Can Digital Rectal Examination Identify the Subtype of Dyssynergic Disorders as Well as High Resolution Anorectal Manometry?
ABSTRACT
Background and Aims
Diagnosing dyssynergic disorders (DD) often requires 3D high-definition anorectal manometry (3D-HRAM), raising concerns about cost, availability, and delayed referral. Digital rectal examination (DRE) offers a reliable, cost-effective alternative for DD diagnosis. This study aimed to assess DRE's capability to classify DD patients into the four subtypes outlined in Rao's classification.
Methods
This retrospective monocentric study involved patients diagnosed with DD through 3D-HRAM. After initial 3D-HRAM performed by one physician, patients underwent a clinical examination, including DRE by a second senior clinician blinded to DD subtypes. Statistical tests measured the correlation between DRE and HRAM in classifying the four DD subtypes.
Results
The study included 200 patients, revealing commendable overall agreement between DRE and 3D-HRAM (Kappa = 0.658). For subtype diagnosis, correlation was substantial for Subtypes I, II, and IV (0.679, 0.741, 0.649, respectively) and moderate for Subtype III (Kappa = 0.325).
Conclusion
DRE demonstrates satisfactory performance in diagnosing the four subtypes of DD. Enhanced training in DRE, emphasizing functional information, has the potential to reduce reliance on additional tests, thereby mitigating economic and organizational impacts.
1 Introduction
Functional anorectal disorders are a common issue in primary care [1], causing a range of debilitating symptoms that significantly affect patients' quality of life. These disorders carry substantial psychological and socio-economic implications [2-4].
The diagnosis and comprehension of the underlying pathophysiological mechanisms often require additional tests. However, the systematic use of these tests is currently under scrutiny due to cost and limited availability. Indeed, a thorough clinical examination, centered on a well-executed digital rectal examination (DRE), has the potential to furnish essential information for diagnosis and etiological orientation.
DRE is a fundamental, readily available, cost-free, and easily performed tool for evaluating anorectal disorders [5]. In the context of anal incontinence, Buch et al. [6] already reported a strong correlation between DRE and conventional anorectal manometry (ARM) in assessing resting and squeeze sphincter pressures as early as 1998. Subsequent studies have consistently supported these findings [7, 8]. Despite the positive results, the systematic use of DRE in clinical practice is inconsistent, with limited emphasis on functional assessment, and it remains less familiar among young physicians [9, 10].
In the context of chronic constipation and, more specifically, in the assessment of dyssynergic disorders (DD), the value of DRE needs clarification, considering the high prescription rates of additional tests. DD are characterized by a lack of coordination between the abdominal and pelvic floor muscles during defecation, leading to a sensation of anal blockage during pushing [2]. In this scenario, 3D high-resolution anorectal manometry (3D-HRAM), standardized by the London classification in 2020 [11], plays a crucial role in classifying patients into four DD subtypes based on the quality of rectal pushing and the state of relaxation or contraction of the anal sphincters [12]. This classification not only enhances our understanding of individual mechanisms but also enables us to better guide rehabilitation, as certain subtypes might be predictive of a good response to biofeedback therapy [13].
On the other hand, data of DRE in this indication are limited. The first study to compare DRE with conventional manometry in the diagnosis of DD was carried out in 2010, finding satisfying results [5]. In 2015, another study using high-resolution manometry as the gold standard reported satisfactory sensitivity and predictive positive value (PPV) of 93.2% and 91%, respectively, for DRE [14]. Despite this suggested value of DRE in the diagnosis of DD, no study has yet evaluated its ability to differentiate patients into the four subtypes of DD. Such assessment could potentially limit the need for 3D-HRAM in these cases, thereby streamlining healthcare pathways and reducing costs.
The aim of our study was therefore to evaluate the performance of DRE in the diagnosis of dyssynergia subtypes, using 3D-HRAM as the gold standard.
2 Methods
2.1 Study Population and Design
This study retrospectively analyzed prospectively collected data from patients referred for 3D-HRAM assessment at AP-HM, Marseille, France. Patients with chronic idiopathic constipation (CIC) per Rome IV criteria were included if diagnosed with DD following 3D-HRAM. A pre-examination questionnaire completed by patients confirmed compliance with the Rome IV criteria, and constipation severity was assessed using the KESS score [15]. The KESS score consists of 12 items that evaluate various aspects of constipation, including the duration of symptoms, the use of laxatives (with or without suppositories or enemas), the need for digital maneuvers, and the frequency of bowel movements. A score of 10/39 or higher is used to diagnose constipation. Additionally, certain questions specifically assess the underlying mechanisms of constipation. The severity of constipation is directly correlated with the KESS score, with a higher score indicating more severe constipation.
