Evolution in coeliac disease diagnosis and management
Declaration of conflict of interest: Jason A Tye-Din has privately or via his institute been a consultant or advisory board member for Anatara, Anokion, Barinthus Biotherapeutics, Chugai Pharmaceuticals, Forte Biosciences, IM Therapeutics, Janssen, Kallyope, Mozart Therapeutics, TEVA, and Topas. He has received research funding from Barinthus Biotherapeutics, Chugai Pharmaceuticals, Codexis, Immunic, Kallyope, Novoviah Pharmaceuticals, Topas, and Tillotts Pharmaceuticals and received Honoraria from Takeda. He is an inventor on patents relating to the use of gluten peptides in coeliac disease diagnosis and treatment.
Abstract
The traditional gut-centric view of coeliac disease is evolving as immune and genetic insights underscore the central importance of a systemic, T cell immune response to gluten in disease pathogenesis. As the field increasingly recognize the limitations of small intestinal histology as the diagnostic standard, data supporting the accuracy of an immune (serologic) diagnosis of coeliac disease - well demonstrated in children - are growing for adults. Novel biomarkers such as interleukin-2 that identify the gluten-specific T cell demonstrate high sensitivity and specificity for coeliac disease and offer the potential for a diagnostic approach that avoids the need for gluten challenge. Asymptomatic disease and manifestations outside the gut pose considerable challenges for diagnosis using a case-finding strategy and enthusiasm for population screening is growing. The gluten-free diet remains a highly restrictive treatment and there is a paucity of controlled data to inform a safe gluten intake threshold. Ongoing symptoms and enteropathy are common and require systematic evaluation. Slowly-responsive disease is prevalent in the older patient diagnosed with coeliac disease, and super-sensitivity to gluten is an emerging concept that may explain many cases of nonresponsive disease. While there is great interest in developing novel therapies for coeliac disease, no drug has yet been registered. Efficacy studies are generally assessing drugs in patients with treated coeliac disease who undergo gluten challenge or in patients with nonresponsive disease; however, substantial questions remain around specific endpoints relevant for patients, clinicians and regulatory agencies and optimal trial design. Novel immune tools are providing informative readouts for clinical trials and are now shaping their design.
Introduction
Coeliac disease (CD) is a prevalent, life-long, immune-mediated illness, elicited by the ingestion of gluten in genetically susceptible individuals and characterized by a variable combination of gluten-dependent clinical manifestations.1 The global seroprevalence of CD is 1.4%.2 Contrasting with the historical view of CD as a pediatric, gut-centric, malabsorptive illness, it is now recognized to occur in patients of all ages and often in those without prominent gastrointestinal symptoms.
Immune and genomic studies highlight the central, pathogenic role of long-lived CD4+ gut-derived gluten-specific T cells that are pro-inflammatory, HLA-restricted, and detectable in the small intestine and circulation of CD patients.3, 4 This supports the view of CD as a systemic, adaptive immune response to gluten rather than a primary gut illness. Indeed, many of the manifestations of CD have their basis in gluten-induced immune (T cell or antibody) responses distinct from the effects of enteropathy-related malabsorption. The diverse presentation of CD that includes extraintestinal manifestations or minimally symptomatic or asymptomatic disease continues to present a challenge for its expeditious detection in the clinic and contributes to the high rate of undetected CD estimated to occur in 50–80% of affected cases in Western countries.5, 6
Although the disease focus has shifted beyond the gut, the gastroenterologist still plays the key role in CD diagnosis and management. Multidisciplinary models of care that incorporate a specialist dietitian and general practitioner may improve follow-up, but their implementation is dependent on local resources, infrastructure, and access to CD expertise. Research to understand how telehealth and smart phone apps, prevalent in the post-COVID era, can be leveraged to support best practice follow-up is needed.7
Mortality in CD is increased, primarily due to malignancies such as non-Hodgkin's lymphoma; however, mortality has reduced in recent decades.8 This may in part result from the wide use of sensitive transglutaminase antibody screening in the past 20 years, leading to the earlier diagnosis of milder phenotype CD and the increasing availability of gluten-free food options. Nevertheless, there are considerable contemporary challenges facing clinicians and patients with CD. These include (i) high rates of undetected CD and limitations of current diagnostic tools employing serology and histology, (ii) shortcomings of the gluten-free diet (GFD) that is onerous, nutritionally restrictive and does not always induce complete mucosal healing or symptom control, (iii) challenges in the definition and management of nonresponsive and refractory CD, and (iv) defining optimal pathways and tools for the development of successful novel therapeutics for CD. These will be discussed in this article.
