Integrated care for geriatric frailty and sarcopenia: a randomized control trial
Abstract
Background
Exercise, nutrition, and psychological interventions may all have positive impacts on frailty and sarcopenia. However, it is not known whether an integrated care programme with all three components can be beneficial and the intensity of such programme is also not certain. In this study, we aim to determine the effectiveness of two levels of integrated care on frailty and sarcopenia.
Methods
A randomized control trial was conducted at two community hospitals in Taiwan. Older adults (65–79 years of age, N = 289) who scored ≥1 on the Cardiovascular Health Study Phenotypic Classification of Frailty (CHS_PCF) were enrolled in the trial. Low-level care (LLC) participants received a 2 h education course on frailty, sarcopenia, coping strategy, nutrition, and demonstration of study exercise programme. Educational multimedia material was distributed as reference for home practice with bi-monthly telephone follow-ups on adherences. High-level care (HLC) participants, in addition to LLC instructions, received six sessions of on-site problem solving therapy and 48 exercise sessions within 6 months. Brief nutrition consultation was also provided during the exercise sessions. Primary outcome was improvement of the CHS_PCF by at least one category (from pre-frail to robust, or from frail to pre-frail or robust) from baseline. Secondary outcomes included changes of individual frailty, and sarcopenia indicators. Assessments were done at 3, 6, and 12 months by trained research assistants blinded to randomization status. Intention-to-treat analysis was applied.
Results
Mean age was 71.6 ± 4.3 years, with 53% females. For the entire cohort, improvement of primary outcome was 35% at 3 months, increased to 40% at 6 months, and remained stable at 39% at 12 months. Improvement rates were similar in both groups. Compared with the LLC group, HLC participants had greater improvements in the following indices: energy expenditure of walking, 5 m walking time, dominant hand grip strength, timed-up-and-go-test, and one-leg-stand time — mainly at 6 and 12 month assessments.
Conclusions
The 6 month integrated care improved frailty and sarcopenia status among community-dwelling elders, with high-intensity training yielding greater improvements. Low-level care could be promoted as a basic intervention, while HLC could be reserved for those at high risk and with high motivation.
Introduction
Frailty is a geriatric condition characterized by increased vulnerability to poor resolution of homoeostasis after a stressor event, which increases the risk of adverse outcomes.1 Sarcopenia is a syndrome of progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes.2 Frailty and sarcopenia are distinct but linked conditions associated with increased risk of falls, disability, and mortality.2, 3 Accordingly, some frailty indexes, such as the Cardiovascular Health Study Phenotypic Classification of Frailty (CHS_PCF),4 include components of sarcopenia measurements, including grip strength and walking speed.
Comprehensive geriatric assessments (CGAs) and subsequent multidimensional intervention programmes have been advocated to target multiple contributing factors of frailty to improve outcomes.1, 5, 6 Other approaches — including exercise, nutritional supplementation, a combination of the previous two modalities, and certain pharmacological agents such as angiotensin-converting enzyme inhibitors — have shown benefits on frailty and sarcopenia.1, 5-7 However, few previous clinical trials have used frailty indicators as their primary outcomes.5
With a 2 × 2 factorial design, our prior pilot randomized control trial of 117 community-dwelling frail older adults founds that (i) the 3 month exercise and nutritional programme resulted in short-term (3 month) frailty status improvement and long-term (12 month) effects on BMD and serum 25 OH Vitamin D, and (ii) problem-solving therapy (PST) also demonstrated limited benefits on frailty, physical performance, and mood.8
In this extended trial, we aim to explore (i) whether the combination of an exercise and nutritional programme with PST into a single integrated-care programme with a longer intervention period (6 months) and larger sample size will result in greater improvements regarding frailty, sarcopenia, and other outcomes; and (ii) whether different intensities of intervention (low level vs high level) will produce different levels of improvements.
