGFP cells (data not shown).
Optimisation of CHO-FMG fusion with human TC
CHO-FMG and HeLa-eGFP cells were mixed at different ratios and fusion was allowed to proceed overnight. The percentage of double-stained cells among total cells (Figure 3a) or among tumour cells (Figure 3b) was evaluated from FACS results. Hybrid yields were calculated by subtracting the percentage of double-stained cells in co-culture samples from those in fusion samples. At the optimal ratio of 1 CHO-FMG per HeLa-eGFP hybrids represented around 10% of total cells and 20% of tumour cells were involved in hybrid formation.
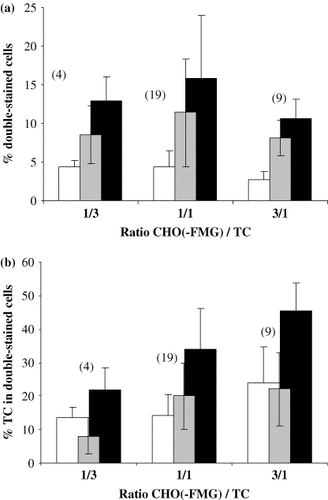
Fusion between CHO-FMG and HeLa-eGFP cells. CHO or CHO-FMG cells were mixed at a ratio of 1 : 3, 1 : 1 or 3 : 1 with HeLa-eGFP cells and incubated overnight. Double-stained cells (PE + eGFP) in co-culture (white bars) and fusion (black bars) samples were detected by FACS. The percentage of hybrids (grey bars) was calculated by subtracting values for co-culture from those for fusion samples. (a) Proportion of double-labelled cells among total. (b) Percentage of HeLa-eGFP cells involved in hybrid formation, calculated as follows: n(PE + eGFP)/n(PE + eGFP) + n(eGFP) where n = number of cells determined by FACS
Fusion of CHO-FMG cells with human DC
Based on the above-described experiments, we fused CHO-FMG cells with human DC. Different ratios of DC to CHO-FMG cells were tested (Figure 4a). Hybrids were labelled with anti-HLA-DR and anti-CHO antibodies and detected by FACS. The number of double-stained cells was always higher in fusion samples containing CHO-FMG as compared to control co-cultures with CHO. In optimal conditions, 3 DC per CHO-FMG cell, 18.4 ( ± 11.5)% of double-stained cells were obtained against a background of 7.6 ( ± 6.3)% of aggregates. This represented an average of 11% of true, FMG-induced hybrids (Figure 4a) which were clearly identified by confocal microscopy through the yellow colour of their membranes (Figure 4b, III).
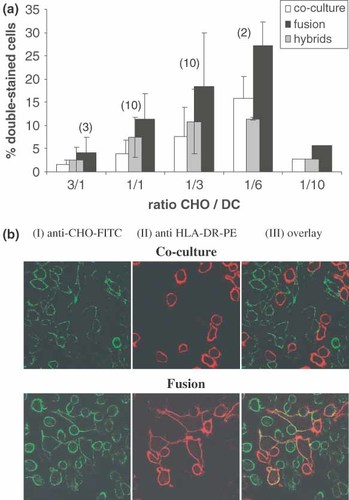
Fusion between CHO-FMG and DC. (a) Cells were mixed at different ratios as indicated, incubated overnight, and stained with a PE-conjugated mouse anti-HLA-DR and a biotinylated mouse anti-CHO antibody followed by avidin-FITC. Hybrid yields were calculated as in Figure 3a. (b) Confocal microscopy of co-culture and fusion samples; magnification: 400×
TC/DC fusion via CHO-FMG
The CHO-FMG model system had allowed generation of hybrids as unambiguously determined by flow cytometry after staining with specific antibodies. We then used the CHO-FMG cell line as an intermediate for TC/DC fusion with the idea that the fusion of a CHO-FMG cell with both TC and DC could lead to hybrid formation. 518-EGFR tumour cells and immature DC (iDC) were mixed at a ratio of 1 : 1 in the presence of different amounts of CHO-FMG or CHO cells. After overnight incubation, the DC and TC fusion partners were stained red with anti-HLA-DR-PE and green with anti-EGFR-FITC antibodies, respectively. The number of double-stained cells was always higher in the presence of CHO-FMG cells as compared to CHO, indicating that TC/DC fusion had indeed occurred (Figures 5 and 6). In order to compare hybrid yields between double (CHO-FMG/TC) and triple (DC/CHO-FMG/TC) fusions, the percentage of hybrids relative to the tumour cell population and not relative to the total cell population was calculated since the latter contains the bulk of CHO-FMG cells. Over 20% of tumour cells were involved in hybrids when twice as many CHO-FMG cells were present (Figure 5b). Hybrid formation was confirmed by light microscopy of Diff-Quik-stained cells (Figure 6b) and by confocal microscopy (Figure 6c). Fusion efficiency was also compared for DC matured on days 5 or 7 and for mature versus immature DC. No significant difference in cell fusion efficiency between cells at different maturation stages could be detected in three independent experiments (data not shown).
