Capsaicin alleviates cisplatin-induced muscle loss and atrophy in vitro and in vivo
Ko-Chieh Huang and Yi-Fen Chiang contributed equally to this work.
Abstract
Background
Cisplatin (CP) is a widely used chemotherapeutic drug with subsequent adverse effects on different organs and tissues including skeletal muscle loss and atrophy as the most common clinical symptoms. The molecular mechanism of cisplatin-induced muscle atrophy is not clearly understood. However, recent significant advances indicate that it is related to an imbalance in both the protein status and apoptosis. Capsaicin (CAP) is one of the major ingredients in chilli peppers. It is a valuable pharmacological agent with several therapeutic applications in controlling pain and inflammation with particular therapeutic potential in muscle atrophy. However, the mechanisms underlying its protective effects against cisplatin-induced muscle loss and atrophy remain largely unknown. This study aims to investigate capsaicin's beneficial effects on cisplatin-induced muscle loss and atrophy in vitro and in vivo.
Methods
The anti-muscle-atrophic effect of capsaicin on cisplatin-induced muscle loss was investigated using in vivo and in vitro studies. By using the pretreatment model, pretreated capsaicin for 24 h and treated with cisplatin for 48 h, we utilized a C2C12 myotube formation model where cell viability analysis, immunofluorescence, and protein expression were measured to investigate the effect of capsaicin in hampering cisplatin-induced muscle atrophy. C57BL/6 mice were administered capsaicin (10, 40 mg/kg BW) as a pretreatment for 5 weeks and cisplatin (3 mg/kg BW) for seven consecutively days to assess muscle atrophy in an animal model for protein and oxidative stress examination, and the grip strength was tested to evaluate the muscle strength.
Results
Our study results indicated that cisplatin caused lower cell viability and showed a subset of hallmark signs typically recognized during atrophy, including severe reduction in the myotube diameter, repression of Akt, and mTOR protein expression. However, pretreatment with capsaicin could ameliorate cisplatin-induced muscle atrophy by up-regulating the protein synthesis in skeletal muscle as well as down-regulating the markers of protein degradation. Additionally, capsaicin was able to downregulate the protein expression of apoptosis-related markers, activated TRPV1 and autophagy progress modulation and the recovery of lysosome function. In vivo, capsaicin could relieve oxidative stress and cytokine secretion while modulating autophagy-related lysosome fusion, improving grip strength, and alleviating cisplatin-induced body weight loss and gastrocnemius atrophy.
Conclusions
These findings suggest that capsaicin can restore cisplatin-induced imbalance between protein synthesis and protein degradation pathways and it may have protective effects against cisplatin-induced muscle atrophy.
Introduction
Chemotherapy entails the administration of anticancer drugs in a standardized regimen specific to each type of cancer. Cisplatin (CP), a platinum-based anticancer drug, is widely used to treat tumours, such as cervical, head-and-neck, prostate, breast, lung, testicular, and ovarian cancers.1 It induces cancerous-cell apoptosis by inhibiting DNA synthesis, metabolized in the liver and excreted by the kidneys.2 While cisplatin can effectively destroy cancer cells, it also has many side effects, including nausea, body weight loss, muscle wasting, fatigue, and impaired functional performance,2, 3 which are considered the hallmarks of cachexia and sarcopenia and in turn significantly influence the treatment outcome.4
Skeletal muscle wasting is a prognostic factor of reduced survival in patients who receive chemotherapy. Muscle wasting is also a marker of poor prognosis in cancer patients, and it decreases their quality of life.5 Accordingly, cisplatin administration to healthy rats could cause some typical symptoms of cancer cachexia, including body weight reduction, skeletal muscle wasting, and weakness.6 A randomized clinical trial indicated that treatment with 100 mg/m2 cisplatin by intravenous injection for 4 weeks decreases the muscle strength and fat mass percentage and reduces cardiovascular health and health-related quality of life (HRQoL) of patients with head-and-neck cancer.7 It has been reported that two muscle-specific ubiquitin E3 ligases, inclusive of muscle ring-finger 1 (MuRF1) and muscle atrophy F-Box (MaFbx), play important roles in muscle wasting induced by cisplatin treatment.8 Cisplatin has also been shown to be associated with low levels of physical activity and a decrease in the quality of life.9 Clinically, those weight loss and muscle mass reduction symptoms are the most debilitating and fastest symptoms in chemotherapy-treated patients.10 Therefore, it has been suggested that, under atrophic conditions, nuclei are eliminated from the symplasm by myonuclear apoptosis as apoptosis could modulate myonuclear loss during muscle atrophy.11 It is important to identify approaches to prevent and attenuate muscle loss and atrophy development during cisplatin therapy.
Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide, CAP), the major active ingredient in chilli peppers, has been widely investigated owing to its extensive bioactivities, such as analgesic, antioxidant, anti-inflammatory, anticancer, and anti-obesity properties.12, 13 Capsaicin supplementation (12 mg) for 10 physically active men increased the resistance exercise performance, improved middle distance running performance, and decreased rate of perceived exertion (RPE) in adults, showing the improvement and enhancement in muscle strength in a clinical trial.14 However, the mechanism for these preventive effects of capsaicin on cisplatin-induced muscle loss and atrophy remain largely unknown.
In the present study, we systematically investigated the effects of capsaicin on cisplatin-induced muscle loss and atrophy by using in vivo and in vitro models and evaluating the signalling pathways involved in protein turnover in skeletal muscle atrophy to thoroughly elucidate the mechanisms of capsaicin in relieving chemotherapy-induced muscle side effects.
Methods
Reagents and antibodies
Reagents and antibodies were purchased from suppliers as follows; high-glucose Dulbecco's modified eagle medium (DMEM) (Gibco, Grand Island, NY, USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) (Sigma, St. Louis, MO, USA), sodium bicarbonate (Sigma), HEPES, free acid (BioShop, Burlington, ON, Canada), penicillin streptomycin solution, ×100 (CORNING, Manassas, VA, USA), foetal bovine serum (FBS, Gibco), horse serum (HS, Gibco), dimethyl sulfoxide (DMSO, Sigma), paraformaldehyde (Sigma), 0.05% trypsin–EDTA 1X (CORNING), bovine serum albumin, BSA (BioShop), protease inhibitors tablet (Roche, Mannheim, Baden-Württemberg, German), phosphatase inhibitor cocktail tablet (Roche), BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA), 30% acrylamide/Bis solution (Bioshop), radioimmunoprecipitation assay (RIPA) buffer (Roche), electrochemiluminescence (ECL) immunoassay (Thermo), polyvinylidene fluoride membranes, PVDF (Millipore, Billerica, MA, USA), Triton X-100 (Sigma), trypan blue (Gibco), crystal violet (Sigma), Capsaicin (Sigma), Cisplatin (Cayman), Testosterone (Sigma), Bafilomycin A1 (BafA1) (Cayman), SB3667971 (Cayman), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Proteintech, Rosemont, IL, USA), anti-rabbit/mouse antibody IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), MaFbx (Proteintech), MuRF-1 (Proteintech), myostatin (Proteintech), Akt (Cell signalling, Boston, MA, USA), p-Akt (Cell signalling), MyH (DHSB, Iowa City, USA), mTOR (Cell signalling), p-mTOR (Cell signalling), Caspase3 (Cell signalling), poly (ADP-ribose) polymerase (PARP) (Cell signalling), Bax (Cell signalling), Bcl-2 (Cell signalling), LC3B (Cell signalling), p62 (Cell signalling), Cathepsin B (Cell signalling), and LAMP1 (Proteintech).
Drug preparation for administration
Capsaicin was dissolved in DMSO and diluted in ddH2O for administration to mice. Cisplatin was freshly dissolved in phosphate buffered saline immediately prior to administration to mice or exposure to cells. The solvent (0.04% DMSO) was administrated in the control group.
Cell culture and treatment procedure
The C2C12 murine myoblast cell line, obtained from Bioresource Collection and Research Center (BCRC) at Food Industry Research and Development Institute (FIRDI) (Taiwan, ROC), was cultured in high-glucose DMEM with 10% FBS at 37°C with 5% CO2. To induce differentiation, 70–80% confluent cells were cultured in a differentiating medium (DM, DMEM supplemented with 2% HS), which was refreshed every 2 days. After 6 days of differentiation, multinuclear myotubes were formed. C2C12 myotubes were pretreated with different doses of capsaicin (10, 25, 50 μM) for 24 h and then treated with cisplatin (40 μM) for 48 h.
Cell viability assay
Cells were plated in a 96-well culture plate at a density of 0.5–1 × 104 cells per well, pretreated with various concentrations of capsaicin for 24 h, and then treated with cisplatin for 48 h. At the indicated time point, cell viability was determined by a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After discarding the medium, 100 μL of the 1 mg/mL MTT was added and incubated for 3 h then aspirated, and DMSO was added to dissolve the crystal formation. ELISA reader (Molecular Devices, San Jose, CA, USA) was used at wavelengths of 570 and 630 nm to detect the absorbance.
