Rad51 siRNA delivered by HVJ envelope vector enhances the anti-cancer effect of cisplatin
Abstract
Background
Every cancer therapy appears to be transiently effective for cancer regression, but cancers gradually transform to be resistant to the therapy. Cancers also develop machineries to resist chemotherapy. Short interfering RNA (siRNA) has been evaluated as an attractive and effective tool for suppressing a target protein by specifically digesting its mRNA. Suppression of the machineries using siRNA may enhance the sensitivity to chemotherapy in cancers when combined with an effective delivery system.
Methods
To enhance the anti-cancer effect of chemotherapy, we transferred siRNA against Rad51 into various human cancer cells using the HVJ (hemagglutinating virus of Japan, Sendai virus) envelope vector in the presence or absence of cis-diamminedichloroplatinum(II) (CDDP, cisplatin). The inhibition of cell growth was assessed by a modified MTT assay, counting cell number, or fluorescence-activated cell sorting (FACS) analysis after Annexin V labeling. The synthetic Rad51 siRNA was also introduced into subcutaneous tumor masses of HeLa cells in SCID mice with or without intraperitoneal injection of CDDP, and tumor growth was monitored.
Results
When synthetic Rad51 siRNA was delivered into HeLa cells using the HVJ envelope vector, no Rad51 transcripts were detected on day 2, and Rad51 protein completely disappeared for 4 days after siRNA transfer. When HeLa cells were incubated with 0.02 µg/ml CDDP for 3 h after siRNA transfer, the number of colonies decreased to approximately 10% of that with scrambled siRNA. The sensitivity to CDDP was enhanced in various human cancer cells, but not in normal human fibroblasts. When Rad51 siRNA was delivered into tumors using the HVJ envelope vector, the Rad51 transcript level was reduced to approximately 25%. Rad51 siRNA combined with CDDP significantly inhibited tumor growth when compared to siRNA or CDDP alone.
Conclusions
Rad51 siRNA could enhance the sensitivity to CDDP in cancer cells both in vitro and in vivo. Our results suggest that the combination of CDDP and Rad51 siRNA will be an effective anti-cancer protocol. Copyright © 2005 John Wiley & Sons, Ltd.
Introduction
Although many different therapeutic strategies or regimens have been developed, there is no definitive treatment for cancer. Every cancer therapy appears to be transiently effective for cancer regression, but cancers gradually transform to be resistant to the therapy. Although strategies have been developed to reverse the resistance, cancer cells develop mechanisms to escape the immune system and anti-neoplastic treatments 1-3. cis-Diamminedichloroplatinum(II) (CDDP) is one of the most widely used anti-cancer drugs 4-6. CDDP inhibits cellular growth by inducing DNA double-strand breaks 7-9. However, cells can use DNA repair machinery to respond to the DNA damage. The levels of DNA repair proteins correlate with resistance to anti-cancer drugs, especially alkylating agents, in human cancer cell lines 10. Two pathways, homologous recombination and non-homologous end joining, are used to repair DNA double-strand breaks 11, 12. BRCA 1 and 2 in a complex with Rad51 are involved in homologous recombination 11-13. Non-homologous repair is performed by the complex of NBS1, MRE11, and Rad50 with the aid of Ku 70, Ku 80, the DNA-dependent protein kinase catalytic subunit, DNA ligase IV, and XRCC4 11, 14. Different studies have drawn conflicting conclusions regarding the pathway used to repair CDDP-induced DNA double-strand breaks in mammalian cells. Initially, non-homologous end joining was believed to responsible for the repair of CDDP-induced DNA damage 15-17. However, CDDP sensitivity was not affected by the level of the Ku70, which is needed for non-homologous end joining repair 18. However, sensitivity to other DNA-damaging agents, such as bleomycin and methyl methanesulfonate, was elevated by suppression of Ku70 18. These findings suggest that non-homologous end joining is not used to repair DNA damage induced by CDDP. Recent evidence suggests that homologous recombination is involved in the repair of DNA double-strand breaks generated by CDDP 19-21. Cancer cells may become resistant to CDDP by increasing the activity of homologous recombination repair machinery. Indeed, a high level of Rad51 is consistent with tumor progression and tumor resistance to cancer therapy 22. Conversely, disabling the DNA repair machinery may enhance the sensitivity of cancers to CDDP.