Evolving diagnostic tools, testing strategies, and noninvasive monitoring
While a tissue-based diagnosis of CD relying on small intestinal histology showing villous atrophy, crypt hyperplasia, and raised intraepithelial lymphocytes remains the gold standard, the shortcomings of this approach are becoming increasingly recognized. Villous atrophy is not pathognomonic for CD and can occur with medications such as olmesartan, infections such as Giardia or viral enteritis, inflammatory disorders such as common variable immunodeficiency, and other immune enteropathies.9 The sampling technique, orientation, and reporting of small intestinal biopsies are crucial for accuracy,10, 11 and there is wide variability in reporting between community and specialist pathology centres.12 While the widely employed approach to assess duodenal histology is based on the categorical Marsh-Oberhuber system, quantitative morphometry that provides a villous height:crypt depth ratio (Vh:Cd) and intraepithelial lymphocyte (IEL) count is favored for clinical trials because of its excellent accuracy and reliability.10, 11 Being more time-consuming, its application in routine practice outside specialty centers is unclear. Quantitative histomorphometric approaches applied in several GCP compliant clinical trials suggest that in treated CD patients, a Vh:Cd of 3.0 or under is the norm.13-17 While this is lower than the Vh:Cd of 3.0–4.0 seen in the normal jejunum based on historical evaluation, contemporary data of normal duodenum using histomorphometry are needed as a more appropriate comparator. In one clinical trial of US and Australasian CD patients on a GFD for at least 12 months, the Vh:Cd was under 2.0 in 60%.18 This could be interpreted to suggest that active disease is more common than previously appreciated in treated CD and only detectable when employing rigorous quantitative histomorphometry. However, matched control data from well-treated CD and healthy populations are necessary to best put these findings into context.
Improvements in CD serology, particularly transglutaminase-IgA, have meant that high-level elevations show excellent positive predictive value for CD in children of over 95%. Revised diagnostic guidelines (ESPGHAN 2020) now support the pediatric gastroenterologist to diagnose CD when the transglutaminase-IgA is >10 times the upper limit of normal, and a second blood test shows a positive endomysial antibody (EMA).19 In Australasia and other places where EMA is less widely employed, it is possible that the deamidated gliadin peptide (DGP)-IgG assessment is a viable alternative to EMA as the second-line test.20 The ESPGHAN 2020 serodiagnostic approach applies to approximately a third of children with positive transglutaminase-IgA but has not yet been consistently recommended for adults with positive serology. However, emerging data are supportive of its utility in adults, and the next few years are likely to see greater adoption of this practice shift outside the pediatric space.21
The shift toward a simple immune-based diagnosis of CD comes at a time when access to endoscopy resources is under considerable strain due to the COVID-19 pandemic, which limited access to endoscopies and for which, in many centers, there remains a considerable backlog. A simple, blood-based diagnostic approach that targets the gluten-specific T cell pathogenic in CD is highly attractive, although current methodologies to detect these cells are laborious and not suited to the clinical pathology laboratory, for example, the HLA-DQ-gluten tetramer-based assay.22 Recently, interleukin-2 (IL-2) was shown to be significantly increased in the blood of people with CD, but not those without, 2–4 h after single bolus oral gluten ingestion and is a marker of activated pathogenic gluten-specific T cells.23 Gluten-induced IL-2 provides a novel approach to differentiate CD patients from healthy controls24 and those with self-reported gluten sensitivity.25 As the elevation of IL-2 correlates with the onset and magnitude of gastrointestinal symptoms to gluten, it is also informative as a symptom biomarker in CD. Recently, this approach was adapted to a whole blood assay system, where blood is combined with gluten peptides “in-tube” with assessment of IL-2 the following day.26 Given the high sensitivity of the assay, it has shown promise as a diagnostic for CD using blood from people who are strictly gluten free, suggesting that one day accurate diagnoses may be possible without the need for oral gluten challenge. Data from prospective validation studies are awaited.