Trypan blue exclusion test of cell number
The dye exclusion test was used to determine the number of viable cells present in a cell suspension. In the protocol presented here, a viable cell would have a clear cytoplasm whereas a nonviable cell would have a blue cytoplasm. Using a haemocytometer counting chamber, cells were counted in each of the four corner quadrants. An average of these four readings was obtained and multiplied by 104 to obtain the number of viable cells per ml in the sample.
Immunocytochemistry
C2C12 myotubes were fixed by 4% paraformaldehyde for 10 min at room temperature, permeated by 0.1% Triton X-100 in PBS for 5 min, and then blocked with 5% bovine serum albumin (BSA) in TBST for 30 min–1 h at room temperature. Myotubes were incubated with anti-MyH (MF-20, 1:100, DSHB) diluted in 5% BSA overnight at 4°C, followed by incubation with Alexa Fluor 488-goat anti-mouse IgG antibody for 1 h at room temperature. Cells were visualized under a fluorescence microscope, and the diameter of myotubes was measured by ImageJ software.
Animals and drug treatments
Male C57BL/6 mice (n = 6, 5 weeks old, weighing 18–22 g) were purchased from the National Laboratory Animal Center (Taipei, Taiwan) and maintained according to a standard animal protocol approved by the Animal Center at Taipei Medical University. Mice were maintained under a 12 h light–dark cycle at 25 ± 1 C and 65 ± 5% humidity. The dose of cisplatin was selected based on the previous study,8 considering the clinical usages of cisplatin were beginning at 15–20 mg/m2 daily for 5 days, equal to 3 mg/kg in mice.15 According to previous results, 10 and 40 mg of capsaicin per kilogram of body weight were given by oral gavage for low- and high-dose groups, respectively.16 According to previous results, the capsaicin doses for animals were revised and administrated at 10 and 40 mg/kg of body weight by stomach intubation using a round-ended needle as low- and high-dose groups.16 Capsaicin (10 and 40 mg/kg of body weight) was administered orally once a day for 5 weeks. Cisplatin (3 mg/kg body weight) and testosterone (15 mg/kg body weight) were injected intraperitonealy (IP) and subcutaneously (SC) once daily for 7 days. Testosterone was used as a positive control to protect against muscle atrophy and enhance muscle mass. After cisplatin administration for seven consecutive days without any mortality, the body weight was measured weekly during the experiment, and the gastrocnemius muscle was resected after sacrifice. All animal studies were conducted according to the protocols approved by the IACUC of Taipei Medical University (Permit No. LAC-2019-0035).
Enzyme-linked immunosorbent assay (ELISA) for pro-inflammatory cytokines
The levels of serum TNF-α in mice were determined using ELISA kits with three replicates per animal (Cayman, Ann Arbor, MI, USA, 500850-96); all procedures followed the manufacturer's instructions. A double-antibody sandwich technique was used to detect the captured TNF-α with HRP-conjugated streptavidin.
Lipid peroxidation determination
The lipid peroxidation in serum and muscle was determined by measuring the level of malondialdehyde (MDA) using 2-thiobarbituric acid-reacting substance test with three replicates per animal (TBARS, Cayman, Ann Arbor, MI, USA, 10009055) and following the manufacturer's instructions. The results were measured on a 532-nm plate reader using a VERSA Max microplate reader (Molecular Devices, San Jose, CA, USA).17
Haematoxylin–eosin (HE) staining
Gastrocnemius muscle samples were fixed, sliced, and stained by Bio-Check Laboratories Ltd. (Taipei, Taiwan). The figures were captured by ×40 magnification using an EVOS® microscope (Thermo Fisher Scientific, USA).
In vivo measurement of forelimb muscle force by grip strength meter
The forelimb muscle force of control and treated mice were measured by means of a grip strength meter (SH-III-20, China). Six mice per group were analysed. Briefly, the mice were gently lifted onto the stage and made to grab the pull-gauge by holding them by the tail. The gauge was then pulled horizontally at a constant rate by the researcher. The grip strength was recorded when the mice released the gauge. Four sequential trials were performed for each mouse. The average of the four trials was taken as the representative value for each animal. The test was performed on day 7 after cisplatin treatment, which was considered the end of the experiment.
Cathepsin B activity assay
The skeletal muscle cathepsin B activity was measured using a fluorometric assay kit (ab65300; Abcam, Cambridge, MA) following the manufacturer's instructions. Briefly, tissues were washed in ice-cold phosphate-buffered saline and then homogenized in extraction buffer. After 30 min of incubation on ice, the extract was centrifuged at 14 000 rpm for 5 min using a BCA protein assay for protein quantification. A measure of 50 μL of the sample was added and kept in the dark at 37°C for 1 h, and the fluorescence was recorded using a Thermo Varioskan Flash (Thermo Electron Corporation, Vantaa, Finland). Cathepsin B activity was measured in triplicate and was expressed as fluorescent units/mg of protein.