The present study focuses on the function of Rad51 as a regulator of CDDP sensitivity. We tested the ability of short interfering RNA (siRNA) to inhibit the expression of Rad51. siRNA has been evaluated as an attractive and effective tool for suppressing the target protein by specifically digesting its mRNA 23, 24. siRNA is superior to antisense oligonucleotides and ribozymes in terms of efficiency and specificity 25, 26. However, finding a suitable delivery system for siRNA has been problematic 27. We have been developing a highly efficient gene delivery system with minimum toxicity by converting viruses into non-viral vectors. We incorporated plasmid DNA into inactivated HVJ (hemagglutinating virus of Japan, Sendai virus) particles to form a HVJ envelope vector. By the strong fusion activity, DNA inside the envelope vector can be directly introduced into the cytoplasm of various types of cells both in vitro and in vivo. The HVJ envelope vector is also very effective for drug delivery 28, 29. siRNA was successfully introduced into pancreatic islet cell lines using the HVJ envelope vector 30. In the present study, siRNA against human Rad51 enhanced the sensitivity of cancers to CDDP both in vitro and in vivo.
Materials and methods
HVJ
HVJ was amplified in chorioallantoic fluid of 10- to 14-day-old chick eggs and was purified by centrifugation and inactivated by UV irradiation (99 mJ/cm2) as previously described 28. Inactivated virus cannot replicate, but its capacity for viral fusion remains intact.
Cell culture
Human cancer cells and normal human diploid fibroblasts (NHDF) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics.
Rad51 cDNA transfer and cell survival assay
The Rad51 open reading frame sequence was subcloned into the expression vector using the Gateway system (Invitrogen, San Diego, CA, USA), amplified, and transfected into HeLa cells (3 × 105 cells) using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. The next day, the cells were passaged in 12-well plates (2 × 104 cells/well). Forty-eight hours after transfection, the cells were treated with 0–4 µg/ml CDDP (Nihon Kayaku, Tokyo, Japan) for 3 h. Then, 48 h later, cell survival was assessed by a modified MTT assay (Dojindo, Tokyo, Japan) as described elsewhere 31.
HVJ envelope vector-mediated siRNA transfection in vitro
An inactivated HVJ suspension (6 × 109 particles) was mixed with 60 µl of 40 µM Rad51 siRNA (5′-GAGCUUGACAAACUACUUC-3′) solution (Dharmacon, Lafayette, CO, USA) and 6 µl of 2% Triton X-100. Scrambled siRNA (5′-GCGCGCUUUGUAGGATTCG-3′) solution (Dharmacon) was used as a control. After centrifugation (18 500 g, 15 min) at 4 °C, the supernatant was removed and HVJ envelope vector that included siRNA was suspended in 120 µl of phosphate-buffered saline (PBS). The incorporation rate of siRNA was approximately 20% of total siRNA initially used. Unincorporated siRNA was reduced to an undetectable level by this process. For in vitro transfection of HVJ that contained siRNA, 1 × 105 cancer cells were seeded in 6-well plates 1 day before transfection. Protamine sulfate (5 µl, 5 mg/ml; Nacalai Tesque, Kyoto, Japan) and 500 µl of medium were added to 20 µl (1 × 109 particles) of HVJ that contained siRNA. Approximately 80 pmol siRNA were delivered to 1 × 105 cells. The cell culture medium was removed, and the HVJ envelope vector was added to each well. Thirty minutes later, the medium containing the vector was replaced with fresh medium.
Western blot analysis
The harvested human cancer cells were lysed in lysis buffer (1% SDS, 20 mM Tris-HCl (pH 8), 135 mM NaCl, 10% glycerol, and a protease inhibitor mixture (Roche, Basel, Switzerland)). After adding 2× sample buffer (0.1 M Tris-HCl (pH 6.8), 4% SDS, 12% 2-mercaptoethanol, 20% glycerol, and 0.01% bromphenol blue), 30 µg of protein were separated by 10% sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% skim milk and subsequently probed with antibodies, anti-human Rad51 (Santa Cruz, Santa Cruz, CA, USA), anti-β-actin (Abcam, Cambrige, UK), and anti-GAPDH (Ambion, Austin, TX, USA). Proteins were detected with horseradish peroxidase labeled anti-goat (Santa Cruz) or anti-mouse (Amersham, Piscataway, NJ, USA) antibodies and the enhanced chemiluminescence reagent (Amersham).