Suboptimal diagnosis of CD remains a global problem. From serology-based population studies, 50–80% of patients are estimated being unaware of their CD diagnosis.5, 27, 28 Diagnostic delay is common, and while a mean delay of up to 13 years has been reported, a delay of only 3 years is still associated with decreased quality of life and excess doctor visits, days of sickness, and use of pharmaceutical agents before diagnosis.29 Despite underdiagnosis, universal screening is contentious due to a paucity of data on both the benefits and harms of screening an asymptomatic population and treating screen-detected disease.5 As a result, case finding in individuals with suggestive signs or symptoms and targeted screening of high-risk groups such as those with a positive family history of CD is recommended.28 However, population studies reveal a high burden of undiagnosed CD in the community that would not be detected through these approaches. Most children and adults with screen-identified CD have previously unrecognized symptoms and many have reduced nutritional indices, bone density, and quality of life that improve with a GFD.28 A mass population CD and type 1 diabetes screening study in Colorado, US, revealed 2.4% of children had previously undiagnosed CD autoimmunity (positive serology) and 90% did not have a positive family history for CD.30 After 1 year of follow-up on a GFD, there were significant improvements in mean symptom severity and frequency, normalization of iron deficiency in half, and improved health-related quality of life scores among caregivers; further, 93% of families reported good or excellent GFD adherence. Notably, while 69% were initially deemed asymptomatic, on extended symptom screening, 93% had identifiable symptoms. Additional data on the value of a population-wide approach will come from Italy with the commencement of a CD and type 1 diabetes screening program in their under-17 population in 2024. This will help address the important questions of cost-effectiveness using a population-based approach and whether the benefits of screening asymptomatic individuals and treating screen-detected CD outweigh the harms.
The GFD limitations and innovations
Adhering strictly to a GFD is challenging and imposes a significant treatment burden on patients comparable with end-stage renal disease.31 Strict dietary adherence varies from 42 to 91%.32 Older age, symptoms after gluten ingestion, better food knowledge, and lower risk of psychological distress are independent predictors of dietary adherence.33 Accidental and unintended gluten exposure, often referred to by patients as being “glutened,” may occur because of cross-contamination, which is common when patients dine out frequently.34, 35 Periodic review to monitor GFD adherence is recommended, but dietary history and CD serology are poor predictors of gluten exposure.7, 36 A noninvasive assay that detects gluten immunogenic peptides (GIP) in stool or urine as markers of dietary gluten exposure offers an objective approach to assess adherence. GIP assessment shows inadvertent gluten ingestion is common in treated CD, ranging from 25 to 89% depending on the frequency of assessment,37, 38 supporting the notion that the GFD is more aspirational than achievable.39 While stool testing is superior to urine testing for detecting intermittent, low-grade gluten exposure,40 questions remain about how to best leverage these highly sensitive tools in the clinic.