Western blot
Western blots were performed as described previously.17 Briefly, C2C12 myotubes or collected mouse tissues were homogenized in RIPA buffer with phosphatase/protease inhibitor. The lysates were centrifuged at 12 000 rpm for 30 min at 4°C. The supernatant was quantified for protein concentration using the BCA protein assay. Equal amounts of protein samples were separated by 10–15% sodium dodecyl-sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The PVDF membranes were blocked in 5% BSA in TBST containing 0.1% Tween 20 for 1 h at room temperature and then incubated with primary antibodies diluted in 5% BSA at 4°C overnight. Myostatin, MuRF-1, MaFbx, MyH, Akt, p-Akt, mTOR, p-mTOR, Caspase3, PARP, Bax, Bcl-2, LC3B, p62, cathepsin B, LAMP1, and GAPDH-HRP. The day after washing, blots were incubated with anti-mouse (1:10 000) and anti-rabbit (1:10 000) IgG horseradish peroxidase-conjugated secondary antibody for 2 h in TBST. An ECL chemiluminescent kit was used to visualize the antibody–antigen interaction, and the chemical luminescence of membranes was detected using an Amersham Imager 600 (GE Healthcare, New York, USA) or eBlot Touch Imager (eBlot Photoelectric Technology, Shanghai, China). The relative band intensity was measured using ImageJ software (NIH, Bethesda, MD, USA). The data were expressed as fold change compared with the untreated group (control group) from three to five independent experiments.
Statistical analysis
Data are expressed as mean ± SD (standard deviation) in vitro. Data are expressed as mean ± SEM (standard error of the mean) in vivo. The Shapiro–Wilk test was used to test the normality. For the data that passed the normality, one-way analysis of variance with Tukey's multiple comparisons test was used as a post hoc test. For the data that did not pass the normality, the Kruskal–Wallis test with Dunn's multiple comparisons test was used as a post hoc test (Supporting information, Table S1). A P value of 0.05 or lower was considered significant in all experiments. All analyses were performed using GraphPad Prism 6.0. Values of P less than 0.05 were considered to be statistically significant.
Results
Capsaicin prevents cisplatin-mediated suppression of muscle C2C12 cell viability
Using C2C12 as an in vitro model, cells were cultured in 96-well culture plates (5,000 cells per well) and then treated for 24 and 48 h with various concentrations of capsaicin. Only at 100 μM for 48 h did capsaicin show potential toxicity as the cell viability significantly decreased compared with the control group (Figure 1A). Cisplatin at various concentrations (10–50 μM), as shown in Figure 1B, significantly decreased the cell proliferation in a dose-dependent fashion. Next, we explored capsaicin's protective effect on cisplatin-induced damage. As shown in Figure 1C, cisplatin treatment significantly decreased myotube cell survival at 40 μM compared with the control, whereas capsaicin mitigated that damage dose dependently. As the dose increased, capsaicin could protect the myotube cell against cisplatin-induced muscle cell damage, especially at 10, 25, and 50 μM. These results indicated that capsaicin could prevent cisplatin-mediated suppression of muscle C2C12 cell viability.
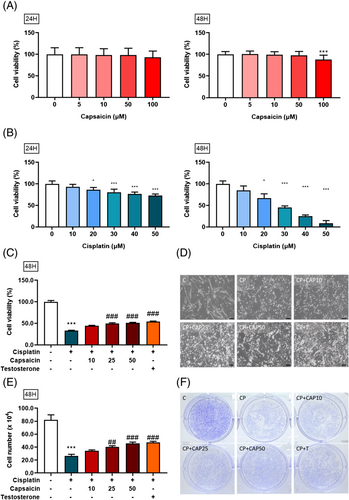
Capsaicin promotes muscle cell survival against cisplatin-induced suppression of C2C12 cell number and morphology change
With the recovery of cell viability under the influence of capsaicin, we further wanted to assess whether the morphology of myotube cells would be affected by cisplatin and salvaged by capsaicin as well. Myotubes were visualized and photographed with a ×100 objective on a microscope. Images were captured randomly in a culture dish for each condition. Cisplatin alone clearly decreased the myotube cell size and length as compared with control, with the induction of cell atrophy at 40 μM. However, pretreatment with different doses of capsaicin could regenerate the cell length, and the myotube cell diameter became longer than in the cisplatin group, especially at 25 and 50 μM (Figure 1D). Similarly, different experiments were performed to evaluate capsaicin's effect on cisplatin-induced cell damage. The cell number was measured by trypan blue assay, as shown in Figure 1E. The results indicate that treatment with cisplatin alone significantly reduced the myotube cell number at 40 μM compared with the control. However, pretreatment with capsaicin at 10, 25, and 50 μM significantly recovered myotube cells compared with the cisplatin group. The results revealed that capsaicin promoted cell survival and inhibited cisplatin-induced damage in myotube cells. Furthermore, the result of cell staining shown in Figure 1F was consistent with the result in cell number (Figure 1E). Finally, using Crystal violet DNA nuclear stain, as a quick and versatile assay for screening cell survival, pretreatment with capsaicin significantly recovered myotube cells as compared with the cisplatin-alone group in a dose-dependent manner (Figure 1F).