Northern blot analysis
Total RNA was isolated from HeLa cells using ISOGEN (Nippon Gene, Toyama, Japan) according to the manufacturer's instructions. Total RNA (15 µg/lane) was separated in a formaldehyde/1.5% agarose gel, transferred to Hybond N+ membrane (Amersham), and then hybridized with 32P-labeled Rad51 and G3PDH cDNA probes.
Colony forming assay
Twenty-four hours after HVJ envelope vector-mediated siRNA transfection to HeLa cells in vitro, the cells were seeded in a 6-cm dish at a density of 103 cells/dish and treated with 0–0.1 µg/ml CDDP for 3 h. After 7 days, the colonies were fixed with methanol and stained with Giemsa (Nacalai Tesque). Then, the colonies were counted. The percentage of colony-forming cells after CDDP treatment was calculated and compared to the untreated control group.
CDDP sensitivity in cultured cells by Rad51 siRNA transfer
Forty-eight hours after transfer of siRNA, the cells were treated with 0.1, 0.3 and 1.0 µg/ml CDDP for 3 h. Then, 48 h later, cell number was counted using a particle counter (Coulter Corporation, Miami, FL, USA). To assess apoptosis, cells treated with Rad51 siRNA and CDDP were harvested and stained with fluorescent isothiocyanate-labeled Annexin V (Becton Dickinson, San Diego, CA, USA) for 20 min at room temperature. The labeled cells were analyzed with FACScan (Becton Dickinson).
In vivo experiments
Viable HeLa cells (5 × 106 cells) were resuspended in 100 µl of PBS and intradermally injected into the right flanks of 6-week-old male SCID mice (Charles River Japan, Yokohama, Japan). The inactivated HVJ suspension (6 × 109 particles) was mixed with 60 µl of 250 µM Rad51 siRNA solution and 6 µl of 2% Triton X-100. Scrambled siRNA solution was used as a control. After centrifugation (18 500 g, 15 min) at 4 °C, the supernatant was removed and the HVJ envelope vector containing siRNA was suspended in 120 µl of PBS. Seven days after tumor inoculation, 100 µl (5 × 109 particles) of HVJ envelope vector containing siRNA were injected into the tumor. Approximately 2.5 nmol siRNA were delivered to the tumor mass in a mouse. The injection was repeated at 2-day intervals until each mouse received a total of three injections. At the time of the second siRNA injection, 200 µg of CDDP were intraperitoneally injected. Tumor size was measured every 2 days, and the tumor volume was calculated using the simplified formula for a rotational ellipse (l × w2 × 0.5). All animals were treated in a humane fashion in accordance with the guidelines of the Animal Committee of Osaka University.
Results
To determine what factors induced by CDDP contribute to the repair of DNA damage, we examined the gene expression of repair genes in cells treated with CDDP. The protein level of Rad51, which is involved in homologous recombination repair, increased 1.57 ± 0.4 times more with CDDP than that without CDDP (data not shown). However, the expression level of Ku70, which is involved in non-homologous end joining, was not changed (0.9 ± 0.3 times) by CDDP treatment.
We examined whether Rad51 expression resulted in resistance to CDDP. To increase the expression of Rad51, HeLa cells were transfected with the human Rad51 gene driven by the cytomegalovirus (CMV) promoter (Figure 1A). When cell proliferation was measured by a modified MTT assay, Rad51-transfected HeLa cells cultured with various concentrations of CDDP were more viable than control cells that had undergone only a mock transfection (Figure 1B). The experiment was repeated three times, and similar results were obtained.
