The impact of sarcopenia on short-term and long-term mortality in patients with septic shock
Abstract
Background
Despite medical advances, septic shock remains one of the main causes of high mortality in critically ill patients. Although sarcopenia is considered a predictor of mortality in septic shock patients, most studies have only investigated short-term mortality, and those on long-term prognosis are limited. We investigated the impact of sarcopenia on long-term mortality in a large patient population with septic shock.
Methods
A retrospective cohort study comprising 905 patients with septic shock was conducted from 2008 to 2019. Sarcopenia was defined based on the measurement of the total abdominal muscle area, assessed using abdominal computed tomography scans. Thereafter, we stratified the patients into two groups—sarcopenia and non-sarcopenia groups—and compared the impact of sarcopenia on short-term (28 days) and long-term (1 year and overall) mortality using multivariable Cox proportional analysis.
Results
A total of 905 patients were included, and the mean age was 65.7 ± 15.1 years. Among them, 430 (47.5%) patients were male and 407 (45.0%) had sarcopenia. We found that the 28 day, 1 year, and overall mortality rates in the sarcopenia group were significantly higher than those in the non-sarcopenia group (13.8% vs. 6.4%, P < 0.001; 41.8% vs. 21.7%, P < 0.001; 62.2% vs. 35.7%, P < 0.001, respectively). Univariable Cox analysis showed that the sarcopenia group had a significant association with the increase in each mortalities compared with the non-sarcopenia group (28 day mortality, hazard ratio (HR) = 2.230, 95% confidence interval (CI) [1.444–3.442], P < 0.001; 1 year mortality, HR = 2.189, 95% CI [1.720, 2.787], P < 0.001; overall mortality, HR = 2.254, 95% CI [1.859, 2.734], P < 0.001). Multivariable Cox analysis showed that both the short-term and long-term mortality rates remained significantly higher in the sarcopenia group than in the non-sarcopenia group, even after adjusting for confounding variables (28 day mortality, HR = 2.116, 95% CI [1.312, 3.412], P = 0.002; 1 year mortality, HR = 1.679, 95% CI [1.291, 2.182], P < 0.001; overall mortality, HR = 1.704, 95% CI [1.381, 2.102], P < 0.001).
Conclusions
Sarcopenia was associated with both short-term and long-term mortality in patients with septic shock. In clinical settings, close attention should be paid to these patients for both short-term and long-term outcomes.
Introduction
Despite medical advances, septic shock remains one of the main issues associated with high mortality in critically ill patients.1 However, sepsis is a heterogeneous disease state, and we need to consider the diverse sources of heterogeneity, including various infection etiologist, individual host co-morbidities, and unique genetics of the patients.2
Sarcopenia is characterized by declining muscle mass, strength, and physical function.3 An increasing number of studies examining the impact of sarcopenia on clinical outcomes in critically ill patients, including those with sepsis, have been published.4-6 Moreover, these data showed a high prevalence (30–70%) of sarcopenia in intensive care units (ICU),7, 8 and sarcopenia was significantly associated with negative clinical outcomes, such as falls, fractures, poor quality of life, mortality, and cognitive dysfunction.9-12
Despite many investigations on the impact of sarcopenia on the adverse outcomes in ICU patients, only a few studies have assessed sepsis,13-15 and those on septic shock are more limited.16, 17 In addition, these previous studies were mostly focused on short-term mortality and enrolled a small number of subjects. Thus, in this study, we explored the association of sarcopenia with both short-term and long-term mortality in a large patient population with septic shock.