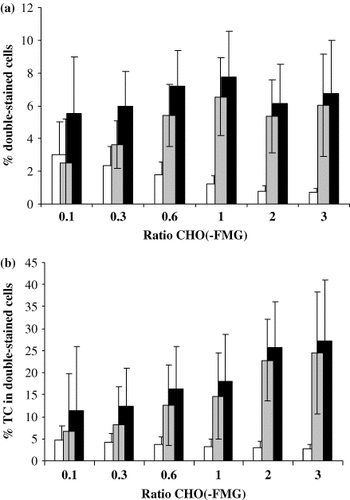
Fusion of 518-EGFR and iDC via CHO-FMG. 518-EGFR and iDC were mixed 105 of each in a 24-well dish and between 104 (ratio 0.1) and 3 × 105 (ratio 3) of CHO or CHO-FMG cells were added. Fusion yields were calculated as in Figure 3. Results are an average from eight experiments
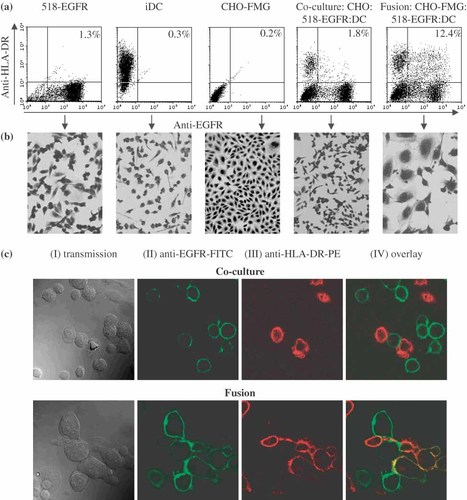
Fusion of 518-EGFR and DC via CHO-FMG. Cells were mixed in a 1 : 1 : 1 ratio and grown overnight. (a) Flow cytometry: cells were stained with FITC-conjugated mouse anti-EGFR and PE-conjugated mouse anti HLA-DR antibodies. (b) Light microscopy of Diff-Quik-stained cells. (c) Confocal microscopy of co-culture and fusion samples; magnification: 400×. The results are from one of the experiments summarised in Figure 5
Discussion
We describe here a novel approach for the generation of hybrids between tumour cells (TC) and dendritic cells (DC), that brings into play a third cell line acting as a ‘fusion agent’. The latter constitutively expresses a fusogenic membrane glycoprotein (FMG) of the gibbon ape leukaemia virus (GALV) and is chosen so as not to be subject to fusion itself. This method has some advantages over conventional methods for the generation of TC/DC hybrids for anti-tumour vaccination, because it can easily be applied to fuse a variety of autologous or allogeneic human TC with autologous or allogeneic DC. Moreover, fusion via a cell line that constitutively expresses GALV-FMG is easy to scale up and it does not require any particular skills or equipment as opposed to PEG or electrofusion 24.
As a model system, we have chosen Chinese ovary hamster (CHO) cells expressing GALV-FMG to promote fusion between an established melanoma cell line and monocyte-derived, human DC. Fusion efficiency between CHO-FMG cells and either DC or TC was in the range of that obtained by electrofusion, around 10% of total cells 56. An average of at least 20% of tumour cells was involved in hybrid formation when equal amounts of CHO-FMG and TC were fused. Interestingly, this yield remained the same when DC were fused to TC via the CHO-FMG cell line at a ratio of 1 : 1 : 2. This yield is probably underestimated because the calculations do not take into account the fact that hybrids are likely to contain more than one tumour cell and because some of the larger hybrids observed by light microscopy may go undetected by flow cytometry given the size limitation inherent to this type of analysis. Indeed, Diff-Quik-stained preparations of DC/CHO-FMG/TC fusions showed a majority of fused cells (Figure 6b), with syncytia containing up to 19 nuclei (median 5). Also, hybrids were easily detected on confocal microscopy pictures, but this method does not allow for quantification.