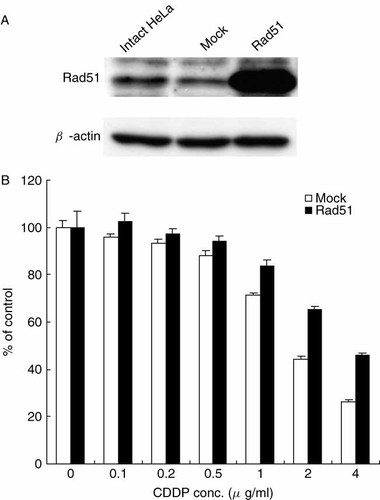
(A) Detection of human Rad51 transcript 48 h after the transfection of human Rad51 cDNA driven by the CMV promoter. Mock sample indicates HeLa cells transfected with a plasmid that did not contain Rad51 cDNA. Intact HeLa indicates HeLa cells that were not transfected. (B) Cell survival was detected by a modified MTT assay after treatment with 0–4 µg/ml CDDP for 3 h. The ordinate indicates the ratio of viable cells treated with various concentrations of CDDP to initial cell number. The mean value ± standard deviation from triplicate samples is shown
To enhance sensitivity to CDDP, we attempted to suppress Rad51 expression with siRNA. When Cy3-labeled siRNA was delivered to HeLa cells using the HVJ envelope vector, the efficiency was 80–100% (data not shown). Rad51 transcripts were not detected by Northern blot analysis 1 day after siRNA delivery, whereas scrambled siRNA did not reduce the transcript level (Figure 2A). We tested five different siRNAs for Rad51, but the only effective siRNA was a 19-mer from no. 321 of the Rad51 mRNA sequence. The other four siRNAs (19-mers from nos. 89, 462, 828, and 989) did not suppress Rad51 expression (data not shown). Two different antisense oligonucleotides against human Rad51 did not reduce the expression of human Rad51 (Figure 2B). These oligonucleotides had the same sequence as mouse Rad51 antisense oligonucleotides that had been used for suppression of Rad51 32. Rad51 protein was not detected by Western blots for 4 days after siRNA transfer. A small amount of Rad51 protein began to reappear on day 5 (Figure 2C). When Rad51 siRNA was introduced into HeLa cells, the growth of the cells was suppressed and the viability was 70% less than cells treated with scrambled siRNA (Figure 3A). The growth of cells treated with scrambled siRNA was not significantly different compared to that of cells treated with HVJ-E containing PBS. When HeLa cells were incubated with 0.02 µg/ml CDDP for 3 h after the delivery of Rad51 siRNA, the survival of the cells was reduced by 90% when compared to equivalent cells that were not exposed to CDDP (Figure 3B). More than 90% of colonies were formed with the same concentration of CDDP when scrambled siRNA was transferred into HeLa cells. Accordingly, with Rad51 siRNA, the number of colonies decreased to approximately 10% of that with scrambled siRNA.
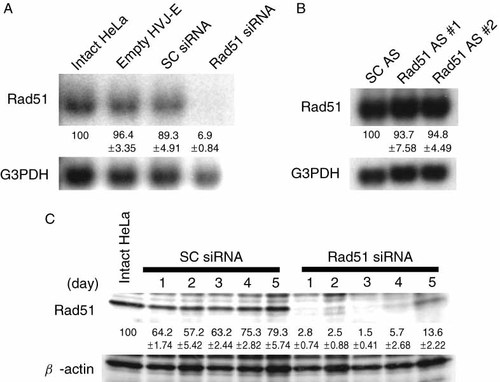
(A) Rad51 transcripts detected by Northern blot analysis 1 day after the delivery of Rad51 siRNA or scrambled (SC) siRNA. Rad51 mRNA in intact HeLa cells and HeLa cells treated with empty HVJ envelope vector were also measured. (B) Rad51 detection by Northern blot analysis 1 day after the delivery of two different antisense oligonucleotides (#1 and #2) against human Rad51 (Rad51 AS) or scrambled oligonucleotides (SC AS). (C) Rad51 protein detected by Western blot on days 1 to 5 after the delivery of either Rad51 siRNA or SC siRNA. These experiments were repeated twice and similar results were obtained. The ratio of Rad51 expression to G3PDH or β-actin expression was calculated by measuring the density of each band using the NIH imager. The percentage of Rad51 expression (mean ± standard deviation) is shown below each lane
