Replication-dependent transgene expression from a conditionally replicating adenovirus via alternative splicing to a heterologous splice-acceptor site
Abstract
Background
Oncolytic viruses are promising anticancer agents because they selectively kill cancer cells and multiply within a tumor. Their oncolytic potency might be improved by expressing a therapeutic gene from the virus genome. In this regard, proper kinetics and level of transgene expression are important. In addition, expression of cytotoxic transgene products should be confined to cancer cells. Here, we developed oncolytic adenoviruses that provide transgene expression dependent on viral replication.
Methods
We constructed an oncolytic adenovirus that expresses luciferase under regulation of the endogenous major late promoter (MLP) via alternative splicing to an inserted splice-acceptor site analogous to that of the adenovirus serotype 40 long fiber gene. Splicing of the luciferase transcript was studied by RT-PCR analysis. Expression was measured in the presence and absence of the flavonoid apigenin, an inhibitor of viral replication.
Results
The inserted splice-acceptor site was properly recognized by the adenoviral splicing machinery. Luciferase expression levels were markedly higher than levels obtained with the cytomegalovirus (CMV) promoter, especially at late stages of infection. Inhibiting adenovirus replication reduced luciferase expression levels dramatically by 4 to 5 logs, whereas expression levels with the CMV-luciferase adenovirus were only moderately affected (2 logs).
Conclusions
Transgene delivery using the endogenous late gene expression machinery resulted in an expression pattern distinct from expression driven by the conventional CMV promoter. The high expression levels and strict coupling of expression to viral replication should be useful for adequate monitoring of replication and might provide a platform for the design of armed conditionally replicating adenoviruses (CRAds) with enhanced oncolytic potency. Copyright © 2005 John Wiley & Sons, Ltd.
Introduction
Tumor-selective replicating viruses hold promise as novel anticancer agents. Their lytic replication cycle allows for amplification of input virus and spread through the tumor mass. The profound knowledge of adenovirus biology and of the interactions of adenoviruses with their host cells has allowed the design of recombinant adenoviruses that selectively replicate in cancer cells. Three strategies have been followed to construct conditionally replicating adenoviruses (CRAds), i.e., controlling expression of an essential early viral gene by using a tumor-specific promoter 1-4 or a tumor-specific mRNA stabilization element 5 and introducing deletions in viral genes encoding proteins that interact with cellular proteins necessary to complete the viral lytic life cycle in normal cells, but not in tumor cells 6-9. An example of the latter class is the CRAd AdΔ24, also called dl922–947, that contains a deletion in the E1A gene that results in an eight amino acid deletion in the pRb-binding region of the adenoviral E1A protein 7, 9. The replication of this CRAd is attenuated in normal cells, because adenoviral replication depends on the interaction between E1A and pRb to induce S-phase progression via activation of E2F. In cancer cells, the pRb pathway is often disrupted and E2F is constitutively active. In these cells, AdΔ24 replicates at least as efficiently as wild-type adenovirus 7, 9.
Although it has been shown that oncolytic viruses are safe, their clinical benefit has so far been modest urging for development of vectors with enhanced oncolytic efficacy 10. Expression of transgenes by the replication-competent virus can aid this development. In this regard, expression of therapeutic transgenes encoding prodrug-converting enzymes, fusogenic membrane proteins or apoptosis-inducing proteins has already been shown to enhance the oncolytic potency of replicating adenoviruses 11-18. In addition, expression of marker genes facilitates the monitoring of oncolytic viruses in vivo using non-invasive means. For example, transgenes encoding secretory peptides, fluorescent proteins, or enzymes that catalyze emission of light upon addition of substrate, have been used to track virus replication and dissemination in vivo 19-21. It is expected that such studies will accelerate and improve the clinical development of oncolytic virotherapy.
Expression of a transgene from a CRAd can be driven by a heterologous promoter or by an adenoviral promoter, the choice of which has important ramifications for the kinetics and level of transgene expression. As with many DNA viruses, lytic adenovirus infections proceed through two phases, early and late, which are delineated by the onset of viral DNA replication. The timing of expression of the early and late adenoviral genes is tightly regulated on the level of transcription, splicing and mRNA transport out of the nucleus 22. The five adenoviral late genes (L1–L5) are all under control of the major late promoter (MLP) and are spliced from the primary transcript to contain a common 5′-end tripartite leader. Expression of pro-apoptotic or cytotoxic transgenes may require a late expression profile, because it has been shown that induction of cell death before completion of DNA replication or encapsidation can compromise virus production 23. Additionally, restriction of transgene expression to the late phase links expression to viral replication. In the context of expressing a toxic transgene product from a CRAd this should improve the safety profile of the virus by preventing expression in normal cells. Furthermore, replication-dependent expression of a marker gene would lead to a more faithful image of replication of the oncolytic viral agent.