Subjects and methods
Study population
We retrospectively analysed adult patients with septic shock who were admitted to the emergency department (ED) of Severance Hospital, a large tertiary care teaching hospital with 2,400 beds in South Korea, from July 2008 to March 2019. Septic shock was defined according to the Surviving Sepsis Campaign guidelines. In our institution, the guidelines designed by Dellinger et al. were used to define septic shock until 201818, 19; from 2019, the Sepsis-3 guidelines have been used to define septic shock.1, 20 Our institution had a clinical pathway correspond to a quality improvement activity for patients visiting the ED who were suspected of having septic shock. This is a clinical process, but not for research, which means it goes through without informed consent and registration. The management process was based on the Surviving Sepsis Campaign guidelines, which mainly consisted of fluid resuscitation, early empiric antibiotic therapy, and vasopressor administration, if needed. Detailed explanations of our clinical pathway for sepsis or septic shock have been described previously.21 According to the clinical pathway workflow, patients fulfilling the following are not candidates for activation of the clinical pathway: (i) age <18 years, (ii) pregnancy status, (iii) acute coronary syndrome, (iv) acute cerebrovascular accident, (v) active gastrointestinal bleeding, (vi) drug overdose, (vii) requirement for immediate surgery, (viii) trauma, (ix) do-not-resuscitation status, or (x) transfer to another institution. Finally, the clinical pathway was activated for patients meeting criteria for septic shock defined in accordance with the Surviving Sepsis Campaign guidelines. In this study, we only reviewed the medical records of patients who were activated in the clinical pathway. In addition, we only enrolled patients with septic shock who had abdominal computed tomography (CT) scans obtained at the ED within 24 h of hospitalization. This study was approved by the institutional review board (IRB) of the Yonsei University Health System Clinical Trial Center (4-2021-0765). The IRB waived the requirement for written consent from the patients, as this study followed a retrospective design and did not contain any personally identifiable information during the study process.
Measurement of muscle area
The total abdominal muscle area (TAMA), which includes the paraspinal and abdominal wall muscles, was measured using abdominal CT images. The cross-sectional TAMA values were calculated at the level of the third lumbar vertebra, as previously described.22, 23 For distinct muscle areas, an image analysis software using an AquariusNET Server (TeraRecon, Foster City, CA, USA) was used; this analysis was based on Hounsfield unit (HU) thresholds. The cross-sectional areas were measured automatically by the sum of the muscle tissue pixels and multiplication of the pixel area. When required, the muscle tissue boundaries were manually checked. The TAMA values were assessed and quantified using thresholds of −29 to 150 HU. The cross-sectional TAMA values were standardized by the square of the height of the subjects and were reported in cm2/m2 as the muscle index. Examples of the TAMA measurements are shown in Figure 1.
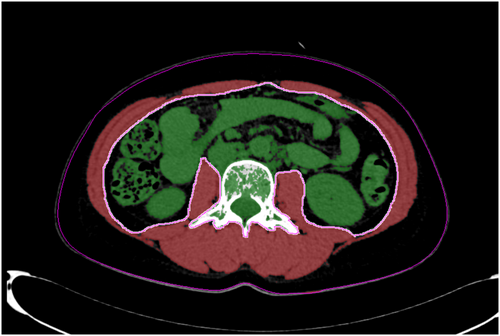
Variables and definitions
We defined sarcopenia as muscle index <45.4 cm2/m2 in men and 34.4 cm2/m2 in women, referring to the results of previous studies.24 The underlying co-morbidities of the patients were defined according to the International Classification of Diseases, 10th revision. The Charlson Comorbidity Index (CCI) was used to categorize patients' overall co-morbidities at ED visits, and Sequential Organ Failure Assessment (SOFA) scores were used to stratify the disease severity. Moreover, we investigated the results of laboratory tests conducted at ED admission. For information on mortality, we used data obtained from the Ministry of the Interior and Safety of South Korea, which handles the information of death for all Koreans.