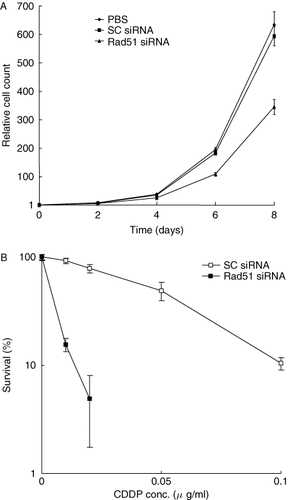
(A) The growth of HeLa cells detected by cell count on days 0 to 8 after the delivery of Rad51 siRNA, scrambled (SC) siRNA or PBS using the HVJ envelope vector. (B) The colony formation of HeLa cells after the delivery of either Rad51 siRNA or SC siRNA. The ordinate indicates the ratio of the number of colonies in the presence of various concentrations of CDDP to the number of colonies without CDDP after the delivery of siRNA. The mean value ± standard deviation from triplicate samples is shown at each point of both experiments. No colonies were observed at 0.05 and 0.1 µg/ml CDDP when Rad51 siRNA was delivered
We tested the effect of Rad51 siRNA on the sensitivity of CDDP in various human cancer cell lines including PANC-1 (pancreatic cancer), AsPC-1 (pancreatic cancer), A549 (lung cancer), DU145 (prostate cancer), MCF7 (mammary carcinoma), and HeLa S-3 (cervical cancer). First, the amounts of Rad51 and Ku70 in these human cancer cells were detected by Western blotting. The protein levels of Rad51 varied among cell lines while Ku70 protein levels were almost similar (Figure 4A). Then, on day 2 after the treatment with CDDP (0.1 µg/ml), the ratio of cell numbers of these cancer cell lines was examined in the presence of Rad51 siRNA or scrambled siRNA introduced using the HVJ envelope vector. Without Rad51 siRNA, more than 80% of the cells were still alive in all the cancer cell lines. Scrambled siRNA did not induce any toxicity in all the cell lines. However, with Rad51 siRNA, Rad51 protein level was reduced to less than 10% of that without siRNA in all the cell lines (data not shown), and all the cell lines were much more sensitive to CDDP. The sensitivity to CDDP increased more than 30% in all cases (Figure 4B). Thus, the enhancement of CDDP sensitivity by Rad51 siRNA appeared to be generally applicable to many cancer cells.
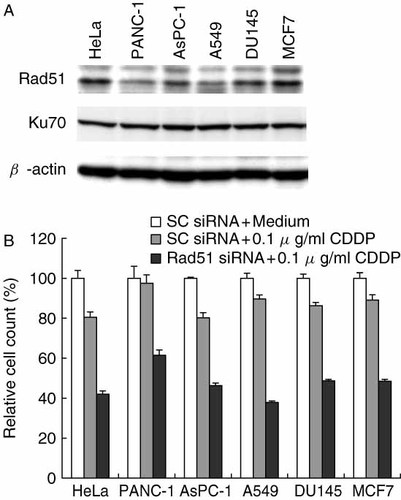
The increase in CDDP sensitivity in various cancer cell lines with Rad51 siRNA. (A) Rad51 and Ku70 protein levels in various cancer cell lines were detected by Western blotting. (B) siRNA was introduced into the human cancer cells using the HVJ envelope vector on day 1 after the inoculation of 105 cells in a 6-well plate. On day 3, cells were incubated with CDDP (0.1 µg/ml) for 3 h, and cell number was counted using a particle counter on day 5. Relative cell count indicates the ratio of cell number (the mean value of triplicate samples) treated with either scrambled (SC) or Rad51 siRNA + CDDP to that treated with SC siRNA + medium
Next, we examined the sensitivity to CDDP in non-cancerous human cells after transfer of Rad51 siRNA. As shown in Figure 5A, the sensitivity to CDDP was not enhanced in NHDF when the concentration of CDDP increased. Then, we compared the apoptosis of NHDF to that of HeLa cells by the treatment with Rad51 siRNA in the presence or absence of 0.1 µg/ml CDDP (Figure 5B). The apoptotic cell ratio was not significantly different between HeLa cells (4.0 ± 1.1%) and NHDF (3.2 ± 0.5%) with Rad51 siRNA in the absence of CDDP. However, in the presence of CDDP, the apoptosis increased to 15.0% in HeLa cells, while it was 4.9% in NHDF.