Ongoing gluten exposure in CD leads to persistence of disease activity, ongoing symptoms, and elevated morbidity due to chronic mucosal disease activity. It is perhaps surprising then, that there is a paucity of high-quality prospective controlled studies that specifically address the “safe” level of gluten exposure in CD, with only one published RCT that aimed to establish a safety threshold for gluten intake.41 Gluten-free claims made on food products sold in Australia and New Zealand need to conform to the Food Standards Australia and New Zealand (FSANZ) guideline that mandates the product contain “no detectable gluten.” This is a nonfixed threshold defined by the sensitivity of the prevailing technology to detect gluten, which is approximately 3 ppm of gluten based on FAOI-accepted assays such as the R5 Ridascreen ELISA. This definition of gluten free differs from the fixed value adopted in Europe (Codex Alimentarius) and the United States (FDA), which is defined as no more than 20 ppm of gluten. There is insufficient controlled data to inform where the level should be. To address this shortcoming, an Australian study is underway that is leveraging the IL-2 assay to assess responses to very low levels of gluten in treated CD patients.42
The nutritional limitations of the GFD have been brought into focus with the mounting data on the higher rates of CD-associated cardiovascular disease (despite the lack of traditional risk factors)43, 44 and metabolic dysfunction including metabolic dysfunction-associated fatty liver disease,45 which could be in part be contributed to by nutritional imbalances in the GFD itself. CD patients, especially those with fatty liver disease, need strict counseling regarding increasing physical activity and optimizing their diet to reduce caloric intake, enrich for unprocessed, naturally gluten-free foods, and minimize highly refined carbohydrates and saturated fat.46
Oats are a highly nutritious cereal that could overcome some of the nutritional limitations of the GFD. Long-term oats ingestion has been associated with improved quality of life.47 While they appear to be safely consumed by most people with CD if they are free of gluten contamination,48 the clinical significance of occasional oats-induced histologic damage and immune activation in CD49-51 is an area of active research. Outside of Australia and New Zealand, contamination-free oats are generally allowed as part of the GFD; however, follow-up is recommended, and in the setting of unexplained symptoms or persistent disease, a trial of withholding oats should be considered.
Poorly responsive disease—Challenges with terminology and pathogenic understanding
Nonresponsive coeliac disease (NRCD) has been defined as a primary failure to respond to at least 6–12 months of a GFD or the secondary re-emergence of symptoms, signs, or laboratory abnormalities typical of CD while still following a GFD.52, 53 Some but not all definitions incorporate the presence of villous atrophy, that is, persistent enteropathy. This variability in definition poses challenges in the clinic and for the standardization of trials of therapies targeting patients with persistently active CD.
NRCD on the basis of ongoing symptoms (not including enteropathy) has been reported to affect up to 30% of adult CD patients on a GFD and appears half as common in children.52-54 Comprehensive evaluation can identify a cause in children and adults in most cases, with the commonest being ongoing gluten intake (Table 1).53 One postulated cause for NRCD, once overt gluten intake and other medical illnesses have been excluded, is “super-sensitivity” to gluten.55 While this notion lacks direct mechanistic data, it is well accepted that there is substantial heterogeneity in the clinical, symptomatic, and immune response to gluten, with effects occurring at different “dose” levels between patients.56, 57 Observations that stricter gluten exclusion diets, such as the “Gluten Contamination Elimination Diet,” may benefit this patient subset help support the notion of gluten super-sensitivity.58 This entity is relevant because several drugs proposed to target NRCD are based on degrading gluten or rendering it less immunogenic (Table 2). To demonstrate the efficacy of these approaches, it will be important to assess patients where gluten exposure is occurring, even if it is low-level amounts in a super-sensitive, otherwise GFD adherent, patient.