Statistical analysis
We compared patients with and without sarcopenia. The independent t-test was used to compare continuous variables between the two groups. The chi-square or Fisher's exact test was used to compare categorical variables. The prognostic factors for mortality were analysed using the Cox proportional hazards model. The proportionality of hazards of Cox's model was verified by tests using residuals and graphs.25 In addition, multivariable regression models were constructed by adjusting confounding variables that were selected based on the clinical significance among the risk factors with P < 0.05 in univariable analysis after checking for multicollinearity. Multicollinearity was checked for the variables of multivariable analysis, including the components of CCI, and was defined as a variance inflation factor of >5.26 Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated from this analysis. We also calculated E-values representing the amount of unmeasured confounding required to explain any association between exposure and mortality.27, 28 Kaplan–Meier (K-M) survival analyses and log-rank tests were performed to compare the short-term and long-term prognoses of patients with and without sarcopenia. Each patient was followed up until death or until the end of the study period (30 September 2020), whichever came first. Statistical significance was set at P < 0.05. All statistical analyses were performed using SAS (version 9.4, SAS Inc., Cary, NC, USA) and R package, version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Among the 905 patients enrolled, 407 (45.0%) had sarcopenia (Figure 2). When we stratified the patients into two groups of sarcopenia and non-sarcopenia groups, there were more male patients and older patients in the sarcopenia group than in the non-sarcopenia group. Moreover, more cases of cerebral vascular disease, dementia, chronic pulmonary disease, ulcer disease, hemiplegia, metastatic cancers, and therefore, higher CCI were found in the sarcopenia group compared with the non-sarcopenia group. For sources of infections, there was no significant difference between the two groups.
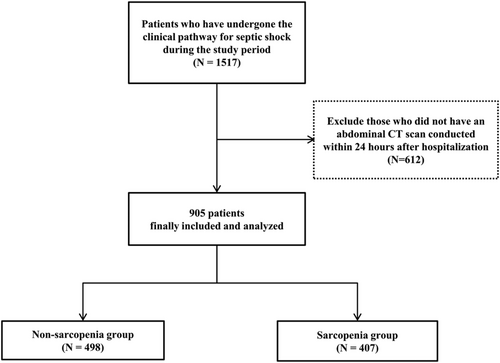
Laboratory findings showed that white blood cell counts, platelet count, serum blood urea nitrogen, and lactate levels were significantly higher and that serum albumin was significantly lower in the sarcopenia group than in the non-sarcopenia group. The SOFA score was higher in the sarcopenia group than in the non-sarcopenia group.
There was no significant difference in the hospital and ICU length of stay as well as the occurrence of complications between the sarcopenia and non-sarcopenia groups. On the other hand, the 28 day, 1 year, and overall mortality rates were significantly higher in the sarcopenia group than in the non-sarcopenia group (Table 1).
| Variables | Non-sarcopenia (N = 498) | Sarcopenia (N = 407) | P-value |
|---|---|---|---|
| Male (N, %) | 160 (32.1) | 270 (66.3) | <0.001 |
| Age (years) | 63.6 ± 15.4 | 68.3 ± 14.4 | <0.001 |
| Co-morbidities (N, %) | |||
| Hypertension | 268 (53.82) | 212 (52.09) | 0.605 |
| Diabetes mellitus | 165 (33.13) | 154 (37.84) | 0.141 |