Capsaicin recovers cisplatin-induced C2C12 cell atrophy on levels of cell morphology and muscle atrophy related protein expression
As shown in Figure 2A, immunocytochemistry (ICC) was used to stain, visualize, and measure the protein expression of MyH (myosin heavy chain), a muscle differentiation marker, which, when stained, appeared as green fluorescence (Alexa Fluor 488). The results showed that the administration of cisplatin at 40 μM significantly attenuated the myotube diameter, with a subsequent significant reduction in the length of the myotube cell. However, pretreatment with capsaicin could restore it and increase its diameter in a dose-dependent manner (Figure 2B). Next, we quantified the fluorescence intensity indicating the protein expression level (Figure 2C). The results indicate that capsaicin is capable of improving muscle atrophy caused by cisplatin, thus reducing the ability of cisplatin to inhibit muscle differentiation and recover its length as well as restore MyH protein expression. In addition, the expression of MaFbx (Figure 2D), MuRF-1 (Figure 2E) and myostatin (Figure 2F) was elevated in cisplatin-treated cells compared with the control group.

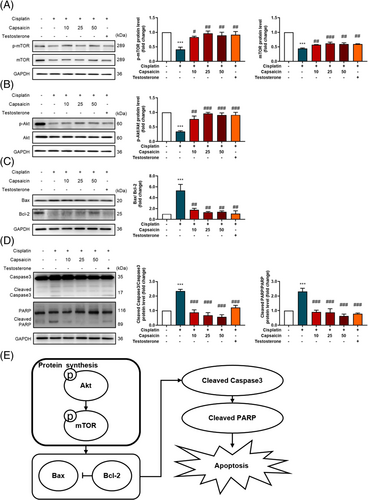
Capsaicin recovers muscle protein synthesis factor and apoptosis-related protein expression
To further investigate the mechanism of cisplatin-induced muscle cell atrophy, the protein expression of the muscle-synthesis- and muscle-degradation-related markers was assessed in myotube cells. The results showed that cisplatin alone significantly reduced the protein expression of muscle-synthesis factor, p-mTOR and mTOR (Figure 3A), and p-Akt/Akt (Figure 3B) compared with the control group. Importantly, capsaicin could ameliorate cisplatin-induced muscle atrophy by recovering p-Akt/Akt ratio and mTOR, which stimulated protein synthesis in skeletal muscle. Furthermore, because apoptosis is associated with subsequent muscle atrophy, in order to investigate the effect of capsaicin on the apoptosis signalling pathway in C2C12 cells, the expression of apoptosis-related proteins was analysed by Western blotting. The results demonstrate that after cisplatin treatment, the expression of apoptosis-related protein Bax/Bcl-2 (Figure 3C) and downstream cleaved caspase3 as well as cleaved PARP (Figure 3D) increased significantly compared with control, while pretreatment with capsaicin could significantly reduce the expression of apoptotic proteins such as cleaved caspase3, cleaved PARP, and the Bax/Bcl-2 ratio. These findings indicate that capsaicin efficiently alleviates cisplatin-induced muscle atrophy through the stimulation of protein synthesis signalling pathway and the reduction of apoptosis (Figure 3E).
