Development and applicability of modified weight loss grading system in cancer: a real-world cohort study
Hailun Xie, Guotian Ruan, and Lishuang Wei contributed equally to this work.
Abstract
Background
The original weight loss grading system (WLGS) was developed in western population, which did not perform effectively in cancer patients from China. This study aimed to develop and validate the modified WLGS (mWLGS) in the prognostic assessment of cancer patients in China.
Methods
A prospective multicentre real-world cohort study involving 16 842 patients diagnosed with cancer was conducted. Cox regression was used to calculate the hazard ratios for overall survival. Logistic linear regression was used to assess the odds ratio for 90-day outcomes.
Results
We calculated survival risks for the 25 mWLGS groups and clustered the approximate survival risks. Finally, we revised the prognostic grading system for mWLGS to include five grades of 0–4. Compared with the original WLGS, the mWLGS had a better prognostic differentiation effect in predicting the prognosis of patients with cancer. The survival rate gradually deteriorated with increasing grade of mWLGS, with the survival rate of grade 0 decreasing from 76.4% to 48.2% for grade 4 (76.4 vs. 72.8 vs. 66.1 vs. 57.0 vs. 48.2%, respectively). The mWLGS provides effective prognostic stratification for most site-specific cancers, especially lung and gastrointestinal cancers. High-grade mWLGS is independently associated with an increased risk of poor quality of life and adverse 90-day outcomes. Multivariate Cox regression analysis showed that the mWLGS was an independent prognostic factor for cancer patients in the validation cohorts.
Conclusions
Compared with the original WLGS, the mWLGS can better stratify the prognosis of cancer patients. mWLGS is a useful tool for predicting survival, 90-day outcomes, and quality of life in patients with cancer. These analyses may provide new insights into the application of WLGS in cancer patients in China.
Introduction
Involuntary cancer-associated weight loss is an important prognostic feature in patients with cancer.1, 2 It is associated with reduced quality of life, physical function, and tolerance to anticancer therapy, and shortened survival in cancer patients.3-5 Cancer-associated weight loss is the main diagnostic criterion for cancer cachexia.6 Cancer-associated weight loss at the initiation of chemotherapy is also associated with decreased chemotherapy response rates and increased toxicity.7, 8 Weight loss is useful to reflect disease severity, but there is great inconsistency in defining the degree of weight loss.9 Moreover, the global obesity epidemic has made the definition and threshold of clinically significant weight loss in cancer patients increasingly unclear. Recent studies have reported the obesity paradox in cancer patients: cancer patients with obesity have a survival advantage in prognostic assessment because of their larger energy reserves.10-12 Therefore, the optimal approach is to assess severity based on weight loss and initial body reserves.
In 2015, Martin et al.13 combined body mass index (BMI) and weight loss to produce a new cancer-related weight loss grading system (WLGS) based on prognostic risk stratification. WLGS was subsequently validated by Vagnildhaug et al.14 They found that WLGS is closely associated with survival, cachexia domains, and disease progression, and that adding certain cachexia domains to WLGS can improve the accuracy of prognosis prediction. Daly et al.15 found that WLGS was helpful in identifying cancer patients with poor quality of life, and independently associated with poor prognosis. However, most studies are from European and American populations, and there are few on the relationship between WLGS and cancer patients in China. When using the original WLGS, we found that it failed to perform effective prognostic stratification for Chinese cancer patients. The differences are likely related to racial differences in body sizes among Asian, European, and North American populations. Therefore, it is necessary to modify WLGS so that it can be applied to the prognostic assessment of cancer populations in China.
Our objective was to develop and apply the modified WLGS (mWLGS) in the prognostic assessment of cancer patients through a prospective multicentre real-world cohort study in China. Additionally, the study aimed to explore the relationship between mWLGS and the quality of life and 90-day outcomes of cancer patients.