| Cerebral vascular disease | 70 (14.06) | 90 (22.11) | 0.002 |
| Chronic liver disease | 47 (9.44) | 32 (7.86) | 0.404 |
| Congestive heart failure | 22 (4.42) | 21 (5.19) | 0.590 |
| Chronic kidney disease | 84 (16.90) | 59 (14.50) | 0.324 |
| Peripheral vascular disease | 3 (0.60) | 1 (0.25) | 0.632 |
| Coronary disease | 72 (14.46) | 73 (17.94) | 0.156 |
| Dementia | 24 (4.82) | 40 (9.83) | 0.004 |
| Chronic pulmonary disease | 26 (5.22) | 39 (9.58) | 0.012 |
| Connective tissue disease | 16 (3.21) | 8 (1.97) | 0.245 |
| Ulcer disease | 19 (3.82) | 32 (7.86) | 0.009 |
| Hemiplegia | 31 (6.22) | 50 (12.29) | 0.002 |
| Cancer | 135 (27.11) | 121 (29.73) | 0.384 |
| Hepatobiliary | 27 (5.42) | 28 (6.88) | 0.447 |
| Gastrointestinal | 45 (9.04) | 38 (9.34) | 0.742 |
| Genitourinary | 33 (6.63) | 28 (6.88) | 0.807 |
| Lung | 7 (1.41) | 9 (2.21) | 0.457 |
| Others | 23 (4.62) | 18 (4.42) | 0.638 |
| Metastatic cancer | 29 (5.82) | 39 (9.58) | 0.033 |
| Solid organ transplantation | 2 (0.40) | 1 (0.25) | 0.253 |
| AIDS | 2 (0.40) | 1 (0.25) | >0.999 |
| CCI | 4.5 ± 3.2 | 5.4 ± 3.1 | <0.001 |
| Source of infections (N, %) | 0.474 | ||
| Pneumonia | 75 (15.06) | 78 (19.16) | |
| Intra-abdominal infections | 122 (24.50) | 94 (23.10) | |
| Genitourinary infections | 205 (41.16) | 157 (38.57) | |
| Skin and soft tissue infections | 17 (3.41) | 15 (3.69) | |
| Primary bacteraemia | 12 (2.41) | 15 (3.69) | |
| Others | 67 (13.45) | 48 (11.80) | |
| SOFA score | 7.7 ± 3.2 | 8.1 ± 3.1 | 0.049 |
| Laboratory findings | |||
| WBC (/mm3) | 13033.7 ± 8,353 | 14330.7 ± 10133.7 | 0.035 |
| Platelet (/mm3) | 192347.4 ± 112568.3 | 215824.3 ± 211555.8 | 0.033 |
| BUN (mg/dL) | 32.9 ± 23.7 | 37.5 ± 30.7 | 0.011 |
| Creatinine (mg/dL) | 2.1 ± 1.9 | 2 ± 1.7 | 0.676 |
| AST (unit/L) | 88.8 ± 158 | 102.4 ± 242.3 | 0.308 |
| ALT (unit/L) | 55.6 ± 110.3 | 63.9 ± 190.4 | 0.417 |
| Total bilirubin (mg/dL) | 1.3 ± 1.6 | 1.2 ± 1.3 | 0.122 |
| Albumin (g/dL) | 3.2 ± 0.7 | 3.1 ± 0.7 | 0.001 |
| Bicarbonate (mEq/L) | 17.6 ± 4.7 | 17.3 ± 5.1 | 0.367 |
| CRP (mg/L) | 138.5 ± 111.3 | 147.2 ± 111.6 | 0.246 |
| Lactate (mg/dL) | 3.5 ± 3.2 | 4.2 ± 3.5 | 0.005 |
| Muscle index (cm2/m2) | 46 ± 19 | 34.6 ± 6.5 | <0.001 |
| Hospital length of stay (days) | 18.8 ± 20.5 | 20.5 ± 37.9 | 0.557 |
| Intensive care unit length of stay (days) | 7.1 ± 8.2 | 6.4 ± 10.9 | 0.235 |
| Mortality (N, %) | 178 (35.74) | 253 (62.16) | <0.001 |
| 28 days | 32 (6.43) | 56 (13.76) | <0.001 |
| 1 year | 108 (21.69) | 170 (41.77) | <0.001 |
| Overall | 178 (35.74) | 253 (62.16) | <0.001 |
| Complications | |||
| Renal failure | 132 (27.10) | 130 (32.66) | 0.072 |
| Liver failure | 23 (4.72) | 19 (4.80) | 0.958 |
| Heart failure | 31 (6.35) | 30 (7.52) | 0.495 |
| ARDS | 11 (2.25) | 15 (3.76) | 0.186 |
| DIC | 24 (4.91) | 19 (4.76) | 0.920 |
- AIDS, acquired immune deficiency syndrome; ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; BUN, blood urea nitrogen;
- CRP, C-reactive protein; CCI, Charlson co-morbidity index; DIC, disseminated intravascular coagulation; SOFA, sequential organ failure assessment; WBC, white blood cell.
- Mean ± standard deviation for the continuous variables; N (%) for categorical variables.