Methods
Trial designs
The proposed study is an extension our prior 3 month pilot RCT on frailty (ended Dec., 2008) with similar methodology.8 Briefly, this 6 month RCT with 12 month follow-up was conducted at one urban community hospital (National Taiwan University Hospital Beihu Branch, BB site) and one rural community hospital (Chung-Kuang, CK site) in Taiwan to determine the comparative effectiveness of high-level vs low-level integrated care on frailty, sarcopenia, and other patient outcomes. (Figure 1). The study was approved in 2009 by the Research Ethics Committee of the National Health Research Institutes (NHRI), Zhunan, Taiwan (Protocol ID: EC0970301, ClinicalTrials.gov: NCT00718432).

Patients and sample size
Older adults from 65 to 79 years of age were referred from outpatient clinics. Based on the results of our prior pilot study, roughly 120 subjects were needed from each site to demonstrate an intervention effect. Assuming a 20% attrition rate, we planned to enroll 150 subjects at each site. The enrollment criteria were very similar to those in the prior pilot study.8
- Scored 3–6 with the ‘Canadian Study of Health and Aging Clinical Frailty Scale (CSHA-CFS) Chinese In-Person Interview Version’.
- Cardiovascular Health Phenotypic Classification of Frailty (CHS_PCF) ≥ 1.
- Nursing home residents.
- Cannot speak any of the following three dialogues: Mandarin, Taiwanese, and Haga.
- Hearing impairment interfering with communication or daily activities.
- Visual impairment interfering with communication or daily activities.
- Cannot complete the screening instrument with the CSHA-CFS Chinese In-Person Interview Version.
- Scored 1, 2, or 7 with the CSHA-CSF Chinese In-Person Interview Version.
- Cognitive impairment defined as 3-item recall ≤ 1.
- Functional Impairment defined as not able to walk for 5 m without assistance.
- Suicidal Ideation defined as Suicide Subscale of the Mini-International Neuropsychiatric Interview (M.I.N.I.) ≥ 6.
- Alcohol abuse disorders active within the last year. (score ≥ 2 on the Chinese edition of Cut down, Annoyed, Guilty, and Eye-opener (CAGE) substance abuse screening tool).
- Organic mental disorders (seizure, brain tumour, brain surgeries), history of schizophrenia or bipolar diagnosed from psychiatrist.
Three-hundred and twenty-three subjects signed the written informed consents and completed first-stage screening with the Chinese Canadian Study of Health and Aging Clinical Frailty Scale (CCSHA_CFS) in-person interview version, modified from the previous Telephone Version (TV)9, 10 (Figure 1) (2009/May to Jul.). Subjects who scored between 3 and 6 on the CCSHA_CFS were invited for second-stage screening with the CHS_PCF (with cut-points modified for the Taiwanese population).4, 8 After comprehensive assessments, 296 (N = 151 at BB site, and N = 145 at CK site) subjects who scored ≥1 on the CHS_PCF were enrolled in the extension trial. (Figure 1) (2009/Aug. to Oct.). Study samples in current study did not overlap with the prior pilot study.
Randomization (2009/Sept. to Nov.)
Subjects were stratified by age (65–74, 75–79) and gender to achieve a balance of baseline characteristics. Within each stratum, a permuted block (4 persons/block) randomization method was used to ensure balanced assignments. Subjects were randomly assigned into either the high-level care (HLC) group or the low-level care (LLC) group. The randomization code was generated at the off-site statistical centre with a computer random number generator. Random group allocation was managed by a project manager not involved in assessment or intervention.
Blinding
Several part-time research assistants were hired to perform baseline and outcome assessments. They were trained by the main study fulltime assistant to ensure reliability of assessments but there were blinded from the randomization status. However, blinding of the designed interventions was not possible for the intervention research assistants and the participants.
Trial interventions
LLC group
LLC group subjects received a 2 h education course at the two participating hospitals. The first hour focused on concepts of frailty, sarcopenia, depression, osteoporosis, healthy diets, and self-coping strategies. The second hour consisted of practice of the study's exercise protocol with a trained exercise specialist. The exercise programme included a 15 min warm up, a 10 min brisk walk, and then gentle stretching. Resistance training (20–30 min) used a rubber band or a bottle of water (0.6–1 L) as a weight for the major muscles of the upper and lower limbs. Balance training was also provided for 10 min, including tandem gaits, standing on one leg, stepping up and down stairs, walking on one's toes, and walking on one's heels. An education booklet and exercise CD were given to all subjects. Subjects were contacted bimonthly to check on how much they had read and watched the study material, and how well they had complied with the suggested diet and exercise protocols.