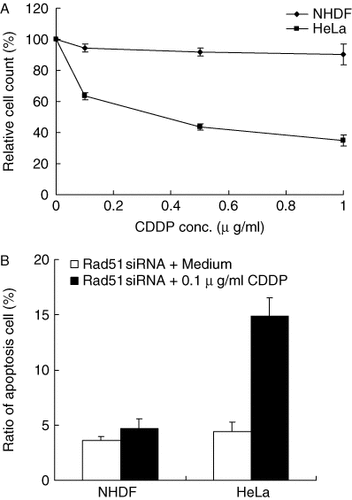
Rad51 siRNA did not enhance the sensitivity to CDDP in NHDF. (A) Forty-eight hours after transfer of Rad51 siRNA, the cells were treated with 0.1, 0.3 and 1.0 µg/ml CDDP for 3 h. Then, 48 h later, cell number was counted using a particle counter. Relative cell count indicates the ratio of cell number (the mean value of triplicate samples) treated with CDDP to that treated with medium alone. (B) To assess apoptosis, cells treated with Rad51 siRNA and CDDP were stained with fluorescein isothiocyanate (FITC)-labeled Annexin V and analyzed with FACScan. The ordinate indicates the ratio of labeled cells treated with Rad51 siRNA + medium or Rad51 + CDDP to that with scrambled siRNA + medium
We examined the ability of CDDP and Rad51 siRNA to suppress tumor growth in SCID mice. First, to test the gene delivery efficiency in vivo, we injected the HVJ envelope vector containing fluorescein isothiocyanate (FITC)-labeled oligodeoxynucleotides (FITC-ODN) into HeLa cell-derived tumors. As shown in Figure 6, the number of FITC-labeled cells and cells stained with Hoechst in randomly selected fields of three independent experiments were counted. They were 1227/2256, 616/1360, and 769/1424 cells. Thus, the delivery efficiency of FITC-ODN to HeLa cell tumors in vivo was 51.5 ± 5.2% (mean ± standard deviation). Next, Rad51 siRNA was delivered to tumors using the HVJ envelope vector. Western blot analysis showed that the level of Rad51 transcript was reduced to approximately 25% of that in intact HeLa tumors (Figure 7). Intraperitoneal injection of 200 µg of CDDP on day 2 transiently suppressed tumor growth, but tumors began to grow again 8 days after the treatment. To enhance the anti-tumor effect of CDDP, Rad51 siRNA delivered by the HVJ envelope vector was injected into the tumors on days 0 and 2. However, the suppression of tumor growth was not significant when compared to CDDP treatment alone (data not shown). Finally, Rad51 siRNA was injected into tumor mass on days 0, 2, and 4, and CDDP was injected into the abdominal cavity on day 2. This combination treatment significantly reduced the growth of HeLa tumors when compared to other treatment groups (Figure 8). Thus, the combination of CDDP and Rad51 siRNA is an effective anti-cancer protocol.