In this paper, we report the construction of a CRAd containing an additional splice-acceptor site in order to allow expression of a transgene under regulation of the endogenous MLP. Expression levels of an inserted luciferase gene were compared to those of control viruses containing luciferase under regulation of the heterologous cytomegalovirus (CMV) promoter.
Materials and methods
Cell lines
A549 lung carcinoma cells, A431 vulva carcinoma cells, SW620 colon carcinoma cells and human embryonic kidney 293 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). T24 bladder carcinoma cells and Saos-2 osteosarcoma cells were obtained from Dr. H. van der Poel (Netherlands Cancer Institute, Amsterdam, The Netherlands) and Dr. F. van Valen (Westfalische Wilhelms-Universität, Munster, Germany), respectively. 911 cells were obtained from IntroGene B.V. (Leiden, The Netherlands). All cell lines were maintained in F12-supplemented Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum (FCS) and antibiotics.
Construction of plasmids
To introduce the splice-acceptor site of the adenovirus type 40 long fiber gene in the deleted E3 region of the Ad5 genome, synthetic oligonucleotides 1 and 2 (see Table 1 for oligonucleotide sequences used in this study) were allowed to anneal and were cloned in pABS.4 (Microbix Biosystems, Toronto, Canada) digested with EcoRI and PstI. The resulting plasmid pABS.4-SA-MCS contains the 32 nucleotide splice-acceptor site, a small multiple cloning site for convenient insertion of transgene cDNA and a polyadenylation (polyA) site (Figure 1A). The cDNA of firefly luciferase was obtained by polymerase chain reaction (PCR) using the pSP-Luc+ vector (Promega, Madison, WI, USA) as template DNA using oligonucleotides 3 and 4, which contain overhanging XbaI and SacI sites, respectively. The PCR product was digested with XbaI and SacI and ligated into pABS.4-SA-MCS digested with the same enzymes to yield pABS.4-SA-Luc. This plasmid was digested with PacI and the fragment containing SA-Luc and the kanamycin resistance gene was inserted into PacI-digested pBHG11 (Microbix Biosystems). A clone with the insert in the orientation that places SA-Luc on the adenovirus R-strand was isolated, and the kanamycin resistance gene was removed by digestion with SwaI followed by self-ligation, yielding pBHG11-SA-Luc. To construct an adenovirus containing the CMV promoter in the deleted E3 region, the human CMV promoter was released from pAdTrack 24 with NheI and BglII and subcloned in pBSK(−) (Stratagene, La Jolla, CA, USA) digested with SpeI and BamHI. Subsequently, this plasmid was digested with XbaI and PstI and the fragment containing the CMV promoter was ligated into pABS.4.SA.MCS digested with XbaI and PstI, thereby replacing the splice-acceptor site with the CMV promoter. This plasmid (pABS.4-CMV-MCS) was used to construct pBHG11-CMV-Luc in a similar way as was done to obtain pBHG11-SA-Luc (see above).
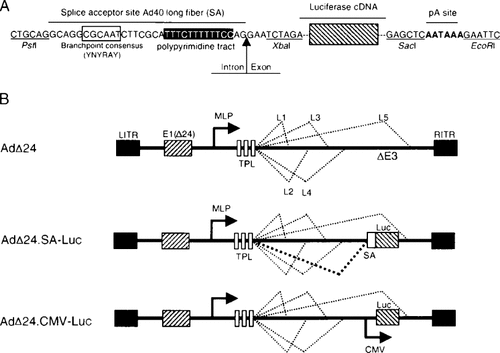
Schematic representation of the synthetic splice-acceptor expression cassette (A) and of the parental CRAd AdΔ24 and the derivative CRAds constructed in this study (B). The major late promoter (MLP) drives the expression of five late genes (L1–L5), of which the mRNA is spliced to a common 5′-end tripartite leader (TPL). To drive luciferase expression in AdΔ24.SA-Luc from the MLP, an expression cassette consisting of a splice acceptor (SA) derived from Ad40 in front of the luciferase cDNA (Luc) and a polyA sequence is inserted in place of the deleted E3 region. In AdΔ24.CMV-Luc, the luciferase gene is under regulation of the CMV promoter
| # | Orientation | Sequence | |
|---|---|---|---|
| 1 | SA | F | GGCAGGCGCAATCTTCGCATTTCTTTTTTCCAGGAATCTAGAGATATCGAGCTCAATAAAG |
| 2 | SA | R | AATTCTTTATTGAGCTCGATATCTCTAGATTCCTGGAAAAAAGAAATGCGAAGATTGCGCCTGCCTGCA |
| 3 | Luciferase | F | GGGTCTAGAGCCACCATGGAAGACGCCAAAAAC |
| 4 | Luciferase | R | CCCGAGCTCCTTACACGGCGATCTTTCCGC |
| 5 | Hexon | F | ATGATGCCGCAGTGGTCTTA |
| 6 | Hexon | R | GTCAAAGTACGTGGAAGCCAT |
| 7 | E1A 13S | F | AATGGCCGCCAGTCTTTT |
| 8 | E1A 13S | R | ACACAGGACTGTAGACAA |