Methods
Study participants
All patients in this study were from the Investigation on Nutrition Status and its Clinical Outcome of Common Cancers Project of China (INSCOC) cohort. The INSCOC cohort was a multicentre prospective study that has been described previously.16, 17 All patients were inpatients, excluding outpatients. The sample inclusion criteria for patients were (i) diagnosed with solid cancers (lung, oesophageal, gastric, hepatic-biliary, pancreatic, colorectal, breast, gynaecological, urologic, nasopharynx, and other cancers); (ii) height and weight measurements recorded during hospitalization; and (iii) complete weight loss information available for at least the past 6 months. Patients aged under 18 years and those who were unwilling or unable to participate due to cognitive impairment were excluded. The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the ethics committees of all participating institutions. Written consent was obtained from all patients. The patient information was de-identified prior to analysis.
Data collection
Baseline data were prospectively collected. Demographic characteristics included age, sex, lifestyle habits (smoking status and alcohol consumption), and family history of cancer. Co-morbidities included hypertension and diabetes mellitus. Pathological information included cancer location and pathological stage, which was evaluated according to the American Joint Committee on Cancer Staging System version 8. Serological data included white blood cells, neutrophils, lymphocytes, platelets, red blood cells, haemoglobin, and albumin. All serum parameters were measured using fasting blood samples collected upon admission in the clinical laboratories of the participating institutions. Treatment information included surgery, radiotherapy, and chemotherapy. Questionnaires were used to record the physical and nutritional status of the patients, including the Patient-Generated Subjective Global Assessment (PG-SGA), Karnofsky Performance Status (KPS), and NRS 2002. Quality of life data were collected on the day of admission using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30 Version 3.0, QoL).18 Hospitalization information included length of hospitalization and hospitalization expenses. The primary outcome of this study was overall survival (OS), defined as the time interval from cancer diagnosis to death or last follow-up. The secondary outcomes were 90-day outcome, defined as the 90-day survival outcome after receiving anticancer therapy, and quality of life, assessed using the EORTC QLQ-C30.
Measurement of modified weight loss grading system
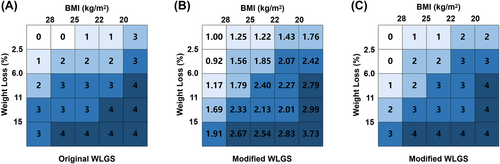
Anthropometric measurements included height, weight, BMI [weight (kg)/height (cm) squared], and involuntary weight loss over the past 6 months. Body weight measurements were accurate to within 0.1 kg using a digital scale. Height measurements were accurate to within 0.5 cm using a wall-mounted sight gauge. Body weight and height were measured within a week of admission. The patient's reported history of weight loss during the previous 6 months was captured by the PG-SGA and verified from the patient's medical records where possible. The percentage of weight loss was calculated as [(current weight in kg − previous weight in kg)/previous weight in kg] × 100. In this study, we used the same categories of weight loss and BMI as the original and re-modified the original WLGS grade by combining weight loss and current BMI according to previous research methods.13 Based on the classification of prognostic risk levels, the population was re-divided into five levels from grade 0 to grade 4.
Statistical analysis
Cox proportional hazards model was used to assess the prognostic risk of the 25 groups cross-classified by weight loss and current BMI. Groups with similar hazard ratios were then clustered and five grades were determined. To assess the prognostic validity of each grade of mWLGS, we constructed survival curves using the Kaplan–Meier method and compared the survival rates of each grade using the log-rank test. Next, we used the C-statistic, continuous net reclassification improvement, and integrated discrimination improvement to compare the prognostic discrimination ability and gain of the combined pathological stages. The χ2 test was used to test the differences in categorical variables in the mWLGS groups, and the nonparametric Wilcoxon rank sum test was used to test the differences in continuous variables in the mWLGS groups. Univariate and multivariate analyses of OS were performed using the Cox proportional hazard model. Hazard ratio with 95% confidence intervals (CI) were calculated. Logistic linear regression was used to assess the relationship between each grade of mWLGS and the odds ratio (OR) for 90-day outcomes. Finally, the population was randomly divided into two internal validation cohorts at a ratio of 7:3 to validate the clinical application value of the mWLGS further. All analyses were performed using R open-source software (4.0.5, http://www.rproject.org). All P values were two-sided, and significance was set at P < 0.05.