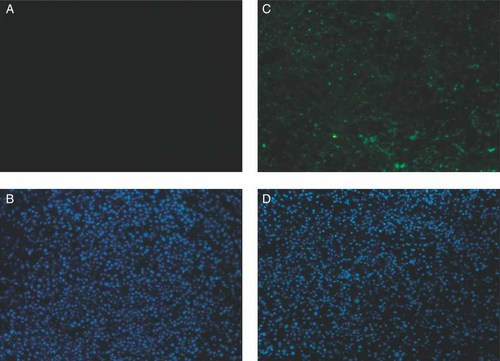
Detection of FITC-labeled ODN in tumors derived from HeLa cells in SCID mice. HVJ envelope vector containing unlabeled ODN (A, B) or FITC-ODN (C, D) was injected into tumors. FITC was detected in A and C. Hoechst 33 258 was used to counterstain the nucleus (B and D). The experiments were repeated three times and representative photos are shown
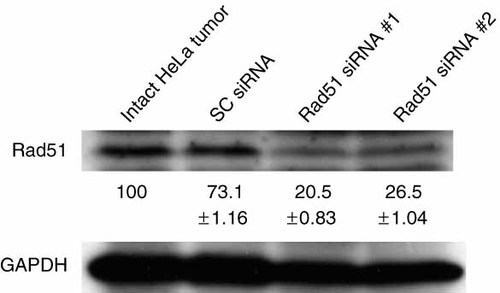
Rad51 transcript was detected by Western blot analysis after the delivery of either Rad51 siRNA or scrambled (SC) siRNA. The samples were isolated from two mice (#1 and #2) injected with the same Rad51 siRNA. This experiment was repeated twice and similar results were obtained. The percentage of Rad51 expression (mean ± standard deviation) below in each lane was calculated as described in Figure 2
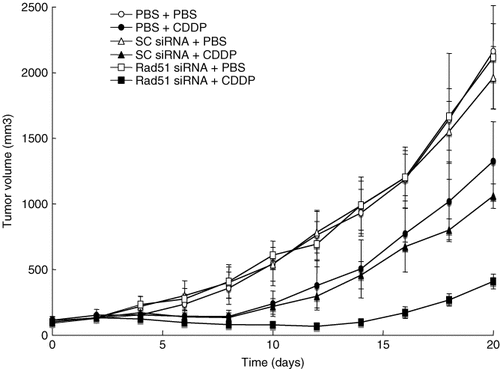
Tumor volume in SCID mice. Intraperitoneal injection of CDDP on day 2 transiently suppressed tumor growth in vivo, but tumors began to grow again 8 days after the treatment. To enhance the anti-tumor effect of CDDP, Rad51 siRNA or scrambled (SC) siRNA was injected on days 0, 2, and 4. In three groups, 200 µg of CDDP were injected into the abdominal cavity on day 2. In a negative control group, PBS was injected into both the tumor mass and peritoneal cavity. Each group contained five mice, and the representative result from three independent experiments is shown
Discussion
We enhanced the sensitivity of cancer cells to CDDP by completely suppressing Rad51 with siRNA. The combination of CDDP and siRNA caused the regression of human tumors in mice. These results support the theory that DNA damage induced by CDDP can be repaired by Rad51. Our results suggest that CDDP-induced DNA damage can be repaired by homologous recombination of DNA double-strand breaks. We succeeded in suppression of Ku70 proteins in HeLa cells using Ku70 siRNA, but the sensitivity to CDDP was not enhanced in HeLa cells (data not shown). An antisense Ku70 study supports our observation 18. Although we have not applied siRNA technology to suppress another factors such as Ku80 and DNA protein kinase (DNA-PK) which are also involved in non-homologous DNA end joining, it has been reported that silencing of DNA-PK or Ku86 by siRNA enhances sensitivity to radiation and anti-cancer drugs such as methyl methanesulfonate and bleomycin, but not to DNA cross-linking agents such as cisplatin and chlorambucyl 32-34. Moreover, cisplatin killing is mediated by kinase activity of the Ku70, Ku80 and DNA-PK complex 35. However, another report indicates that novel inhibitors of DNA-PK, vanillins, sensitize cells to cisplatin 36. Thus, the involvement of DNA-PK in cisplatin sensitivity is still controversial. A comparative study of Rad51 siRNA and DNA-PK siRNA in cisplatin sensitivity should be conducted.
siRNA very effectively suppressed Rad51 expression. A previous study found that antisense oligodeoxynucleotides against mouse Rad51 enhanced the radiosensitivity of malignant glioma 37. Although the target sequence of the antisense oligonucleotides is the same in humans and mice, the antisense oligonucleotides to human Rad51 did not suppress human Rad51 mRNA (Figure 2). As shown in Figure 2, Rad51 protein completely disappeared for 4 days after the siRNA transfer. We have never observed such complete loss of target protein using either antisense oligonucleotides or ribozymes. However, only one of five siRNA constructs effectively suppressed Rad51 expression. The system for predicting effective siRNA sequences should be improved.