Persistent symptoms
|
Persistent enteropathy
Immune
Infective
Medications
|
| Therapy | Targeted mechanism | Route | Study subjects and design | Study phase/clinical trial code | Gluten challenge | Outcome measures |
|---|---|---|---|---|---|---|
| KAN-101 | Antigen specific immune tolerance | Intravenous | Well-treated CD patients | Phase 1b/2; NCT05574010 (ACeD-it) | Yes | Primary: Part A: AEs; Part B: Change in IL-2 pre- and post-gluten challenge; Secondary: PK |
| Phase 2a; NCT06001177 (SynCeD study) | Yes | Primary: Vh:Cd; Secondary: IL-2, IEL count, AEs, ADA, PK | ||||
| TPM502 | Antigen specific immune tolerance | Intravenous | Well-treated CD patients | Phase 2a; NCT05660109 | Yes | Primary: AEs; Secondary: IL-2, Symptoms (CeD PRO, Global Symptom Survey) |
| DONQ52 | HLA-gluten peptide blockade | Subcutaneous | Well-treated CD patients | Phase 1; NCT05425446 (Lily Study) | No | Primary: AEs; Secondary: PK, ADA |
| Ritlecitinib | JAK3/TEC kinase inhibitor | Oral | Well-treated CD patients | Phase 2; NCT05636293 | Yes | Primary: Vh:Cd; CeD PRO; Secondary: Serology, IEL count |
| Bovine Colostrum | Reduce gluten absorption | Oral | Well-treated CD patients | Phase 1; NCT05555446 | Yes | Primary: Urine GIP; Secondary: CDSD score |
| ZED1227 | TG2 inhibition | Oral | NRCD defined by persistent symptoms and enteropathy | Phase 2b; EudraCT: 2020–004612-97 | No | Primary: Histologic improvement and CD symptom improvement (CDSD); Secondary: Duodenal and serum inflammation, AEs, QoL |
| TAK-062 | Gluten degradation | Oral | Symptomatic CD patient with enteropathy (Vh:Cd < 2.5) | Phase 2; NCT05353985 | Yes (SIGE bar) | Primary: Change in CDSD GI symptom severity score; Secondary: Vh:Cd, AEs, ADA |
| L-tryptophan supplementation | Promote aryl hydrocarbon receptor signaling to support gut integrity | Oral | Persistent CD symptoms | Exploratory; NCT05576038 | No | Primary: CSI score; Secondary: QoL, anxiety and depression scales, GSRS, duodenal indole production |
| PRV-015 (AMG- 714/ordesekimab) | Anti IL-15 | Subcutaneous | NRCD defined by persistent symptoms and positive CD serology | Phase 2b; EudraCT: 2020–000649-16 (PROACTIVE) | No | Primary: Improvement in abdominal symptoms (CeD PRO); Secondary: Safety, tolerability, PK |
| Tofacitinib | JAK inhibition | Oral | Refractory coeliac disease type 2 | Phase 2; EudraCT: 2018–001678-10 | No | Primary: Reduction in aberrant IELs; Secondary: Histologic and clinical/symptom response |
- ADA, anti-drug antibodies; AEs, adverse events; CDSD, Celiac Disease Symptom Diary; CD, coeliac disease; CSI, Celiac Symptom Index; GSRS, Gastrointestinal Symptom Rating Scale; GIP, Gluten Immunogenic Peptide; IEL, intraepithelial lymphocyte; JAK, janus kinase; PK, pharmacokinetics; PRO, patient-reported outcome; SIGE, simulated intermittent gluten exposure; TG2, transglutaminase-2; Vh:Cd, villous height-crypt depth ratio.