Results
Descriptive characteristics
In total, 16 842 patients had BMI and weight loss data at baseline. Of the patients, 54.9% were men, mean age 57.66 (±11.77) years. Lung cancer was the most common cancer, accounting for 23.1% of the cases, followed by colorectal (18.7%), breast (13.1%), and gastric (11.8%) cancer. The number of patients with stages I–IV was 1930 (11.5%), 3738 (22.2%), 4571 (27.1%), and 6603 (39.2%), respectively. Cancer patients with an mWLGS of grades 0, 1, 2, 3, and 4 were 5.5%, 31.6%, 31.6%, 19.2%, and 12.1%, respectively. Compared with breast, urologic, gynaecological, and nasopharynx cancers, high mWLGS (≥grade 2) was more common in patients with oesophageal, gastric, pancreatic, and colorectal cancers. From grade 2 onwards, the incidence of Cachexia increased rapidly (3.4 vs. 1.1 vs. 13.2 vs. 84.3 vs. 100%, respectively). In addition, high mWLGS was significantly associated with male, advanced age, poor lifestyle habits (smoking and alcohol consumption), advanced pathological stage, high inflammatory state, malnutrition, low quality of life, and poor outcomes (Table S1).
Comparison of original weight loss grading system and modified weight loss grading system
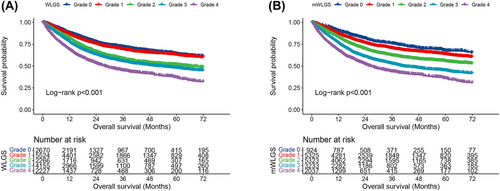
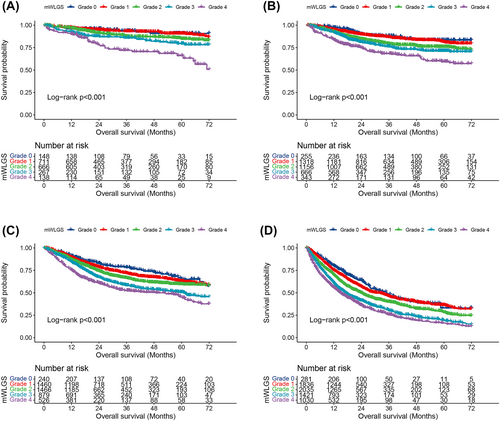
We calculated the survival risks for the 25 mWLGS groups with reference to groups with WL < 2.5% and BMI ≥ 25 and clustered the approximate survival risks. Finally, we revised the prognostic grading system of the mWLGS using five grades of 0–4 (Figure 1). A comparison of the original WLGS and the mWLGS is shown in Figure S1. Next, we compared the ability of the original WLGS and mWLGS to predict the survival risk of patients with cancer. As shown in Figure 2A, we found that grades 0/1 and 2/3 of the original WLGS did not provide good prognostic stratification of cancer patients. In contrast, mWLGS had a good prognostic differentiation effect in predicting the prognosis of patients with cancer. The survival rate gradually deteriorated with an increase in the grade of mWLGS, with the survival rate of grade 0 decreasing from 76.4% to 48.2% for grade 4 (76.4 vs. 72.8 vs. 66.1 vs. 57.0 vs. 48.2) (Figure 2B). In the subgroup analysis, we found that mWLGS still showed good prognostic discrimination for patients with the same pathological stage, with a significant step-down effect from grades 0 to 4 (Figure 3). Multivariate-adjusted subgroup analysis showed that mWLGS could further effectively distinguish the risk of poor prognosis in patients with the same pathological stage, and with the progression of mWLGS grade, the risk of poor prognosis showed a stepwise upward trend (Figure S2).
In terms of the discrimination index, the prognostic prediction accuracy of the mWLGS was better than that of WLGS, BMI, and weight loss. For the C-statistic, mWLGS improved by 0.004 (0.000, 0.007) compared with WLGS. For the continuous net reclassification improvement, the proportion of correct reclassification of mWLGS was 9.5% (7.1%, 12%) higher than that of WLGS. For integrated discrimination improvement, the prediction ability of mWLGS was also improved by 0.5% (0.1%, 0.8%) compared with that of WLGS (Table S2). In the model performance, after the addition of tools to the pathological stage for predicting all-cause mortality, we found that mWLGS also resulted in the optimal benefit for pathological stage (Table S3).