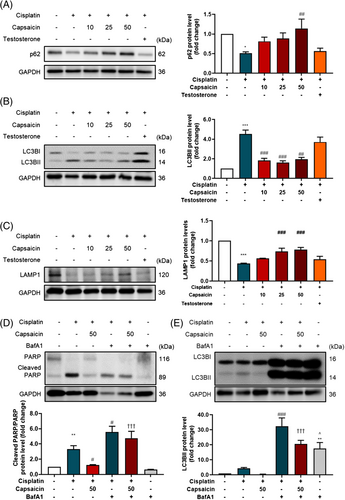
Capsaicin recovers cisplatin-induced autophagy dysfunction
Muscle atrophy accompanied lysosome fusion dysfunction.18 The protein expression of the autophagy-related markers was assessed in myotube cells. The results showed that cisplatin alone significantly reduced the protein expression of p62 (Figure 4A) and increased the LC3BII protein expression (Figure 4B) compared with the control group. However, the lysosome marker LAMP1 with the reduction in LAMP1 (Figure 4C) shows that cisplatin treatment impaired the lysosome function in autophagy progression. Lysosome fusion inhibitor Bafilomycin A1 (BafA1) was used to evaluate the lysosome fusion function in muscle atrophy. The results show that the inhibition of lysosome fusion could exacerbate cisplatin-induced apoptosis (Figure 4D), and autophagosome synthesis (Figure 4E), which was barely recovered by capsaicin.
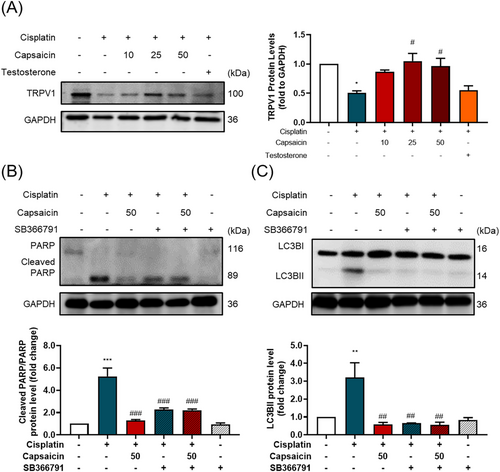
The role of TRPV1 channel in cisplatin-induced muscle atrophy and the recovery effect of capsaicin
To discover the potential role of capsaicin in muscle atrophy modulation, we examined the TRPV1 channel protein expression; the protein expression of TRPV1 was decreased by cisplatin and recovered by capsaicin (Figure 5A). Capsaicin, as a TRPV1 agonist, may effectively modulate TRPV1 signalling transduction, which may cause the muscle hypertrophy effect.19 The intervention of TRPV1 antagonist SB366791 not only disturbed capsaicin's effect on cisplatin-induced damage but also subsided the cisplatin-induced harmfulness (Figure 5B,C), which indicates that the TRPV1 modulations are important in muscle atrophy and capsaicin's effect.
Capsaicin can alleviate cisplatin-induced body weight loss in vivo
We utilized an in vivo model, with a flow chart shown in Figure 6A. Mice were divided into five groups, including a control group, cisplatin group (intraperitoneal injection, 3 mg/kg), low-dose capsaicin group (oral administration, 10 mg/kg), high-dose capsaicin group (oral administration, 40 mg/kg), and testosterone group (subcutaneous injection, 15 mg/kg). Mice were pretreated with capsaicin for 5 weeks. At the fifth week, cisplatin was treated for 1 week. Mice were weighed every week over the experiment. The results show that there was no significant weight change after capsaicin treatment. After cisplatin injection, the weight of the cisplatin group was significantly lower than that of the control group. However, pretreatment with either low- or high-dose capsaicin (10 and 40 mg/kg body weight) could significantly recover this body weight loss compared with cisplatin alone group (Figure 6B), indicating that capsaicin can alleviate the body weight loss caused by the chemotherapy drug cisplatin.
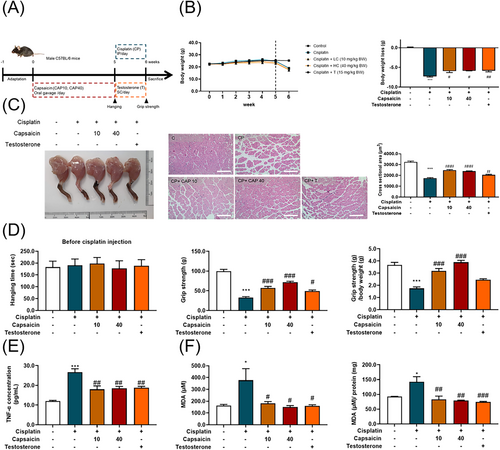
Capsaicin can ameliorate cisplatin-induced muscle loss and muscle strength in vivo
To examine the effect of capsaicin on cisplatin-induced muscle loss and atrophy, we used the gastrocnemius muscle figure, gastrocnemius muscle cross-sectional area, and the histological examination (Figure 6C). Pretreatment with capsaicin for 5 weeks did not alter the muscle strength, measured by the forelimb hanging test, and after cisplatin injection, the forelimb grip strength and the normalization to body weight were measured (Figure 6D). Cisplatin treatment decreased the gastrocnemius muscle fibre cross-sectional area and weakened the forelimb grip strength compared with that of the healthy mouse control. However, the administration of capsaicin could significantly minimize the loss of the myofibre area induced by cisplatin-induced mice. Additionally, we observed that capsaicin significantly enhanced the forelimb grip strength compared with the cisplatin group. These data indicate that capsaicin administration could recover cisplatin-induced muscle side-effect conditions, at least in part, by preventing muscle loss and atrophy.