| 9 | TPL | F | TAACCAGTCACAGTCGCAAG |
| 10 | Penton | R | ACATTTGGCATGTTGGTATGC |
| 11 | Luciferase | R | AATAACGCGCCCAACACCG |
Recombinant adenoviruses
CRAds were made by homologous recombination in 293 cells between the pXC1 (Microbix Biosystems) derivative pXC1-Δ24, which carries a 24-bp deletion corresponding to amino acids 122–129 in the CR2 domain of E1A necessary for binding to the Rb protein 7, with pBHG11-SA-Luc, pBHG11-CMV-Luc or pBHG11. This way, the CRAds AdΔ24.SA-Luc, AdΔ24.CMV-Luc and the parental AdΔ24 were generated. The non-replicating control virus Ad.CMV-Luc lacks the E1 and E3 regions and contains an expression cassette in place of the E1 region consisting of the CMV promoter derived from pCEP4 (Invitrogen, Carlsbad, CA, USA) and the luciferase cDNA from pGL3-Basic (Promega) ([ 25]; generously provided by Dr. M. Yamamoto, University of Alabama at Birmingham, AL, USA). The CRAd Ad5-Δ24E3 containing the 24-bp E1A CR2-deletion and a wild-type E3 region was described earlier ([ 26]; generously provided by Dr. K. Suzuki, University of Alabama at Birmingham, AL, USA).
Viruses were plaque purified and propagated on A549 cells. High-titer batches were produced by double CsCl-gradient banding. The E1Δ24 mutation and SA-Luc or CMV-Luc transgene insertion were confirmed by PCR on the final products. Physical virus particle (vp) titers were determined by OD260 measurement. Total vp yields per cell were 10 767 for AdΔ24, 5133 for AdΔ24.SA-Luc and 5200 for AdΔ24.CMV-Luc, respectively. Functional virus titers were determined in two ways, i.e., plaque-forming unit (PFU) titers were determined by limiting-dilution plaque titration on 293 cells according to standard techniques and infectious unit (IU) titers were determined using the Adeno-X rapid titer kit (BD Biosciences Clontech, Palo Alto, CA, USA) on 911 cells. The vp/PFU and vp/IU ratios were 4 and 40 for AdΔ24, 536 and 92 for AdΔ24.SA-Luc, and 11 and 20 for AdΔ24.CMV-Luc, respectively. Infection experiments were normalized on the basis of PFU titers.
Assay for oncolytic activity of CRAds on cancer cells
Infections with viruses were performed in 96-well plates nearly confluent with cells at various multiplicities of infection (MOIs) for 2 h at 37 °C followed by replacement of the infection medium with fresh medium. Two (A549 and SW620) or three (A431) weeks after infection, viability of the cells was determined by a colorimetric WST-1 cell viability assay (Roche Diagnostics, Mannheim, Germany). To this end, the culture medium was replaced by 100 µl of 10% WST-1 in culture medium. Depending on the cell type, the formation of the formazan dye was allowed to proceed for 30–60 min at 37 °C after which the A450 was measured on a Bio-Rad (Hercules, CA, USA) model 550 microplate reader. WST-1 conversion was expressed as a ratio of the conversion by uninfected control cells, after subtraction of background values of WST-1 incubated in the absence of cells.
Western blot analysis
Cells were seeded in 6-well plates at a density of 8 × 105 cells/well. The next day, the cells were infected with AdΔ24 or AdΔ24.SA-Luc at 20 PFU/cell for 1 h and incubated with fresh medium at 37 °C and 5% CO2. The cells were harvested and lysed in 400 µl reporter lysis buffer (Promega) at 12, 24 and 48 h after infection. Lysates were cleared by centrifugation, and protein concentrations were determined using the BCA protein assay kit (Pierce, Rockford, IL, USA). Luciferase activity in the cell lysates infected with AdΔ24.SA-Luc was determined using luciferase assay reagent (Promega). Subsequently, equal amounts (10 µg) of protein were separated on a NuPAGE Novex 10% Bis-Tris gel (Invitrogen) and transferred to a polyvinylidene difluoride membrane (Bio-Rad). Immunoblots were processed according to standard procedures, using primary antibodies for E1A 13S (Santa Cruz Biotechnology, Santa Cruz, CA, USA), fiber (4D2; Lab Vision, Fremont, CA, USA), or β-actin (AC-15; Sigma, St. Louis, MO, USA), followed by anti-IgG-HRPO conjugate (DAKO) and ECL detection reagent (Amersham Biosciences, Piscataway, NJ, USA).