Association between modified weight loss grading system and overall survival in overall and specific-site cancers
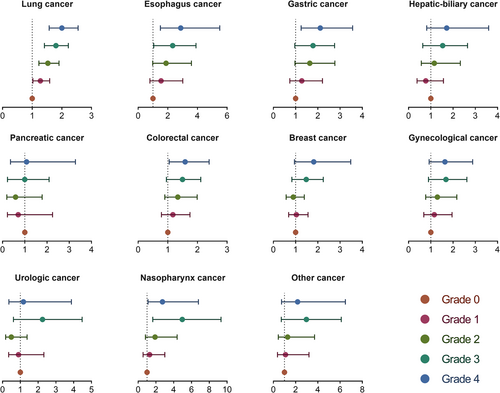
The median OS of the entire cohort was 20.2 months. At the last follow-up, 10 924 (64.9%) of 16 842 patients were still alive. The median OS of grade 0 decreased from 26.1 months to 16.0 months for grade 4 (log-rank: P < 0.001). In univariate analysis, high mWLGS was a risk factor affecting the prognosis of patients with cancer. In the multivariate analysis, grades 1–4 were all independently associated with decreased survival of cancer patients, and with the increase in grades, the risk of poor prognosis increased progressively to 1.206, 1.477, 1.787, and 2.193, respectively (Table 1). The mWLGS still provides effective prognostic stratification for most site-specific cancers, especially lung and gastrointestinal cancers (Figure 4).
| mWLGS | Model a | P value | Model b | P value | Model c | P value |
|---|---|---|---|---|---|---|
| Grade 0 | Ref | Ref | Ref | |||
| Grade 1 | 1.244 (1.079, 1.434) | 0.003 | 1.126 (0.976, 1.298) | 0.103 | 1.206 (1.046, 1.391) | 0.010 |
| Grade 2 | 1.655 (1.438, 1.904) | <0.001 | 1.424 (1.237, 1.639) | <0.001 | 1.477 (1.282, 1.701) | <0.001 |
| Grade 3 | 2.290 (1.985, 2.641) | <0.001 | 1.780 (1.543, 2.054) | <0.001 | 1.787 (1.548, 2.064) | <0.001 |
| Grade 4 | 3.069 (2.653, 3.551) | <0.001 | 2.180 (1.883, 2.524) | <0.001 | 2.193 (1.893, 2.542) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 |
- Model a: No adjusted. Model b: Adjusted for age, sex, and TNM stage. Model c: Adjusted for age, sex, TNM stage, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoking, drinking, and family history.
Association between modified weight loss grading system and quality of life
Elevations in mWLGS were significantly associated with worse scores on many functional scales (physical, role, emotional, cognitive, social, and global health), symptom scales (fatigue, nausea and vomiting, pain, decreased appetite, and dyspnoea), and total EORTC QLQ-C30 scores (Table S4). The mWLGS grades differed significantly across all the EORTC QLQ-C30 quality of life score items. The largest differences in symptom scales between grades 0 and 4 were in sleep disturbance (0.0 vs. 33.3, P < 0.001), appetite loss (0.0 vs. 33.3, P < 0.0001), fatigue (0.0 vs. 22.22, P < 0.001), and pain (0.0 vs. 16.67, P < 0.001). Of note, deterioration of the function and symptom scales of the EORTC QLQ-C30 was most common in grades ≥ 2. Importantly, an increase in mWLGS was associated with a worsening of the total EORTC QLQ-C30 score (66.67 in grade 0 vs. 50.00 in grade 4). In the multivariate logistic regression, mWLGS was independently associated with total EORTC QLQ-C30 scores below the median (<66.6). Although grade 1 was not associated with a worse total EORTC QLQ-C30 score than grade 0, grade 2 (OR = 1.538, 95% CI = 1.312–1.804, P < 0.001), grade 3 (OR = 1.992, 95% CI = 1.312–1.804, P < 0.001), and grade 4 (OR = 3.622, 95% CI = 3.041–4.314, P < 0.001) were independently associated with an increased risk of poor quality of life (Table S5).