When siRNA was delivered using the HVJ envelope vector, the efficiency was almost 100% in cultured cells, and Rad51 expression was completely prevented for 4 days after the delivery. siRNA very effectively suppresses gene expression, especially when an efficient delivery system is used. However, even when the HVJ envelope vector was used, the efficiency of a single siRNA injection into a tumor was only 50%. One limitation of synthetic siRNA is that its effect is transient, probably because the siRNA is gradually diluted after cell division. The use of lentivirus vector or retrovirus vector to insert siRNA expression DNA into the host chromosome has been proposed 38, 39. However, we believe that a combined treatment of synthetic siRNA and CDDP is sufficient for cancer treatment, because the cells that received Rad51 siRNA and CDDP in this study died in a few days. An important factor in the success of the combination treatment is the consecutive delivery of synthetic siRNA. Indeed, three injections of Rad51 siRNA into the tumor were more effective for tumor regression than two injections. The immunogenicity of the HVJ envelope vector is much less than that of native HVJ because of the inactivation of the viral genome. Consecutive injection is feasible with this vector system 28.
Rad51 siRNA enhanced the sensitivity to another anti-cancer drug, bleomycin, which can induce DNA double-strand breaks. The enhancement of bleomycin sensitivity by Rad51 siRNA was almost similar to that in a CDDP experiment (M. Ito and Y. Kaneda, unpublished data). It has been reported that Rad51 is also involved in the sensitivity of cancers to other anti-cancer drugs, such as etoposide (VP16) and imatinib mesylate (Gleevec) 40, 41. Since only Rad51 siRNA decreased cancer cell viability (Figure 4A), Rad51 siRNA can also enhance the sensitivity of cancer cells to other drugs which do not induce DNA double-strand breaks. This experiment is being performed in our laboratory. Furthermore, although Rad51 expression levels varied from cell line to cell line, all the cancer cells became very sensitive to CDDP in combination with Rad51 siRNA. The sensitivity of the cancer cell lines to CDDP did not appear to be related to the endogenous Rad51 protein level. These results suggest that the combination of CDDP with Rad51 siRNA will be generally applicable to various human cancers.
The enhancement of CDDP sensitivity by Rad51 siRNA was observed only in HeLa cells, not in NHDF. Similarly, apoptosis by Rad51 siRNA and CDDP increased in HeLa cells, but not in NHDF. The discrepancy of CDDP sensitivity by Rad51 siRNA between NHDF and HeLa cells may be due to the difference of the CDDP uptake by the two cell lines. Indeed, the equitoxic dose of CDDP in NHDF and HeLa cells was 1.2 and 0.5 µg/ml, respectively, in our case (M. Ito and Y. Kaneda, unpublished data). Another possibility is that cell cycle difference between both cells may affect the sensitivity to CDDP in the presence of Rad51 siRNA. The precise mechanism of this different sensitivity to CDDP remains to be solved.
However, in human gene therapy, we should be very careful regarding the toxicity of Rad51 siRNA. As shown in Figure 5B, Rad51 siRNA alone induced apoptosis in both HeLa cells and NHDF, although the apoptotic cell ratio was much lower in the absence of CDDP. This may be consistent with the fact that Rad51 knockout mice are embryonic lethal 42. To minimize the adverse effects to normal tissues, tumor-selective targeting is indispensable for cancer treatment. There are two ways to achieve selective targeting. One is the insertion of tumor-specific molecules to vectors, and another is the modification of vector size and charge. We have already reported that HVJ-cationic liposomes targeted tumor nodules in mouse peritoneum by intraperitoneal injection 43. We are now constructing targeting vectors by modifying the HVJ envelope vector with polymers or tumor-specific single-chain antibodies.
When delivered by tumor-targeting vectors, siRNAs against genes resistant to cancer therapy hold great promise to become very effective anti-neoplastic therapeutics in combination with chemotherapy or radiotherapy.
Acknowledgements
We thank Atsuko Okuno and Konomi Higashidani for their excellent technical assistance. This work was supported by grant 15300163 for Y.K. from the Ministry of Education, Culture, Sports, Science and Technology of Japan.