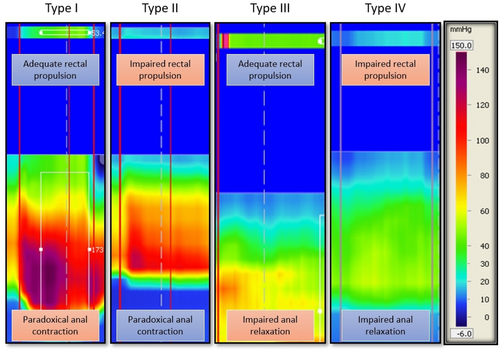
After 3D-HRAM diagnosis, the second practitioner, informed of DD diagnosis but blinded to subtype, conducted consultations. Detailed medical histories, treatments, and constipation profiles were reviewed to correlate with the manometric diagnosis of DD. Patients subsequently underwent DRE for pelvic floor functional assessment and were classified into four subtypes derived from 3D-HRAM patterns (refer to Figure 1). This information was documented for each patient.
Inclusion criteria were age > 18, Rome IV-defined CIC, confirmed DD by 3D-HRAM, completed DRE with reported data classifying patients into four DD subtypes, completed KESS score, and availability of clinical data. Exclusion criteria were age < 18, non-compliance with Rome IV criteria, absence of DD on 3D-HRAM, unavailable DRE date, unavailable severity scores, history of proctological surgery, and DRE and 3D-HRAM assessment by the same examiner.
2.2 Digital Rectal Examination
DRE was performed by only one experienced senior coloproctology referent, as described by Tantiphlachiva et al. [5] in the first study assessing DRE in DD diagnosis in comparison of ARM. The patient was installed in a left lateral decubitus position, with hip and legs flexed at 90°. After patient consent, the examination started with an anoperineal inspection, during which attention was paid to hemorrhoidal abnormalities, anal fissure, cutaneous lesion, and spontaneous rectal prolapse.
The next step was to insert a gloved and lubricated index finger into the rectum, first to examinate the rectal wall, looking for the presence of palpable masses or polyps, presence of a rectocele, arguments for intussusception, the onset of pain, the presence of stenosis, stools and, in this case, their consistency. Resting and squeezing pressures were then assessed.
To determine the dyssynergic nature of defecation, the patient was asked to make a bear-down attempt, following the same procedure performed during 3D-HRAM. The quality of the rectal push was then described as adequate or impaired (with the hand of the practitioner on the abdomen of the patient during pushing effort), as well as the degree of anal sphincter relaxation. For the latter, we differentiated three cases: adequate relaxation, impaired anal relaxation, and paradoxical contraction. A normal response was defined as adequate rectal push and adequate sphincter relaxation. The other cases were in favor of a DD diagnosis. The outcomes found during DRE were recorded by the examiner and used to classify patients according to the four subtypes of dyssynergia, based on Rao's classification [12] (see Figure 1).
2.3 3D High-Definition Anorectal Manometry
3D-HRAM was performed according to the usual guidelines described by Tae Hee Lee et al. [16]. For all exams, the probe used was the Mano-Scan 3D (Sierra Scientific Instruments, Los Angeles, CA, USA).
- –
Type I: The patient can generate an adequate pushing force (rise in intra-abdominal pressure) along with a paradoxical increase in anal sphincter pressure.
- –
Type II: The patient is unable to generate an adequate pushing force (no increase in intrarectal pressure) but exhibit a paradoxical anal sphincter contraction.
- –
Type III: The patient can generate an adequate pushing force (increase in intrarectal pressure) but either has absent or incomplete (< 20%) anal sphincter relaxation (i.e., no decrease in anal sphincter pressure).
- –
Type IV: The patient is unable to generate an adequate pushing force and demonstrates an absent or incomplete anal sphincter relaxation.