Transfection and adenovirus infections for luciferase activity assay
A549 cells at 50–70% confluence in 96-well plates were transfected with 100 ng pBHG11-SA-Luc or pBHG11-CMV-Luc together with 5 ng pRL-CMV (Promega) using Lipofectamine Plus (Invitrogen) according to the manufacturer's protocol. Two days after transfection, cells were lysed with passive lysis buffer (Promega) and activities of firefly and Renilla luciferase were determined using the dual-luciferase reporter assay system (Promega) according to the manufacturer's protocol. From each sample, the firefly luciferase activity was divided by the Renilla luciferase activity to normalize for differences in transfection efficiency.
Infections with viruses were performed in 96-well plates nearly confluent with cells at a MOI of 20 PFU/cell for 2 h at 37 °C followed by replacement of the infection medium with fresh medium with different concentrations of apigenin. Luciferase activity of cell lysates harvested at indicated time points was determined using the luciferase assay reagent (Promega).
Quantification of adenoviral genomic DNA by real-time PCR
Infection with Ad5-Δ24E3 was performed in 96-well plates nearly confluent with A549 cells at a MOI of 20 PFU/cell for 2 h at 37 °C followed by replacement of the infection medium with fresh medium with 0, 25, 50, or 75 µM apigenin. Thirty-two hours after infection, cells were lysed in 50 µl reporter lysis buffer (Promega) followed by four freeze/thaw cycles. The lysate was treated with proteinase K to remove virion protein components that interfere with the PCR reaction. A real-time PCR was performed with 2 µl of ten-times diluted lysate using the reaction mix of the Lightcycler FastStart DNA Master SYBR Green I (Roche, Indianapolis, IN, USA) according to the manufacturer's protocol. Thermal cycling conditions were one denaturation step at 95 °C for 10 min, followed by 40 cycles of 95 °C 0 s, 55 °C 10 s and 72 °C 14 s. After each cycle, fluorescence was measured and from this data the crossing point was determined. Primers 5 and 6 were used that are specific for the hexon region in the adenoviral DNA. A standard curve for quantification of copy numbers of unknown samples was made using known amounts of the plasmid pBHG11. The PCR was carried out using a LightCycler system (Roche). Data was analyzed with LightCycler software and plotted as viral DNA copies divided by the number of cells originally infected.
Quantification of adenoviral mRNA by quantitative RT-PCR
Nearly confluent A549 cells in 24-well plates were infected with AdΔ24.SA-Luc at a MOI of 20 PFU/cell for 2 h at 37 °C, followed by replacement of the infection medium with fresh medium. At indicated time points total RNA was isolated using TRIZOL reagent (Invitrogen). RNA was treated with DNAse followed by purification with the RNeasy kit (Qiagen, Valencia, CA, USA). One microgram of purified total RNA was used to obtain cDNA using the Cloned AMV first-strand synthesis kit (Invitrogen) with Oligo(dT) 20 primers following the manufacturer's instructions. This cDNA was diluted 50 times and a volume of 2 µl was used in the reaction mix of the Lightcycler FastStart DNA Master SYBR Green I kit (Roche) according to the manufacturer's protocol. For detection of E1A 13S, primers 7 and 8 were used. Primer 8 is designed to anneal to the splice junction specific for the 13S isoform. For detection of penton mRNA and luciferase mRNA, the same forward primer (primer 9) was used that anneals to a sequence in the tripartite leader, whereas the reverse primers were specific for the penton-coding sequence (primer 10) and luciferase-coding sequence (primer 11), respectively. To calculate relative mRNA levels, for each primer combination a standard curve was made by serial dilution of one cDNA sample. For this purpose, the 8 h post-infection sample was used for the E1A PCR and the 48 h post-infection sample was used for the penton and luciferase PCR. The values obtained were normalized by arbitrarily setting the E1A mRNA level at 8 h post-infection to 1 and setting the penton and luciferase values at 24 h post-infection to 1.