Association between modified weight loss grading system and 90-day outcome
In this study, 829 (4.9%) patients experienced a 90-day outcome. Multivariate-adjusted logistic regression analysis showed that the mWLGS was independently associated with 90-day outcomes. As the grade of the mWLGS increased, the risk of 90-day outcomes gradually increased. Compared with grade 0, grade 2 (OR = 2.166, 95% CI = 1.326–3.540, P = 0.001), grade 3 (OR = 2.474, 95% CI = 1.507–4.063, P < 0.001), and grade 4 (OR = 4.337, 95% CI = 2.639–7.127, P < 0.001) were independently associated with an increased risk of adverse 90-day outcome (Table 2).
| mWLGS | Model a | P value | Model b | P value | Model c | P value |
|---|---|---|---|---|---|---|
| Grade 0 | Ref | Ref | Ref | |||
| Grade 1 | 1.269 (0.772, 2.088) | 0.347 | 1.113 (0.674, 1.838) | 0.676 | 1.175 (0.710, 1.946) | 0.530 |
| Grade 2 | 2.512 (1.549, 4.072) | <0.001 | 2.076 (1.275, 3.381) | 0.003 | 2.166 (1.326, 3.540) | 0.002 |
| Grade 3 | 3.337 (2.049, 5.435) | <0.001 | 2.444 (1.493, 4.000) | <0.001 | 2.474 (1.507, 4.063) | <0.001 |
| Grade 4 | 6.281 (3.861, 10.218) | <0.001 | 4.239 (2.592, 6.934) | <0.001 | 4.337 (2.639, 7.127) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 |
- Model a: No adjusted. Model b: Adjusted for age, sex, body mass index, and TNM stage. Model c: Adjusted for age, sex, body mass index, TNM stage, tumour types, surgery, radiotherapy, chemotherapy, hypertension, diabetes, smoking, drinking, and family history.
- mWLGS, modified weight loss grading system.
Randomized internal validation of modified weight loss grading system
According to the 7:3 ratio, we randomly divided the entire cohort into validation cohort A (11 790) and validation cohort B (5052). As shown in Table S6, the clinicopathological data of the two cohorts were independent. We found that mWLGS could effectively differentiate the prognosis of patients in both validated cohorts A (Figure S3A) and B (Figure S3B), and the risk of poor prognosis showed a gradually increasing trend with the progression of grades. In validation cohort A, compared with grade 0, grade 1 (OR = 1.198, 95% CI = 1.010–1.420, P = 0.038), grade 2 (OR = 1.462, 95% CI = 1.234–1.731, P < 0.001), grade 3 (OR = 1.848, 95% CI = 1.556–2.193, P < 0.001), and grade 4 (OR = 2.129, 95% CI = 1.786–2.537, P < 0.001) were independently associated with poor prognosis in patients with cancer (Table S7). Multivariate Cox regression analysis showed that the mWLGS was an independent prognostic factor for cancer patients in validation cohort B (Table S8).
Discussion
The severity of weight loss should be assessed according to the weight loss and body reserves. The potential benefit of high initial body weight has not been considered in previous risk assessment studies of patients with cancer or treatment-related weight loss. Therefore, Martin et al.13 systematically developed a cancer-related WLGS system that includes both weight loss and BMI dimensions. Since then, WLGS has been reported to be associated with the prognosis of many cancer patients.15, 19 However, our study found that the original WLGS did not effectively differentiate the prognosis of cancer patients, especially those with grades 0/1 and 2/3. It is well known that Asian, European, and American populations have significant differences in physique and BMI, which may explain why the original WLGS failed to exert an effective prognostic stratification effect. Therefore, we modified the WLGS to make it more suitable for the cancer population in China. Compared with the original WLGS, the mWLGS can better stratify the prognosis of cancer patients. Our findings underscore that mWLGS is a strong independent prognostic factor for cancer patients, and that the prognosis of cancer patients progressively worsens with the progression of mWLGS. Our study also confirmed that the mWLGS was independently associated with the prognosis of patients with cancer in the internal validation cohorts. Moreover, the mWLGS also showed excellent prognostic stratification ability among different site-specific cancers.