2.4 Statistical Analysis
Main characteristics of the sample were described as mean and standard deviation or median and interquartiles for continuous parameters, as number for subtypes. Agreement between DRE and 3D-HRAM was assessed using Kappa correlation coefficients for: (i) dyssynergia subtypes (four modalities); (ii) age classes (≤ and > 41 years); (iii) KESS score (≤ and > 20). The number of DRE misclassifications according to 3D-HRAM Rao classification were provided par dyssynergia subtypes.
2.5 Regulatory Aspects and Ethics
This study has been declared compliant with the European General Data Protection Regulation (EGDPR) and the recommendations of the French Supervisory Authority, the “Commission Nationale de l'Informatique et des Libertés.” In accordance with EGDPR guidelines, no consent was required for the use of study data, as this is a retrospective study involving patients whose care adhered to the department's standard practices. Data were anonymized and collected from the AP-HM computerized file, after approval by the AP-HM's Data Protection Officer (DPO). Registration PADS number: 2018_18.
2.5.1 Competing Interest Declaration
None of the authors of this study have any conflicts of interest related to the work.
2.5.2 Funding Declaration
No funding is to declare concerning this study.
2.5.3 Data Availability
The data supporting these results are not publicly available due to their sensitive nature. However, they can be made accessible upon request to the corresponding author.
3 Results
3.1 Population
A total of 1500 patients were screened, but 200 patients meeting the selection criteria were included in the study, each having undergone both 3D-HRAM and DRE. Data collection spanned from January 2019 to January 2020. The demographic characteristics of the patients, presented in Table 1, show a mean age of 56.16 ± 15 years, a median BMI of 23 kg/m2 [20–35], and a median disease duration of 120 months [12–660]. The patients' median KESS score was 20 [7–39], indicating varied levels of constipation severity among participants.
| Parameters | Value |
|---|---|
| Age (years), [mean ± SD] | 56 [16 ± 15] |
| BMI (kg/m2), median [range] | 23 [20–35] |
| Duration of disease (months), median [range] | 120 [12–660] |
| KESS score, median [range] | 20 [7–39] |
| Number of vaginal deliveries, median [range] | 2 [0–7] |
| DD subtypes (based on 3D-HRAM), number of patients | |
| Subtype I n (%) | 44 (22) |
| Subtype II n (%) | 114 (57) |
| Subtype III n (%) | 6 (3) |
| Subtype IV n (%) | 36 (18) |
| 3D-HRAM variables | |
| Resting pressure mean ± SD (mmHg) | 82.414 ± 26.43 |
| Squeeze pressure mean ± SD (mmHg) | 191.99 ± 64.01 |
| Rectal pressure during pushing maneuvers median [interquartile] (mmHg) | 35.18 [18.90; 39.90] |
| Percentage of anal pressure relaxation median [interquartile] | −25.00 [−52.75; −3.00] |
- Abbreviations: 3D-HRAM, 3D high-resolution anorectal manometry; BMI: body mass index; DD, dyssynergic disorders.
3.2 Correlation Between DRE and 3D-HRAM
The classification of patients according to the four DD subtypes by both methods (DRE and 3D-HRAM) is shown in Table 2. The overall agreement between the two techniques was substantial, with a kappa coefficient of 0.658, indicating a notable level of concordance. For the identification of specific DD subtypes, DRE showed strong agreement with 3D-HRAM for subtypes I, II, and IV, with kappa values of 0.679, 0.741, and 0.649, respectively (see Table 4). In contrast, the agreement for Subtype III was moderate, with a kappa of 0.325 (Table 3).
| Rao subtypes according to DRE | 3D-HRAM Rao subtypes | Total | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| I | 26 | 0 | 1 | 0 | 27 | |
| II | 1 | 92 | 0 | 3 | 96 | |
| III | 17 | 0 | 5 | 0 | 22 | |
| IV | 0 | 22 | 0 | 33 | 55 | |
| Total | 44 | 114 | 6 | 36 | 200 | |
- Abbreviations: 3D-HRAM, 3D high-resolution anorectal manometry; DRE, digital rectal examination.
| Agreement between DRE and 3D-HRAM | ||
|---|---|---|
| DD subtypes | Overall population (N = 200) | |
| Kappa | p | |
| Type I | 0.679 | < 0.0001 |
| Type II | 0.741 | < 0.0001 |
| Type III | 0.325 | < 0.0001 |
| Type IV | 0.649 | < 0.0001 |
- Abbreviation: DD, dyssynergic disorders.