Results
Construction of a CRAd expressing firefly luciferase via an exogenous splice acceptor
To allow regulation of a transgene by the endogenous MLP, we made use of the genome organization found in human subgroup F adenoviruses (Ad40 and Ad41). These viruses naturally express two fiber genes (long and short) instead of the one fiber gene found in most adenoviruses 27. The splice-acceptor site derived from the down-stream long fiber gene of Ad40 (SA) was placed in front of firefly luciferase cDNA, followed by a polyA site (Figure 1A). This cassette was placed directly upstream of the fiber gene in the deleted E3 region of the CRAd AdΔ24 11 to form AdΔ24.SA-Luc. Due to the introduction of the exogenous splice acceptor, it is expected that the luciferase cassette will be spliced onto the tripartite leader exons of the major late transcript (Figure 1B). In addition, we made an AdΔ24.CMV-Luc control CRAd that contains luciferase under the control of the CMV promoter inserted at the same locale. Figure 1B shows a schematic overview of the viruses constructed in this study.
Oncolytic activity of AdΔ24.SA-Luc
To analyze the ability of the CRAds to replicate in and lyse tumor cells, A549, SW620 and A431 cells were infected with AdΔ24, AdΔ24.SA-Luc and AdΔ24.CMV-Luc at different MOIs. The cells were subsequently cultured in vitro to allow lateral spread of viral progeny through the cell monolayer by multiple rounds of infection before cell viability was determined (Figure 2). All three CRAds caused dose-dependent cell death, with AdΔ24.SA-Luc and AdΔ24.CMV-Luc being slightly more effective than AdΔ24 on SW620 and A431 cells, but not on A549 cells. This suggested that introduction of the splice-acceptor site from the Ad40L fiber gene into the genome of an Ad5-derived CRAd did not decrease its oncolytic activity.
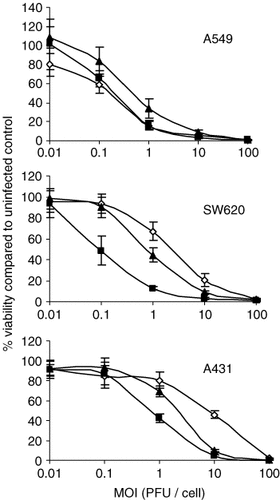
Oncolytic potency of AdΔ24.SA-Luc (closed squares) compared to parental virus AdΔ24 (open diamonds) and AdΔ24.CMV-Luc (closed triangles). A549, SW620 and A431 cells were infected at indicated multiplicities of infection (PFU/cell). Two (A549 and SW620) or three (A431) weeks after infection cell viability was measured using a WST-1 conversion assay and compared with the viability of uninfected control cultures. Data are the mean values ± SD from a representative experiment performed in quadruplicate
Kinetics and level of AdΔ24.SA-Luc transgene expression
The kinetics of luciferase transgene expression from AdΔ24.SA-Luc was determined and compared with expression kinetics of the early E1A gene and the late fiber gene. A549 and SW620 cells were infected with AdΔ24.SA-Luc or parental AdΔ24 and cell extracts were collected at different time points after infection. Western blot analysis was performed using antibodies detecting E1A 13S and fiber (Figure 3A). Despite the introduction of an additional splice-acceptor site immediately upstream of the L5 transcript encoding the fiber protein, the level of fiber expression by AdΔ24 and AdΔ24.SA-Luc was comparable. Expression of E1A reached maximal levels earlier from AdΔ24.SA-Luc than from AdΔ24. Significant levels of the early E1A protein were present at 12 h post-infection in cells infected with AdΔ24.SA-Luc, while levels of the late fiber protein were still undetectable. The luciferase activity in these cell extracts mirrored the expression pattern of the fiber protein with levels less than 0.5% at 12 h post-infection compared to the levels present at 24 h post-infection (Figure 3B).