Clinicopathological staging helps to determine prognosis and guide subsequent treatment of patients with cancer, such as radiotherapy, chemotherapy, or targeted therapy.20 However, even at the same pathological stage, the prognosis of cancer patients is still different, suggesting that additional evaluation of other factors is needed to improve the accuracy of the prognostic prediction. In this study, we found that mWLGS could effectively stratify the prognosis of patients with the same pathological stage, suggesting that mWLGS can be an effective supplement to pathological stage in prognostic assessment. High mWLGS is not only an independent risk factor for long-term prognosis but is also independently associated with adverse 90-day outcomes in cancer patients. Compared with grade 0 patients, patients with grade 4 had a more than three-fold higher risk of developing an adverse 90-day outcome. Vagnildhaug et al.14 found significant deterioration in all cachexia domains with increasing WLGS, especially above grade 2. In this study, we also found that high mWLGS (≥grade 2) were closely associated with cachexia progression. Cachexia is widely recognized as closely related to poor prognosis in cancer patients. We further found that compared with patients with grades 0/1, those with ≥grade 2 had a significantly greater survival reduction (3.6 vs. 6.7 vs. 9.1 vs. 8.8%, respectively).
The quality of life of cancer patients has recently received increasing attention.21, 22 The EORTC QLQ-C30 is a commonly used tool to assess the quality of life of patients with cancer. The mWLGS was able to identify patients at risk for low EORTC QLQ-C30; in particular, grade 4 mWLGS was significantly associated with increased symptom burden and decreased functional areas. The function and symptom scales of the EORTC QLQC30 deteriorated significantly with an increase in the mWLGS. For sleep disturbance, appetite loss, fatigue, and pain, the median score increased by more than 10 points from grade 0 to grade 4, whereas for physical function, role function, and cognitive function, there was a drop of more than 10 points between grades 0 and 4, which are clinically significant differences. Importantly, multivariate logistic regression indicated that high mWLGS was independently associated with poor quality of life.
However, this study has some limitations. First, the mWLGS was developed based on the Chinese population, and whether it can be extended to other populations still requires further evidence. Second, measurements of body composition were not examined in this study; therefore, the components of weight loss (skeletal muscle versus adipose tissue) were unknown. In addition, we did not record whether the patients received oral nutritional supplements or any medications that might affect appetite and weight gain. Finally, although the randomized internal validation of this study achieved satisfactory results, external studies are needed in the future.
Overall, mWLGS is a useful tool for predicting survival and quality of life in patients with cancer and can effectively differentiate risk differences for poor prognosis. The mWLGS may be helpful in identifying and predicting the grading of cancer patients with poor prognosis, providing a valuable reference for individualized treatment of cancer patients. This study provides valid evidence for the further application of WLGS in cancer patients in China. To the best of our knowledge, this study has the largest sample population in terms of WLGS application, and it is the first to modify WLGS to make it more suitable for the population of cancer patients in China. These are the strengths of the present study.
Conclusions
Compared with the original WLGS, the mWLGS can better stratify the prognosis of cancer patients. mWLGS is a useful tool for predicting survival, 90-day outcomes, and quality of life in patients with cancer. These analyses may provide new insights into the application of WLGS in cancer patients in China.
Acknowledgements
We thank all the participants in this study, the entire team of the Investigation on Nutrition Status and Its Clinical Outcome of Common Cancers (INSCOC) past and current investigators and organizers from different study centres and hospitals for their contributions in various aspects of the INSCOC study, including input in study design, review of study protocols, recruitment of the patients, data collection and data sorting, entry and, checking, as well as retrieving miss data. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.23
Funding
This study was supported by the National Key Research and Development Program to Dr. Hanping Shi (No. 2017YFC1309200 and No. 2022YFC2009600) and Young Elite Scientists Sponsorship Program by CAST (2022QNRC001).
Conflict of interests statement
None declared.