Univariable analysis for the 28 day, 1 year, and overall mortality
Next, we performed univariable Cox proportional analysis to assess the 28 day, 1 year, and overall mortality. The sarcopenia group showed a significant association with an increase in 28 day, 1 year, and overall mortality when compared with the non-sarcopenia group (28 day mortality, HR = 2.230, 95% CI [1.444–3.442], P < 0.001; 1 year mortality, HR = 2.189, 95% CI [1.720, 2.787], P < 0.001; overall mortality, HR = 2.254, 95% CI [1.859, 2.734], P < 0.001) (Table 2). Similarly, the K-M curve showed that the 28 day, 1 year, and overall mortality rates in the sarcopenia group were significantly higher than those in the non-sarcopenia group (Figure 3).
| Variables | 28 day | 1 year | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | |||||||
| HR, 95% CI | P-value | HR, 95% CI | P-value | HR, 95% CI | P-value | HR, 95% CI | P-value | HR, 95% CI | P-value | HR, 95% CI | P-value | |
| Sarcopenia vs. None | 2.230 (1.444, 3.442) | <0.001 | 2.116 (1.312, 3.412) | 0.002 | 2.189 (1.720, 2.787) | <0.001 | 1.679 (1.291, 2.182) | <0.001 | 2.254 (1.859, 2.734) | <0.001 | 1.704 (1.381, 2.102) | <0.001 |
| Female vs. Male | 0.903 (0.594, 1.371) | 0.630 | 1.576 (0.995, 2.498) | 0.053 | 0.617 (0.486, 0.784) | <0.001 | 0.953 (0.735, 1.236) | 0.717 | 0.581 (0.479, 0.703) | <0.001 | 0.886 (0.719, 1.092) | 0.256 |
| Age (per 1 year increase) | 1.033 (1.016, 1.051) | <0.001 | 1.026 (1.005, 1.047) | 0.015 | 1.035 (1.025, 1.045) | <0.001 | 1.019 (1.007, 1.030) | 0.001 | 1.038 (1.029, 1.046) | <0.001 | 1.018 (1.009, 1.028) | <.001 |
| CCI (per 1 unit increase) | 1.098 (1.034, 1.165) | 0.002 | 1.013 (0.937, 1.096) | 0.742 | 1.165 (1.128, 1.202) | <0.001 | 1.102 (1.059, 1.146) | <0.001 | 1.180 (1.036–1.179) | 0.002 | 1.119 (1.084, 1.156) | <0.001 |
| SOFA (per 1 unit increase) | 1.252 (1.177, 1.331) | <0.001 | 1.152 (1.073, 1.237) | <0.001 | 1.149 (1.107, 1.192) | <0.001 | 1.070 (1.028, 1.114) | 0.001 | 1.124 (1.091, 1.158) | <0.001 | 1.048 (1.013, 1.083) | 0.006 |
| Albumin (per 1 g/dL increase) | 0.392 (0.299, 0.515) | <0.001 | 0.425 (0.307, 0.588) | <0.001 | 0.408 (0.349, 0.476) | <0.001 | 0.447 (0.374, 0.535) | <0.001 | 0.497 (0.436, 0.565) | <0.001 | 0.56 (0.484, 0.648) | <0.001 |
| CRP (per 1 mg/L increase) | 1.002 (1.001, 1.004) | 0.005 | 1.000 (0.998, 1.002) | 0.730 | 1.001 (1.000, 1.002) | 0.122 | - | 1.000 (0.999, 1.001) | 0.778 | - | ||
| Lactate (per 1 mg/dL increase) | 1.165 (1.119, 1.212) | <0.001 | 1.166 (1.108, 1.226) | <0.001 | 1.114 (1.085, 1.144) | <0.001 | 1.102 (1.068, 1.138) | <0.001 | 1.092 (1.067, 1.118) | <0.001 | 1.077 (1.048, 1.107) | <0.001 |
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CCI, Charlson co-morbidity index; CRP, C-reactive protein; MAP, mean arterial pressure; SOFA, sequential organ failure assessment; WBC, white blood cell.
- Multivariate analysis was conducted with adjustment for age, sex, CCI, SOFA, serum albumin, CRP (for only 28 day mortality), and lactate levels.
- Multicollinearity was checked for the variables of multivariable analysis, including the components of CCI.