Capsaicin alleviates cisplatin-induced cytokine secretion, and oxidative stress
By using an ELISA kit to analyse the TNF-α secretion (Figure 6E), it was found that capsaicin and testosterone significantly decreased TNF-α secretion. Moreover, alleviated grip strength and the myofibre area were assessed by histological examination. Because the muscle loss and weakness may be related to oxidative stress,20 we examined both the serum and muscle MDA concentrations (Figure 6F); capsaicin and testosterone could effectively decrease the MDA level, showing the ability to modulate oxidative stress.
Capsaicin administration in cisplatin-induced mice decreases muscle degradation-related protein expression
It has been reported that, cisplatin-induced inflammatory cytokines such as IL-6, TNF-α, and IL-1 are critical inducers of muscle atrophy.21 To further characterize the muscle loss and atrophy induced by cisplatin, the protein expression of the myostatin (Figure 7A), MaFbx, and MuRF-1 (Figure 7B) were assessed in the gastrocnemius muscle. As reported previously, the expression of myostatin, MaFbx, and MuRF-1 were elevated in the cisplatin-treated group. Consistent with its preventive effect on muscle wasting, capsaicin administration reduced the levels of MaFbx, MuRF-1, and myostatin. These findings indicate that capsaicin efficiently suppresses cisplatin-induced inflammatory responses and alleviates muscle breakdown.
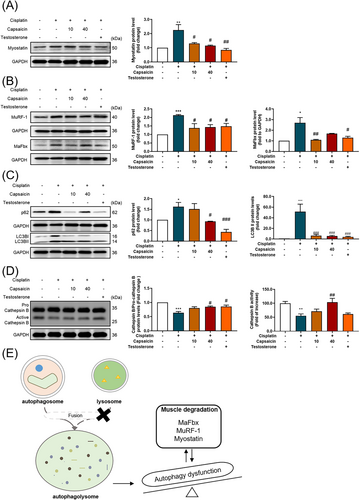
Capsaicin recovers lysosome-induced autophagy dysfunction in cisplatin-induced muscle atrophy animal model
Autophagy is considered a major homeostasis regulator of protective mechanisms against the side effects of chemotherapy.22 To explore that notion, we examined the autophagy-related protein expression of p62 and LC3B (Figure 7C); the results show an autophagolysosome fusion dysfunction.23 Through the examination of lysosome protein expression, the results prove that the fusion dysfunction may be related to the cisplatin-induced cathepsin B protein expression and cathepsin B activity (Figure 7D), causing autophagy dysregulation (Figure 7E). These results indicate that capsaicin treatment could improve cisplatin-induced lysosome dysfunction and enhance the regulation of autophagy.
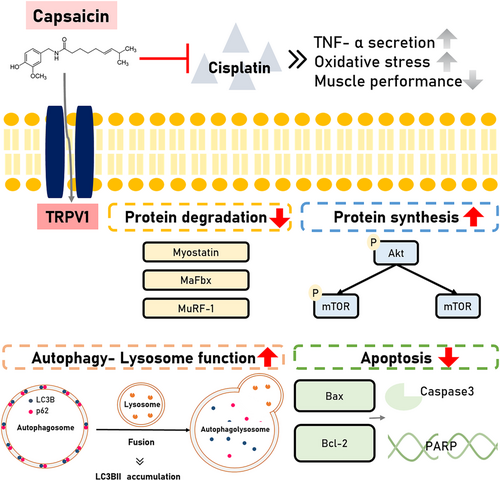
Impact of capsaicin administration in cisplatin-induced muscle atrophy
Different in vivo and in vitro models indicated that capsaicin had its ability on cisplatin-induced muscle atrophy (Figure 8). Both models revealed the capsaicin's effect on muscle or protein degradation-related protein expression through TRPV1 modulation, lysosome function recovery , the decrease of oxidative stress, and cytokine secretion to recover the muscle performance.