The prevalent misclassifications with DRE were between Types I and III, on the one side, and types II and IV, on the other (refer to Table 2).
3.3 Comparative Analysis by Age and Symptom Severity
Table 4 presents the agreement between DRE and 3D-HRAM according to patient age and constipation severity assessed by the KESS score. Among patients aged 41 and younger, a higher correlation was observed across all subtypes compared to patients over 41 years old. In contrast, the KESS score did not significantly impact the agreement between DRE and 3D-HRAM, highlighting the stability of DRE performance regardless of subjective symptom severity.
| DD disorders | Age > 41 | Age ≤ 41 | KESS score > 20 | KESS score ≤ 20 | ||||
|---|---|---|---|---|---|---|---|---|
| (N = 149) | (N = 51) | (N = 95) | (N = 105) | |||||
| Kappa | p | Kappa | p | Kappa | p | Kappa | p | |
| Type I | 0.637 | < 0.001 | 0.835 | < 0.0001 | 0.561 | < 0.0001 | 0.718 | < 0.0001 |
| Type II | 0.695 | < 0.001 | 0.873 | < 0.0001 | 0.783 | < 0.0001 | 0.698 | < 0.0001 |
| Type III | 0.296 | < 0.0001 | 0.485 | < 0.0001 | 0.341 | < 0.0001 | 0.313 | < 0.0001 |
| Type IV | 0.609 | < 0.0001 | 0.806 | < 0.0001 | 0.747 | < 0.0001 | 0.549 | < 0.0001 |
4 Discussion
The present study demonstrates for the first time the ability of DRE to classify patients with DD according to the four subtypes described in Rao's classification, using 3D-HRAM as the gold standard. Our results show a good correlation between DRE and 3D-HRAM in classifying patients into Subtypes I, II, and IV. For Subtype II, a moderate correlation was found between the two assessment modalities.
The DRE stands as a fundamental clinical tool and cornerstone of proctological examinations. However, a notable decline in its utilization is observed [18], primarily due to the misconception of its low diagnostic value and the emergence of increasingly sophisticated complementary examinations. Consequently, DRE is often conducted with limited focus on anorectal function assessment, prompting frequent reliance on additional physiological tests. Yet, well-conducted DRE can furnish valuable data sufficient for a reliable diagnosis in most anorectal functional disorders. Studies dating back to 1989, such as Hallan et al. [19], demonstrated a strong correlation between DRE and conventional ARM, particularly in assessing sphincter tones. Subsequent research has supported these findings. In the context of DD, Tantiphlachiva et al. [5] reported promising diagnostic parameters for DRE, including a sensitivity of 75%, specificity of 83%, and a PPV of 97%. Similarly, Soh et al. [14] found comparable results, using 3D-HRAM as reference. Given the lack of studies evaluating DRE's ability to differentiate DD subtypes, our study aimed to address this gap, demonstrating a good correlation between DRE and 3D-HRAM in diagnosing subtypes I, II and IV, and a moderate correlation for Subtype III diagnosis. These findings emphasize the crucial role of functional assessments provided by DRE in evaluating DD. Hence, systematic DRE is essential in suspected cases of DD, involving assessments of sphincter function, thrusting behavior and quality.
Current guidelines advocate for the systematic use of supplementary tests when pelvic floor disorders are suspected [20]. These tests serve multiple purposes: confirming the diagnosis, elucidating the underlying pathophysiological mechanisms by categorizing patients into the four subtypes outlined in Rao's classification, and ultimately guiding treatment decisions. This is further supported by the variability in the diagnostic value of DRE, which can depend on the clinician's level of experience. But as recently demonstrated by Dekker et al. [21] DRE diagnostic value correlate well with other functional tests when performed by an experienced clinician. Enhanced training, particularly emphasizing functional assessment via DRE, has the potential to reduce reliance on additional tests. This approach takes into account factors such as test availability, cost constraints, and delays in patient referral and management. By optimizing the diagnostic process through more proficient DRE skills, clinicians could streamline patient care pathways and enhance overall healthcare efficiency and resource allocation.