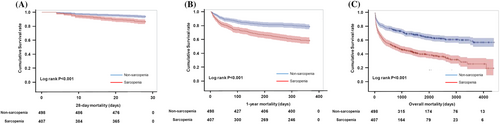
In addition, a 1 year increase in age was significantly associated with an increase in 28 day, 1 year, and overall mortality by 3.3%, 3.5%, and 3.8%, respectively. Women had significantly lower 1 year and overall mortality rates than men. Moreover, CCI and SOFA scores were also significantly related to increased 28 day, 1 year, and overall mortality. However, the HRs of serum albumin were 0.392 (0.299, 0.515) for 28 day mortality, 0.408 (0.349, 0.476) for 1 year mortality, and 0.497 (0.436, 0.565) for overall mortality, whereas that of serum lactate were 1.165 (1.119, 1.212) for 28 day mortality, 1.114 (1.085, 1.144) for 1 year mortality, and 1.092 (1.067, 1.118) for overall mortality. For C-reactive protein (CRP) level was significantly associated only with the increase in the 28 day mortality, but not the 1 year and overall mortality (Table 2). Furthermore, the 28 day, 1 year, and overall mortality rates were significantly decreased by 3.4%, 2.4%, and 2.5%, respectively, with a 1 cm2/m2 increase in the muscle index (Table 3). The results of univariable analysis of other variables for the overall mortality are shown in the Supporting Information, Table S1.
| Variables | 28 day | 1 year | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | |||||||
| HR, 95% CI | P-value | HR, 95% CI | P-value | HR, 95% CI | P-value | HR, 95% CI | P-value | HR, 95% CI | P-value | HR, 95% CI | P-value | |
| Muscle Index (per 1 cm2/m2 increase) | 0.96 (0.942, 0.99) | 0.006 | 0.969 (0.945, 0.994) | 0.014 | 0.976 (0.963, 0.99) | 0.001 | 0.978 (0.964, 0.992) | 0.002 | 0.975 (0.964, 0.986) | <0.001 | 0.974 (0.963, 0.986) | <0.001 |
| Female vs. Male | 0.903 (0.594, 1.371) | 0.630 | 1.036 (0.594, 1.371) | 0.876 | 0.617 (0.486, 0.784) | <0.001 | 0.722 (0.561, 0.931) | 0.012 | 0.581 (0.479, 0.703) | <0.001 | 0.653 (0.532, 0.801) | <0.001 |
| Age (per 1 year increase) | 1.033 (1.016, 1.051) | <0.001 | 1.025 (1.004, 1.046) | 0.020 | 1.035 (1.025, 1.045) | <0.001 | 1.019 (1.008, 1.031) | 0.001 | 1.038 (1.029, 1.046) | <0.001 | 1.018 (1.009, 1.027) | <0.001 |
| CCI (per 1 unit increase) | 1.098 (1.034, 1.165) | 0.002 | 1.018 (0.942, 1.100) | 0.657 | 1.165 (1.128, 1.202) | <0.001 | 1.101 (1.059, 1.145) | <0.001 | 1.180 (1.036–1.179) | 0.002 | 1.119 (1.083, 1.155) | <0.001 |
| SOFA (per 1 unit increase) | 1.252 (1.177, 1.331) | <0.001 | 1.147 (1.069, 1.230) | <0.001 | 1.149 (1.107, 1.192) | <0.001 | 1.073 (1.030, 1.117) | <0.001 | 1.124 (1.091, 1.158) | <0.001 | 1.052 (1.017, 1.088) | 0.003 |
| Albumin (per 1 g/dL increase) | 0.392 (0.299, 0.515) | <0.001 | 0.425 (0.308, 0.587) | <0.001 | 0.408 (0.349, 0.476) | <0.001 | 0.452 (0.379, 0.539) | <0.001 | 0.497 (0.436, 0.565) | <0.001 | 0.569 (0.492, 0.657) | <0.001 |
| CRP (per 1 mg/L increase) | 1.002 (1.001, 1.004) | 0.005 | 1.000 (0.999, 1.002) | 0.628 | 1.001 (1.000, 1.002) | 0.122 | - | 1.000 (0.999, 1.001) | 0.778 | - | ||
| Lactate (per 1 mg/dL increase) | 1.165 (1.119, 1.212) | <0.001 | 1.164 (1.107, 1.224) | <0.001 | 1.114 (1.085, 1.144) | <0.001 | 1.103 (1.068, 1.138) | <0.001 | 1.092 (1.067, 1.118) | <0.001 | 1.077 (1.048, 1.107) | <0.001 |
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CCI, Charlson co-morbidity index; CRP, C-reactive protein; MAP, mean arterial pressure; SOFA, sequential organ failure assessment; WBC, white blood cell.