When considering villous atrophy as part of NRCD, it is important to recognize the limitations of current data. Reported rates of mucosal healing after periods of 6 months–10 years of a GFD is highly variable and after 2 years ranges between 12 and 79%.6, 18, 59-67 These data are impacted by the histologic reporting method (discussed above), the large number of retrospective studies (which are limited by selection bias), how mucosal recovery was defined, variations in GFD duration or adherence, variations in patient age, incomplete follow-up (many patients are not re-biopsied), and the presence of slow responders. Slowly-responsive CD describes the important group of patients with enteropathy at 12 months on a GFD who, given enough time (in some cases 2 years or more), eventually achieve full mucosal and serologic remission. Slowly-responsive CD acknowledges that delayed healing is common and is more frequent in people diagnosed with CD at an older age, with patients reporting no more symptoms than patients who experience mucosal recovery.68 By way of comparison, over 80% of children with CD have mucosal healing on a GFD within a 12-month time frame.69 One Finnish study showed persistent villous atrophy at 12-month follow-up biopsy was not associated with increased risk of long-term complications such as malignancies, osteoporosis, and increased mortality after a median follow-up of 16 years, with the authors noting that in their highly GFD adherent patient cohort at least 96% of patients achieve complete recovery on long-term treatment.68 These findings indicate that mucosal re-biopsy to confirm mucosal healing, if it is to be performed, will be more informative at 2 years than one.7 For the CD patient with persistent enteropathy who has replete nutritional parameters, stable bone density, and no active symptoms, it may be appropriate to simply support strict dietary adherence and monitor clinical state and histology.68 This conservative approach may change if safe and effective therapeutics for persistently active CD become available.70
In contrast to slowly-responsive CD, true persistent (nonrecovering) villous atrophy is a concern because it is associated with greater morbidity such as increased risk of hip fractures71 and lymphoproliferative malignancy,72, 73 as well as elevated mortality,73 although not all studies have shown this latter association.74 Compared with the general population, CD patients with persistent villous atrophy have a 3.78-fold (CI 2.71–5.12) increase in lymphoproliferative malignancy, while those with mucosal healing have a 1.5-fold (CI 0.77–2.62) increase, although the absolute risk of lymphoproliferative malignancy in CD remains low at 70 per 100 000 person-years.75 Risk factors for delayed recovery include the presence of severe enteropathy or malabsorption at diagnosis, a classical gastrointestinal presentation, lack of clinical response, use of some medications, and poor GFD adherence.73, 76 A scoring system based on these risk factors shows promise in predicting patients at risk of persistent villous atrophy.73 Concerns around the negative clinical impact of persistent villous atrophy underscores the need for close patient follow-up and the development of effective therapeutic interventions.70
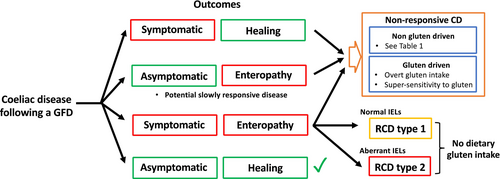
Refractory coeliac disease (RCD) is defined by persistent enteropathy with malabsorptive symptoms and no gluten intake for 12 months or longer. It is uncommon, affecting approximately 0.3–4% of CD patients.55 RCD pathogenesis is thought to relate to autonomous inflammation driven by interleukin-15 independent of gluten intake.77 RCD is divided into RCD type 1 and type 2, the latter characterized by aberrant duodenal intraepithelial lymphocytes that frequently carry somatic gain-of-functions mutations in the JAK1–STAT3 pathway.78 RCD type 2 is associated with more substantial malabsorptive symptoms and more severe and extensive enteropathy.79 It carries a poor 5-year survival rate (44–58%),80-83 with a 50% conversion to enteropathy-associated T cell lymphoma within 5 years that carries a dismal 8% 5-year survival rate.80, 84 In contrast to RCD type 2, RCD type 1 lacks positive diagnostic biomarkers, which poses a challenge in distinguishing it from NRCD (Table 3). Both present in the same way with ongoing symptoms and enteropathy, with the main distinctions being RCD has substantial malabsorptive symptoms and gluten intake is not the driver (Fig. 1). However, the rating of symptoms is subjective and determining whether gluten intake is occurring is difficult. Coeliac serology is a poor marker of dietary adherence, and the presence of enteropathy gives no clues as to whether the driver was gluten or RCD, except when RCD type 2 has developed and aberrant cells are identifiable. GIP testing could prove useful to distinguish mucosal disease due to gluten intake from true RCD; however, this approach needs validation.85 Steroids are a first-line treatment for RCD, and topical budesonide administered in an open-capsule format can improve symptoms and enteropathy in both NRCD and RCD.86 However, novel therapies, especially for RCD type 2, remain an important need.