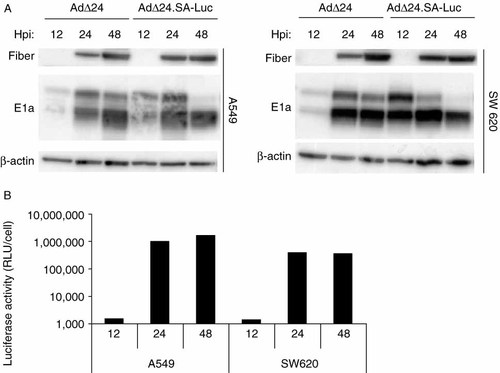
Western blot analysis and luciferase activity in cell extracts of A549 or SW620 cells infected with AdΔ24 or AdΔ24.SA-Luc Luc at 20 PFU/cell and harvested at different hours post-infection (hpi). (A) Blots were probed with antibodies detecting E1A 13S, fiber and the loading control β-actin. (B) Luciferase activity in the cell extracts of cells infected with AdΔ24.SA-Luc was measured and plotted as relative light units (RLU) per cell
Correct use of the inserted splice-acceptor site was verified using RT-PCR on RNA isolated from A549 lung cancer cells at different times after infection with AdΔ24.SA-Luc. The PCR reaction on the cDNA was performed using a forward primer specific for the tripartite leader and a reverse primer specific for luciferase. As shown in Figure 4A, a PCR product of the expected length was obtained, indicating proper splicing to the inserted splice-acceptor site. Band intensity increased from 8 to 24 h post-infection corroborating the kinetics observed with the luciferase activity measurements. To quantify the amount of luciferase mRNA, real-time RT-PCR was performed. The amount was compared to mRNA amounts of the immediate early E1A gene and the late penton gene in the same sample (Figure 4B). At 8 h post-infection the E1A mRNA level was higher than at later time points. In contrast, at 8 h post-infection, penton and luciferase mRNA levels were only 0.1% and 1% from their levels at 24 h post-infection, respectively. These results indicate that luciferase expression followed the pattern of the late penton gene and confirm the results of the Western blot analysis.
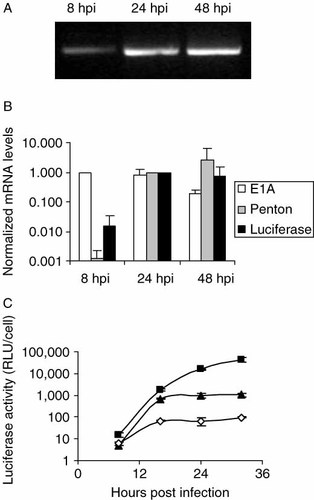
Kinetics and efficiency of transgene expression from AdΔ24.SA-Luc measured by quantitative RT-PCR and luciferase enzyme activity. (A) RT-PCR of RNA isolated at different hours post-infection (hpi) from A549 cells infected with AdΔ24.SA-Luc at 20 PFU/cell. The cDNA was used as template for a PCR reaction using a forward primer specific for the tripartite leader and a reverse primer specific for luciferase, resulting in a specific amplification product of 325 bp. (B) Quantitative RT-PCR on RNA isolated as in (A) with splice-specific primers for E1A 13S, penton base and luciferase. Data shown represents the average of two independent experiments performed in duplo + SD. (C) A549 cells were infected with AdΔ24.SA-Luc (closed squares), AdΔ24.CMV-Luc (closed triangles) or Ad.CMV-Luc (open diamonds) at 20 PFU/cell and luciferase activities were determined at different time points. Data shown is the mean ± SD from a representative experiment performed in triplicate
Next, transgene expression levels from AdΔ24.SA-Luc were compared to those of AdΔ24.CMV-Luc and of the replication-deficient adenovirus vector Ad.CMV-Luc 25. A549 cells were infected with these viruses and the luciferase activity was determined at several time points after infection (Figure 4C). Early after infection, luciferase expression from all three viruses was low. At 16 h post-infection, replication-defective virus Ad.CMV-Luc had reached maximal transgene expression. The CRAd AdΔ24.CMV-Luc exhibited a similar expression profile, but reached a higher expression level than Ad.CMV-Luc, which may be due to template amplification. Strikingly, luciferase expression by AdΔ24.SA-Luc continued to rise over the duration of the experiment, reaching at least 50-fold higher levels than AdΔ24.CMV-Luc at 32 h post-infection. Hence, MLP-driven luciferase expression was particularly high during late stages of infection.
AdΔ24.SA-Luc transgene expression is dependent on viral replication
A prerequisite for the expression of adenoviral late-phase genes is the occurrence of virus DNA synthesis, as was shown in studies using inhibitors of adenoviral replication 28. We sought to determine whether this was also true for luciferase expression by AdΔ24.SA-Luc. This was done in two ways. First, we transfected A549 cells with the pBHG11 plasmids containing SA-Luc or CMV-Luc expression cassettes that were used to construct the AdΔ24.SA-Luc and AdΔ24.CMV-Luc viruses, respectively. They contain a nearly full-length adenovirus genome except for a deletion from map units 0.5–3.7 encompassing the E1A region 29. In the absence of E1A complementation DNA replication cannot occur. Under this circumstance, luciferase expression from SA-Luc was approximately 20-fold lower than from CMV-Luc (Figure 5A).