- Multivariate analysis was conducted with adjustment for age, sex, CCI, SOFA, serum albumin, CRP (for only 28 day mortality), and lactate levels.
- Multicollinearity was checked for the variables of multivariable analysis, including the components of CCI.
Impact of sarcopenia on the 28 day, 1 year, and overall mortality
To determine the impact of sarcopenia on mortality, we conducted a multivariable Cox proportional analysis. The sarcopenia group showed a significant association with the increase in 28 day, 1 year, and overall mortality compared with the non-sarcopenia group even after adjusting for age, sex, CCI, SOFA score, serum albumin, lactate levels, and CRP value (CRP value was only adjusted for 28 day mortality) (comparison between sarcopenia and non-sarcopenia for 28 day mortality, HR = 2.116, 95% CI [1.312, 3.412], P = 0.002; for 1 year mortality, HR = 1.679, 95% CI [1.291, 2.182], P < 0.001; for overall mortality, HR = 1.704, 95% CI [1.381, 2.102], P < 0.001) (Table 2). The HRs could be explained by an unmeasured confounder that was associated with both exposure and mortality with an HR of 3.65 for 28 day mortality, 2.22 for 1 year mortality, and 2.25 for overall mortality (E-value), above and beyond the measured confounders, but weaker confounding could not do so.
Furthermore, we investigated the effect of muscle index on the increase in mortality. The HRs for 28 day, 1 year, and overall mortality were 0.969 (0.945, 0.994), 0.978 (0.964, 0.992), and 0.974 (0.963, 0.986), respectively, indicating that the increase in muscle index had a protective effect on mortality in patients with septic shock (Table 3). This result was also found to be concordant in TAMA (Table S2).
Discussion
The depletion of muscle mass may be a predictor for the poor prognosis in cancer, transplantation, ICU-admitted patients and septic patients.7, 13, 14, 29-31 Moreover, we have demonstrated the association between muscle mass reduction and adverse outcomes, mostly with the following pathophysiological background: skeletal muscle mass has been increasingly recognized as an important proxy for the physiologic reserve. First, skeletal muscle mass plays a highly important role in glucose disposal, protein synthesis, and mobility. Second, it also plays a protective role against infection.4, 32-34
Consistent with previous studies,13-16, 29 we also found that the depletion of muscle mass was significantly associated with an increase in 28 day, 1 year, and overall mortality in patients with septic shock. However, to the best of our knowledge, the current study is the first to delineate the impact of sarcopenia on long-term overall mortality among a large patient population with septic shock. Most studies in critically ill patients, including those with sepsis, have shown the impact of sarcopenia on short-term mortality (usually 28 day or 90 day mortality). Lee et al. presented the impact of 6 month mortality, but we needed to investigate the longer impact of sarcopenia, and they picked logistic analysis instead of Cox proportional analysis.35 In addition, Cox et al. recently reported the impact of sarcopenia on 1 year mortality in a prospective cohort study,15 but only 47 septic patients were enrolled. In contrast, we explored the impact of sarcopenia on the long-term mortality of 905 patients with septic shock and followed them for up to more than 10 years (median follow-up duration was 36.5 months).
In many patients, malnutrition and depletion of muscle mass usually appear simultaneously with a combination of reduced nutrient intake, body weight, and physical activity, leading to decreased muscle mass, strength, and physical function.34 Such depletion in the muscle mass is a main factor for the suppression of amino acid and protein synthesis in response to biological changes in the immune system36, 37; this indicates that sarcopenic patients may be more susceptible to newly developed infection and worsening current infection status due to poor immune system than non-sarcopenic patients.