| NRCD | RCD type 1 | RCD type 2 | |
|---|---|---|---|
| Presentation | Persistent symptoms | Persistent malabsorptive-type symptoms | Severe, progressive symptoms with malabsorption (cachexia, B symptoms); poor response to therapy |
| Frequency | Common | Uncommon | Very uncommon |
| Pathology | Normal small intestine or enteropathy | Enteropathy with normal IEL phenotype; mild “reactive” mesenteric lymphadenopathy | Enteropathy with abnormal IEL phenotype; extensive and severe villous atrophy, ulcerative jejunoileitis, small spleen |
| Treatment | Identify causes other than gluten ingestion; gluten-contamination elimination diet; immunosuppression | Immunosuppression | Immunosuppression, chemotherapy, stem cell transplantation |
| Prognosis | Heterogeneous condition; main impact is on quality of life | Good 5-year survival rate of 80–100% | Poor 5-year survival rate of 40–50%; high risk of EATL: 50% within 5 years; 5-year survival with EATL: 8–10% |
- EATL, enteropathy-associated T cell lymphoma; IEL, intraepithelial lymphocyte; NRCD, nonresponsive coeliac disease; RCD, refractory coeliac disease.
The road to novel therapies for CD
The shortcomings of the GFD as an effective treatment for CD have driven considerable industry interest in the development of novel therapies. Despite this enthusiasm, no drug has yet demonstrated efficacy in a Phase 3 registrational trial. Industry are faced with multiple challenges for successful drug development in CD, and unlike the established IBD clinical trial field, there is a lack of consensus on appropriate endpoints to support efficacy and drug registration and how clinical trials should be designed.87-89 While small intestinal histology is accepted as a key marker of disease activity, the use of patient-reported clinical outcome measures is considered vital by regulatory bodies in evaluating treatment response and providing insights into how patients perceive their well-being and functionality.90-92 PROs are often employed in acute gluten challenge studies but fail to capture vomiting, a common symptom with acute (but less so with chronic) gluten exposure.93 There is a need to develop PROs that accurately reflect true CD-related symptoms including those occurring outside the gut.94 Accurately measuring extraintestinal symptoms is important as they can have a significant impact on patient functioning and quality of life, and their pharmacological treatment will become appealing to drug developers if they can be accurately measured. One example is brain fog, which describes a heterogeneous constellation of cognitive symptoms encompassing memory deficits, attention problems, mental fatigue, and slowed information processing.95 Unfortunately, the lack of formal descriptors has hampered efforts to assess this symptom complex. Recent work has helped to clarify CD patients' descriptions and experience of brain fog, underscoring that it is common and impactful, and defined an assessment scale that can inform subsequent PRO development.95 PROs suitable for the pediatric CD population, where symptomatology may differ from adults, are also needed.
Current drugs in the preclinical or clinical development pipeline for CD aim to reduce the immunogenic gluten load, block the gluten-specific immune response, induce immune tolerance to gluten, or target RCD. Treatments may provide adjunctive support to the GFD and, in some cases and depending on their efficacy, potentially allow larger amounts of gluten to be safely ingested. A drug that can shift the gluten dose–response curve to minimize gluten-mediated effects will still have a considerable positive impact on patient quality of life even if unrestricted amounts of gluten cannot be consumed. A list of actively recruiting trials is summarized in Table 2. The most advanced in development, latiglutenase (IMGX003), a gluten degrading enzyme, is planned for assessment in Phase 3 trials in 2024.17 Broadly, two clinical trial approaches have been utilized to date: assessment of well-treated CD patients with incorporation of a gluten challenge to test therapies aiming to protect against the effects of gluten exposure, and trials targeting CD patients with persistent disease activity despite a GFD, to determine efficacy in inducing symptomatic and histologic remission. Gluten challenge can be undertaken for 2 weeks or longer when histology is a key readout, but can be shortened to a single, lower dose when the IL-2 immune readout is employed.56 Recently, the use of low-dose gluten challenge several times per week has been employed to simulate intermittent gluten exposure (SIGE). This strategy may be useful to test whether the drug can prevent the effects of “real-world” low-level gluten exposure commonly experienced by people following a GFD. It may also be employed to minimize the Hawthorne (observer) effect noted in prior CD drug trials, when subjects with persistent symptoms and/or enteropathy improve as a result of trial participation and adhering to a stricter GFD; here the goal is to stabilize gluten intake and symptoms caused by gluten but not exacerbate them.