To identify patient subgroups where DRE performs optimally, we conducted comparative analyses based on age and the severity of constipation as indicated by the KESS score. Our results demonstrate a stronger correlation between DRE and 3D-HRAM across all subtypes in patients under 41 compared to those aged 41 and older, likely due to age influencing ARM results independently [22]. Conversely, the KESS score had minimal impact on the agreement between DRE and 3D-HRAM (see Table 4). However, assessing the KESS score remains valuable, particularly for tracking treatment response during follow-up.
The management of DD primarily relies on biofeedback therapy, yielding favorable outcomes in most cases. However, research of response predictive factors is not conclusive. A study by Andrianjafy et al. [13] in 2019 found that patients with Subtypes II and IV, along with a low KESS score, exhibited a better response to biofeedback. Interestingly, our study showed a strong correlation between DRE and 3D-HRAM for these subtypes, both characterized by impaired rectal thrust. However, recent research by Shah et al. [23] challenges the prognostic value of DD subtypes in predicting biofeedback response. According to their findings, squeeze pressure and duration were the only relevant parameters for predicting clinical outcomes.
In our study, DD diagnosis relied solely on the clinical presentation of constipation and confirmation through 3D-HRAM. Because similar manometric patterns are found in healthy volunteers [24], current recommendations advise additional complementary examinations (balloon expulsion test (BET), colonic transit time, or defecography) to validate the diagnosis, posing a potential limitation to our study. Indeed, while our study focused on a population of patients with constipation exhibiting a defecatory disorders profile, Blackett et al. [25] recently demonstrated that other pathophysiological mechanisms, beyond dyssynergia, may also play a role in chronic constipation associated with defecatory disorders. However, despite their utility in the diagnostic process, the accuracy and relevance of additional tests can also be questioned, particularly because they do not always clarify the underlying pathophysiological mechanisms and may potentially yield false negatives [26, 27]. Furthermore, the study by Blackett et al. [25] reaffirmed the reliability of high resolution manometric assessment, even when multiple diagnostic tests were performed, while noting some nuances in its agreement with additional tests. To streamline treatment pathways, reduce costs, and expedite patient care, we designed our study under the premise that identifying a DD pattern on 3D-HRAM in a patient with a high probability of DD based on clinical history should be considered conclusive.
It should be noted that only female patients met the inclusion criteria for this study. Consequently, no male participants were included. While differences in ARM data between sexes exist, these do not appear to affect our results. The literature primarily reports higher resting pressures in men and differences in rectal distension perception, but no specific abnormalities related to DD mechanisms [28]. Therefore, the lack of male participants in our study seems unlikely to affect the conclusions.
The study, however, is subject to several limitations. First, the lack of utilization of a scoring system like the DRESS system [29] for functional assessment could affect the reproducibility of our findings. Second, while the involvement of a single expert examiner adds strength to the study given the demonstrated impact of examiner experience on the diagnostic value of DRE [21, 30], it may also limit the reproducibility of results. In this study, DREs were performed exclusively by a highly experienced examiner with over 10 years of specialization in functional coloproctology. This examiner had extensive practice in comparative assessments between DRE and ARM, ensuring both consistency and reliability in DRE evaluations. This high level of expertise provided a strong basis for accurately assessing DD subtypes but may also limit the extrapolation of results to less experienced practitioners. Future studies using the DRESS system and involving multiple experienced examiners could help standardize DRE procedures and allow for an assessment of inter-examiner reproducibility.
Although DRE is a valuable clinical tool, its precision is limited, as evidenced by the misclassification occurrences in our results. Additionally, the study is limited by the diagnostic approach used for DD, as we only relied on 3D-HRAM data, while current guidelines recommend additional rectal evacuation tests, such as BET or defecography. Although 3D-HRAM demonstrates excellent performance in diagnosing DD, this may impact our results, as some of the patients included in the study might not have shown patterns consistent with a DD diagnosis upon further diagnostic procedures. Lastly, the retrospective nature of this study limits the control over potential biases and data completeness.
In conclusion, both existing literature and our study suggest that DRE can serve as an acceptable diagnostic tool in the context of DD. Therefore, advocating for a broader utilization of DRE is warranted, potentially necessitating enhanced education for both senior and junior clinicians on proper examination techniques, functional assessment, and the recognition of DD features.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
I, Philippe Onana Ndong, the primary and corresponding author of this manuscript, affirm that I had unrestricted access to all study data and take full responsibility for its accuracy and integrity. Any questions regarding data access, analysis methods, or materials can be directed to me as the corresponding author.