However, there is no clear mechanism to explain the impact of sarcopenia on long-term mortality. Cox et al. and Voron et al. reported the long-term impact of sarcopenia in their patients,15, 38 but they could not provide a clear association between sarcopenia and long-term mortality. However, several studies reported that sarcopenia was more prevalent in older patients (≥60 years), but its presence was not correlated with severe co-morbidities,4, 39 indicating that sarcopenia was a part of the patient's frailty but not the patient's morbidity. Such frailty might work on the progression of long-term clinical outcomes without correction. Although we could not delineate the mechanism for the association between sarcopenia and long-term mortality due to the limitations of a retrospective cohort study, we were able to show that sarcopenia is associated with short-and long-term mortality in patients with septic shock.
However, for future studies, we need to consider the following data: some studies showed the improvement of poor clinical outcomes in postoperative patients with sarcopenia through the early detection and treatment of sarcopenia using different strategies, which included a specific nutritional intervention with protein and branched-chain amino acid supplementation.40-42 Although there was no clear mechanism for their association, we would expect clinical improvement with a specific nutritional intervention in sarcopenic patients with septic shock. Therefore, an interventional study design with the various strategies mentioned above may potentially improve the long-term prognosis among sarcopenic patients with septic shock.
Moreover, several studies have attempted to reveal the pharmacokinetics of drugs in cachectic patients, including those with sarcopenia; these studies have demonstrated that changes in body structure and function may influence the pharmacokinetics of drugs, although no general guidelines exist for drug dose adjustments in cachectic patients.43, 44 However, in this study, we wondered whether several drugs, including antibiotics, for the treatment of septic shock would reach the optimal target dose. Although we could not investigate the pharmacodynamic kinetics in this study, we surmised that inappropriate doses of drugs might result in an increase in mortality in patients with sarcopenia and septic shock based on the above theory.
This study has several limitations. First, because this was a retrospective cohort study, the risk of selection bias was inevitable. Based on the clinical pathway activation, patients not activated in the pathway were not a focus in this study. Therefore, we do not know how many patients were not recognized in the clinical pathway. Also, septic shock patients who did not undergo abdominal CT scans were not included. To compensate for this, we investigated various relevant co-morbidities and sources of infections and adjusted them by using the CCI representing the co-morbidities as a variable in multivariable analysis. Second, we did not investigate the variation of sarcopenia during hospital stay. However, the aim of the study was to explore the effect of sarcopenia at baseline on the long-term mortality among septic shock patients. Third, we conducted a study with data from a single center in Korea; therefore, the results should be interpreted with caution, while also considering the ethnicity of the study population. Fourth, we used muscle index to define sarcopenia,22-24 although there are different ways to measure it. Therefore, new studies with diverse methods to measure sarcopenia will be needed in the future. Despite these limitations, the strength of this study is that it is the first to delineate the association between sarcopenia and long-term mortality in a large patient population with septic shock.
In conclusion, sarcopenia was associated with both short-term and long-term mortality in patients with septic shock. Therefore, closer attention should be paid to these patients for long-term outcomes. We need to explore newly designed studies with diverse strategies to improve the long-term clinical outcomes in sarcopenic patients with septic shock.
Author contributions
N.S.K. contributed to the conception and design of the study. H.J.O., J.H.K., J.Y.A., S.J.J., N.S.K., J.Y.C., J.-S.Y., and Y.G.S. contributed to data acquisition and analysis. H.J.O., J.H.K., and H.R.K. performed statistical analysis of the data. H.J.O. and J.H.K. wrote the first draft. N.S.K., J.Y.C., J.-S.Y., and Y.G.S. contributed to the review and editing of the manuscript. The manuscript was approved by all authors. N.S.K. is responsible for the overall content.
Acknowledgement
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.45
Conflict of interest
All authors declare that they have no conflict of interests.
Funding
This research did not receive any funding from agencies in the public, commercial, or not-for-profit sectors.