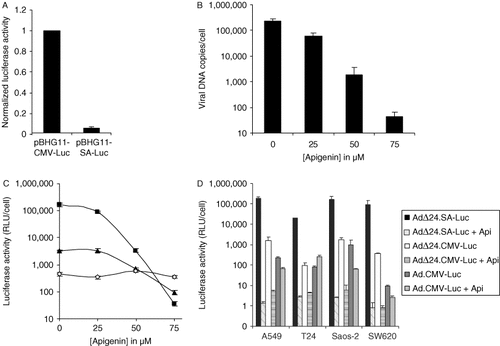
Influence of adenoviral replication on the transgene expression from the CRAds. (A) Luciferase activity from the splice-acceptor site cloned in the near full-length adenoviral plasmid pBHG11. A549 cells were transfected with 100 ng of pBHG11-SA-Luc or pBHG11-CMV-Luc and luciferase values were determined 48 h after transfection. Values were normalized for transfection differences by co-transfection with 5 ng of a plasmid encoding Renilla luciferase. The value obtained with pBHG11-CMV-Luc was arbitrarily set to 1. Data shown is the average of four independent experiments each performed in triplicate. The error bar indicates the standard deviation. (B) Effect of apigenin on adenoviral replication. A549 cells were infected with Ad5-Δ24E3 at 20 PFU/cell and cultured in the presence of increasing amounts of apigenin. Thirty-two hours after infection, the cells were lysed and the number of adenoviral genomes was determined using real-time PCR with primers specific for the hexon region. Data shown represent the average of two independent experiments performed in duplicate. The error bars indicate the standard deviation. (C) Effect of apigenin on transgene expression in A549 cells that were infected with AdΔ24.SA-luc (closed squares), AdΔ24.CMV-Luc (closed triangles) or Ad.CMV-Luc (open diamonds) at 20 PFU/cell. After infection, cells were cultured in the presence of increasing concentrations of apigenin. Thirty-two hours after infection cells were lysed and the luciferase activities determined. Data are the mean values ± SD from a representative experiment performed in triplicate. (D) Effect of apigenin on transgene expression in several cancer cell lines. A549, T24, Saos-2 and SW620 cells were infected at 20 PFU/cell and cultured without apigenin or with 75 µM apigenin (+Api). Thirty-two hours after infection cells were lysed and the luciferase activities determined. Data are the mean values ± SD from a representative experiment performed in triplicate
Second, we compared expression levels from AdΔ24.SA-Luc and AdΔ24.CMV-Luc in the presence or absence of an inhibitor of adenoviral replication. Apigenin is a potent cytostatic agent that in human cancer cells induces a G2/M cell-cycle arrest, presumably by inhibiting the activity of casein kinase II 30-33. Because adenoviral replication relies on progression of host cells through the S-phase we anticipated that apigenin would inhibit adenoviral replication. To test this, we infected A549 cells with the CRAd Ad5-Δ24E3 26 and treated the cells with increasing concentrations of apigenin. The virus was allowed to replicate for 32 h after which the cells were harvested and the amount of adenovirus genomes was determined by quantitative PCR (Figure 5B). This experiment showed that apigenin indeed potently inhibited CRAd replication in a concentration-dependent manner, reducing adenovirus DNA copy numbers between 3 and 4 logs at 75 µM. Next, A549 cells were infected with AdΔ24.SA-Luc, AdΔ24.CMV-Luc or Ad.CMV-Luc in the presence of increasing amounts of apigenin. Apigenin reduced transgene expression of both CRAds, whereas transgene expression of the replication-defective virus was unaffected (Figure 5C). The latter strongly suggested that apigenin did not affect luciferase expression per se. A striking difference was found between the apigenin-mediated reduction of luciferase expression from AdΔ24.SA-Luc and AdΔ24.CMV-Luc. At 75 µM, apigenin reduced transgene expression by AdΔ24.SA-Luc approximately 4600-fold, whereas transgene expression by AdΔ24.CMV-Luc was reduced only 35-fold. These results were confirmed on three other cell lines (Figure 5D). In all cell lines, the expression of luciferase from AdΔ24.SA-Luc was markedly higher compared to control viruses expressing luciferase from the strong CMV promoter. However, in the presence of apigenin, absolute levels expressed by AdΔ24.SA-Luc dramatically decreased to levels below those of the control viruses. Expression from AdΔ24.SA-Luc was highly sensitive to apigenin with reductions of 4 to 5 logs, whereas luciferase expression by AdΔ24.CMV-Luc was reduced by approximately 2 logs, and expression from non-replicating Ad.CMV-Luc was hardly affected by apigenin in most cell lines (Figure 5D).