Discussion
In this study, we demonstrated cisplatin's effect on muscle-atrophy-related protein expression and lysosome dysfunction. Notably, pretreatment with capsaicin could effectively improve cisplatin-induced muscle atrophy through the regulation of protein synthesis and protein degradation. Muscle atrophy occurs in the bypass period in sarcopenia, and as a rapid and irreversible stage, which may need early intervention to prevent these circumstances.24 The model we used was to support the pretreatment effect of capsaicin on muscle atrophy, but in clinical application may need co-treatment to alleviate the chemotherapy-induced muscle atrophy. Our results also supported that the co-treatment with capsaicin could minimize apoptosis, autophagy, and muscle-degradation-related protein expression in vitro study, and significantly decrease the weight loss and increase the grip strength compared with cisplatin group in in vivo study (Figures S1 and S2).
Muscle wasting is a devastating cisplatin-related side effect contributing to decreased quality of life and survival.25 Muscle atrophy progress may precipitate the circulation of catabolic factors such as TNF-α secretion activation26 and subsequently trigger the accumulation of oxidative stress.27 Consistence with the present results, cisplatin induces TNF-α level elevation and increases the oxidative stress in serum and animal muscles through TBARS assessment.
Capsaicin is known as the agonist of TRPV1; TRPV1 plays an important role in the muscle exercise performance and improves the energy metabolism in skeletal muscle.28, 29 Capsaicin may activate TRPV1 channel-mediated signalling transduction, including the phosphorylation of mTOR and MAPK signalling transduction,30 to further modulate muscle hypertrophy.19 Capsaicin's antitumour activity was also shown with strong anticancer activity by targeting multiple signalling pathways, including the activation of apoptosis, cell-growth arrest, and inhibition of metastasis.31 Moreover, the modulation of TRPV1 as a main target in cisplatin-induced toxicity and a cancer-suppression effect was also shown.32 Our results indicate that capsaicin treatment significantly increases TRPV1 protein expression and activates the downstream signalling pathway. By using a TRPV1 antagonist, we found out that cisplatin's and capsaicin's effects are both decreased by the blockage of the TRPV1 channel.
Cisplatin not only changed the protein degradation/synthesis progress but also altered the autophagy regulation. In a cisplatin-induced muscle atrophy model, LC3B II and p62 were increased along with the downregulation of Akt.6 In muscle atrophy progression, the decrease in lysosome blocked the autophagolysosome fusion and limited the autophagy progression.18 A CT-26-bearing cancer cachexia model showed that a decrease in cathepsin activity modulates autophagy dysfunction in skeletal muscle.33 Cisplatin-induced hepatotoxicity studies also show decreased cathepsin B in cisplatin-treated hepatocytes.34 Lysosome dysfunction also acts as one pathology of the effects of chemotherapy drugs on anticancer properties with disrupted autophagy influx,35, 36 causing ROS accumulation to decrease cancer cell viability.37
In the present study, capsaicin treatment caused the recovery of the lysosome and achieved autophagy balance. Cisplatin injection decreases the active-form cathepsin B protein expression and activity. A deficiency in cathepsin B activity causes unusual morphology and reduced survival in the myoblast and differentiation with impaired cellular fusion.38 Using lysosome fusion inhibitor BaFA1 indicated that cisplatin treatment could increase the autophagosome,23 but the reduction in lysosome marker LAMP1 protein expression caused lysosome fusion dysfunction and impaired the progression of autophagy. With capsaicin treatment, the lysosome dysfunction could recover and the autophagy progress was repaired. This study shows the effects of capsaicin in cisplatin-induced muscle atrophy in healthy conditions. However, chemotherapeutic drugs are only used in cancer patients rather than in healthy ones. Thus, the tumour-bearing animal will be further analysed in the future. It is worthwhile to explore whether capsaicin has recovery effects against tumours with chemotherapeutic drugs-induced muscle wasting to strengthen the clinical usage ability.
In conclusion, capsaicin could ameliorate cisplatin-induced muscle atrophy by stimulating protein synthesis in skeletal muscle and downregulating protein degradation-related protein expression. Additionally, capsaicin could downregulate apoptosis-related markers, which are induced by cisplatin, while restoring the autophagy-related lysosome dysfunction through TRPV1 modulation. These findings suggest that capsaicin may be a protective agent against chemotherapy-induced muscle loss and atrophy, contributing to gaining insight into the new molecular modulation and potential usage of capsaicin in the future.
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.39
Funding
This study was supported by the grants (MOST109-2314-B-038-059, MOST109-2628-B-038-015, MOST110-2314-B-038-158, MOST110-2628-B-038-018 and MOST110-2811-B-038-543) from the Ministry of Science and Technology, Taiwan; and (NSTC111-2811-B-038-022, NSTC111-2314-B-038-006 and NSTC111-2628-B-038-019) from the National Science and Technology Council, Taiwan.
Conflicts of Interest
The authors declare no conflict of interest.