All fusion methods generate a background of double-stained cells in co-culture controls. These probably represent mainly aggregates 57 and not debris taken up by the other cell partner because they are also observed for fusions that do not involve cells with a capacity for phagocytosis. It is indeed equivalent for fusions among TC only and fusion between TC and DC, at least in our system. It can however not be excluded that some background is generated through uptake of tumour debris. Therefore, fusogenic treatments that induce cell death, like electrofusion or PEG treatment, could be a source of artefacts. Fusion via the expression of a viral protein does not induce significant cell death at least during the periods of time that are required for hybrid formation. Syncytia formed by GALV-FMG were reported to be stable and metabolically as well as transcriptionally active for several days 43. Loss of viability only occurs by day 5 after fusion 58. In our experiments, eGFP activity was more than 10-fold lower in hybrids compared to unfused cells. Given the long half-life of eGFP (∼26 h) 59, downregulation of its expression cannot account for this decrease, which is more likely explained by dilution of the eGFP in a larger cell volume following fusion with CHO-FMG cells.
For FMG fusion to be applicable in clinical trials, either autologous TC transduced with the FMG gene in vitro or an allogeneic FMG-expressing cell line could be fused to autologous DC derived from the patient. The autologous tumour cell approach is likely to be hampered by poor transfection efficiencies of primary tumours. 518-EGFR cells transfected with the pCR3.1GALV plasmid or infected with the AAV-GALV vector expressed low levels of FMG. The number of FMG-expressing cells strongly decreased 48 h after transduction and was no longer detected in three experiments out of four (data not shown). Parallel transduction with the eGFP gene yielded similar expression levels but in a greater number of cells. This expression did not decrease after 48 h (data not shown). The inefficient FMG expression in 518-EGFR cells could be due to the lethal effect of GALV-FMG-induced fusion in susceptible cells. Alternatively, FMG could be sequestered by the Pit1 receptor in the reticulum and therefore not reach the cell surface in these cells. In CHO cells, levels of FMG and eGFP expression were equivalent 24 and 48 h after transduction (data not shown). However, even in one experiment, in which 70% of pCR3.1GALV-transfected CHO cells expressed the FMG, fusion with 518-EGFR cells was less efficient than with the CHO-FMG cell line as judged by light microscopy and no significant hybrid formation was observed by flow cytometry (Figure 2a). This is probably due to the 10-fold lower FMG expression level in transduced cells (Figure 2c).
A threshold number of FMG-expressing cells seem also to be required for efficient fusion. As judged by experiments in which increasing numbers of CHO cells were added to CHO-FMG cells before fusion with HeLa-eGFP, at least 40% of FMG-expressing CHO cells were required to see a clear difference between background and fusion (4% of hybrids). When the proportion of FMG-expressing cells was increased to 80 or 100%, the numbers of hybrids reached 10 and 15%, respectively (results not shown). These results suggest that the number of FMG-expressing cells is a limiting parameter for the generation of hybrids via GALV-FMG. Fusion of VSV-G FMG transfected B16 mouse melanoma also generated only around 4% of hybrids unless Polybrene was added, in which case this figure rose to 38% 28.
The establishment of an allogeneic FMG-expressing human cell line is rendered difficult by the lethality induced by FMG expression. A stable cell line that expresses GALV-FMG under the control of a heat-inducible promoter has been described 60. Although background expression of GALV-FMG was very low at 37 °C, it could compromise the long-term stability of these cells. Results from our attempts at establishing an inducible cell line indicate that even low background expression of FMG can have a deleterious effect (data not shown).
While vaccination with DC/TC hybrids is an interesting approach to ameliorate TAA presentation, it may be insufficient to overcome anergy that is associated in some cases with tumour development 4. The tri-parent hybrid system offers the possibility to modulate the immune response by the addition of appropriate genes to the intermediate, fusogenic, cell line. How the CHO component of the tri-parent hybrid influences the outcome of the immune response has yet to be evaluated. In our model system the CHO-FMG cell line has been chosen as a fusion agent partly because of the ease with which they can be grown in large amounts. It may however be necessary to replace CHO cells by human cells that are not susceptible to GALV-FMG-induced fusion. Potential candidates could be sought among cells that are not infected by GALV 61.
Acknowledgements
We thank Sandrine Hospied, Jacques Leroux and Laurence Torset for technical assistance with antibody production. SCC was supported by Télévie (FNRS) and a grant from the Fondation David et Alice van Buuren (Belgium). CS was supported by a grant from FRIA. We thank the Vector Core of the University Hospital of Nantes, supported by the Association Française contre les Myopathies (AFM), for providing the AAV-GALV vectors. Moreover, we thank Françoise Bex (Laboratoire de Microbiologie, CERIA) for important help with confocal microscopy.