Discussion
Transgene expression by oncolytic viruses can be used to monitor lytic replication and to improve the oncolytic efficacy. In this study, we designed a CRAd that expresses a transgene driven by the endogenous MLP via alternative splicing to an introduced splice-acceptor site. This CRAd expressed high levels of transgene, exceeding expression levels from a control CRAd containing the strong CMV promoter during late stages of infection. Most importantly, transgene expression from the endogenous MLP was tightly coupled to the replication of the virus.
CRAds derive their tumor-specificity from mutations in the viral genome that lead to attenuation of viral replication in non-cancerous cells. Therefore, CRAds with the transgene under control of the MLP will show reduced expression in non-tumor cells and this may reduce toxicity caused by the transgene. Transgene toxicity is a particular concern upon systemic virus delivery. Notably, high liver toxicity was already reported following systemic infusion of replication-deficient adenoviral vectors carrying prodrug-converting enzymes under control of a constitutive promoter 34-36. In addition, even intratumoral or loco-regional administration of adenoviruses can cause liver damage. In this regard, intraprostatic administration of a replication-competent adenovirus expressing a CD/TK fusion protein from the constitutively active CMV promoter caused a mild liver and gall bladder inflammation 37. These symptoms were exacerbated by double prodrug therapy, suggesting that prodrug conversion occurring in non-target tissue caused the toxicity.
For the expression of pro-apoptotic or cytotoxic transgenes, it may be important to restrict transgene expression to the late phase of the virus life cycle, because induction of cell death before completion of DNA replication or encapsidation may compromise virus production 23. In this context, low levels of transgene expression early after infection may be more important than obtaining high levels of transgene expression late after infection. The levels of transgene expression obtained with the AdΔ24.SA-Luc virus were much lower (i.e., 3 to 4 logs) at an early time point (8 h) compared to a late time point (32 h). However, expression was not silent at the early time point, suggesting that AdΔ24.SA-Luc may not be suitable to deliver highly toxic transgenes. The new CRAd seems more useful for transgenes that require high expression levels to exert their therapeutic effect and for monitoring purposes.
Two different approaches to obtain transgene expression that follows the kinetics of adenoviral genes have been reported previously, i.e., replacement of non-essential genes harbored in the E3 region, thereby retaining native transcriptional regulators 38-41, and fusion to adenoviral genes via introduction of an internal ribosome entry site (IRES) 12, 42, 43. In several of these studies, the level of expression obtained was compared to that of a non-replicating adenovirus expressing the same gene under the CMV promoter. Comparison with our results is possible because the same cell line, comparable MOIs and comparable time points after infection to measure expression were used. Expression by replacing genes in the E3 region was approximately 2-, 3- and 10-fold higher than of the non-replicating virus when the transgene was placed in the ADP, 6.7K/gp19K and E3B region, respectively 38-40. Expression via an IRES placed after E1A was similar to expression of a non-replicating control virus, while placing the IRES after the E2B and fiber genes resulted in 7-fold and 27-fold higher expression levels, respectively 43. In the present study, using the splice-acceptor site of the Ad40 long-fiber gene to allow transgene expression from the native MLP, we found levels approximately 400-fold higher than the non-replicating control virus at late time points. Recently, a very similar way to drive transgene expression from a replication-competent adenovirus as described herein was reported 42. In that study, the splice acceptor of the Ad41 long-fiber gene was used. Strikingly, it was found that expression was inferior compared to expression from an IRES behind the fiber gene. This suboptimal functioning of the splice-acceptor site appears to contrast our results, where expression levels exceeded even those of a CRAd containing the strong CMV promoter. The major difference in the virus design between the two studies is the insertion site for the expression cassette. We placed the transgene upstream of the fiber gene, while Fuerer and et al. inserted it downstream of the fiber gene. The exact position of the splice-acceptor site in the genome may thus be an important determinant for efficient splicing.
Taken together, we have constructed a CRAd backbone that allows high-level, replication-dependent transgene expression. Our findings in vitro encourage the use of this virus in an in vivo tumor xenograft model testing for tumor-selective expression. This backbone might find utility in designing armed CRAds expressing therapeutic transgenes. In addition, the CRAd expressing luciferase made herein can be used to accurately monitor virus replication using bioluminescence imaging. It could, therefore, be exploited in optimizing CRAds for tumor-selective replication.
Acknowledgements
We thank Dr. M. Yamamoto for providing Ad.CMV-Luc, Dr. K. Suzuki for Ad5-Δ24E3 and Jeroen Mastenbroek for expert technical assistance. This research was supported by grants from the VU University Stimulation Fund (USF), the Pasman Foundation, the Schumacher-Kramer Foundation and by a research fellowship of the Royal Netherlands Academy of Arts and Sciences (KNAW) to Victor van Beusechem.