The first therapy aiming to induce immune tolerance in CD to enter Phase 2 trials, Nexvax2, consisted of an adjuvant-free gluten peptide mix.96 While injections of Nexvax2 into CD participants modified T cell responses to these gluten peptides, treatment did not translate into protection from symptoms induced by a large oral dose of gluten. The trial highlighted the challenge of meaningfully modifying responses to gluten when long-lived gluten-specific T cells are well established in CD. The drug development program did identify the value of IL-2 as a readout of the gluten-specific immune response,23, 56 and this has shown value as an immune readout in clinical trials when measured in serum97 and in whole blood.98 Further, as IL-2 is a symptom biomarker for gluten, coupling food challenges with the IL-2 readout can be leveraged to differentiate true gluten-induced symptoms from those that are due to functional disorders or other causes.99
The future
The limitations of small bowel histology as the gold standard diagnostic tool for CD are increasingly recognized and, at the same time, acceptance of an immune-based, serologic diagnosis of CD has gained considerable traction in the clinic. Assessment of IL-2 in blood after single dose gluten challenge or in vitro in a whole blood assay can sensitively detect the pathogenic gluten-specific T cell in CD and may eventually simplify CD diagnosis in people following a GFD unable to undertake a gluten challenge.
A definition of CD centered around the gut-derived gluten-specific T cell, and not the small bowel, fits with our understanding of CD as a systemic T cell driven immune illness and may well identify patients immunoreactive to gluten without overt or significant enteropathy who still medically benefit from a GFD. This notion is already supported by clinical studies identifying patients with “minimal enteropathy CD” who benefit from a GFD.100 The clinical approach to diagnosis may further evolve when results from population screening studies inform on whether this is a cost-effective strategy and one that can improve outcomes for CD patients who are asymptomatic.
Improved management of persistent symptoms and enteropathy has become an attractive goal for drug developers, but there is a need to better define what nonresponsive disease is and how to differentiate it from RCD type 1. In trials of drugs targeting NRCD that aim to degrade or render gluten less immunogenic, there is the conundrum of identifying people following a GFD without overt, substantial gluten exposure but still consuming enough gluten to consistently drive symptoms and enteropathy. Biomarkers to identify whether the patient has become refractory to gluten removal, that is, whether RCD has developed, are needed to better stratify patients for trials and inform clinical management.
Controlled gluten challenge in treated CD participants is an important and highly informative component of clinical trials, although standardization of protocols is necessary, for example, the use of low FODMAP formulations to avoid confounding symptomatic responses.88 Gluten challenge in NRCD could be via a low-dose gluten SIGE strategy to overcome the Hawthorne effect; however, it may not be appropriate to challenge patients struggling to achieve symptomatic and mucosal remission with large amounts of gluten.88 Although protection against gluten-induced symptoms is considered an important efficacy endpoint for drug trials in CD, PRO measures are subjective and lack validation to assess gluten challenge induced symptoms, making the development and validation of standardized PROs a pressing need. The strong and consistent link between gluten-induced IL-2 release and adverse symptoms is informing mechanistic studies to understand the immune and neuro-enteric interactions underlying symptoms to gluten in CD, and this may translate into better ways to monitor and treat these symptoms. Ongoing refinements in immune-based tools are necessary to support better approaches to diagnosing CD and undertaking safe and efficient drug trials.
Acknowledgment
Open access publishing facilitated by The University of Melbourne, as part of the Wiley - The University of Melbourne agreement via the Council of Australian University Librarians.