HLC group
HLC group subjects also received the same 2-h on-site education course. In addition, they were invited to take the designed 6 month group exercise course at the participating hospital, which comprised 48 exercise sessions and six PST11 sessions. Briefly, our PST consisted brief form of evidence-based psychotherapy that was developed in Britain for medical professionals. It focuses on helping people solve the ‘here-and-now’ problems contributing to their mood-related conditions and increase their self-efficacy. Previous studies have shown that this psychotherapy can lead to improvement of both mental and physical health.11 The research team also inquired about the subjects' dietary compliance and responded to their dietary questions during the exercise sessions. For example: if subjects were not clear about how to get high calcium diet suggested from the booklet, study team member would provide answers when they came to the training sessions.8 Including the educational classes, the entire intervention period is roughly 7 months from 2009/Nov. to 2010/Jun.
The major differences between two groups is that LLC subjects received only one session of face to face teaching class then all the interventions were done at home while HLC subjects added twice weekly practices at the participating hospitals. The content of exercise programme is the same for two groups but only HLC subjects received on site practice guidance from exercise specialist. Also, LLC subjects only were asked to read the booklet for healthy diet and frailty related information but the HLC subjects can consult research team members on diet or other issues during the on-site training time.
Measurements and procedures
Baseline assessments were completed before randomizations. Outcomes were assessed at 3 months, 6 months (end of intervention), and 12 months after initiation of on-site exercise programmes (from 2010/Feb. to 2010/Dec.).
Baseline assessments
Frailty-related characteristics were collected during the screening stages. Later, comprehensive assessments were performed to collect data on demographic characteristics, frailty, sarcopenia indices, and other health-related characteristics, such as comorbidities. Important primary and secondary outcomes are listed below.
Primary and secondary outcomes
The primary outcome was improvement of CHS_PCF by at least one level (from pre-frail to robust, or from frail to pre-frail or robust) from baseline assessments.4 Secondary outcomes included interval changes of the following indicators between baseline and repeated assessments. In the frailty index domain, we included improvement of each of the five indicators from the CHS_PCF.4 We also create several continuous variables from four frailty indicators (excepting weight loss) to further explore the intervention effects. Specifically, we created an exhaustion score by summing the levels of exhaustion from two questions (maximum 6). From the measures of physical activity (the Taiwan International Physical Activity Questionnaire),12 we estimated the weekly energy consumption of walking. Five-metre walking time (slowness) and grip strength (weakness) were measured as continuous variables rather than as categorical variables. For the sarcopenia domain, fat free mass (FFM)/height2 (Inbody 3.0) was used as a surrogate for muscle mass. Other sarcopenia indicators included 5 m walking time (also frailty indicators), dominant hand grip strength, timed-up-and-go-test, and left-leg-stand time. For 5 m walking time, we draw a line on the ground for 9 m with 4 marks on 0, 2, 7, and 9 m point. Then we asked the participants to walk at their usual speed and timed between 2 and 7 m marks. We used dynamometer (JAMAR 5030 J1, Hydraulic Hand Dynamometer; Sammons Preston, Chicago, IL) to measure the dominant hand grip power for 3 times and the average number of the two best measures was used. For time-up-and-go test, participants were timed during the periods of standing up form a chair with no chair arms, walking for 3 m, turning, and walking back to sit down. Participants were also timed when they stood up on their left leg until maximum of 30 s.
Approaches to analysis
Data were collected on study sites and entered online into a password-protected central database located at NHRI by trained research assistants; this was done with coding to permit blinding to group allocation during statistical analysis. All statistical analyses were conducted using SAS software, version 9.3 (SAS Institute Inc., Cary, NC). Analysis was conducted at baseline and at 3, 6, and 12 month follow-up assessments in accord with the ‘intention-to-treat’ principle (ITT). If data on outcomes were missing, the last observations were carried forward. Summary statistics, including mean and standard deviation, were provided for continuous variables if they have normal distribution, such as weight and height. Otherwise, median and interquartile range were presented when appropriate. Frequencies and proportions were used to summarize discrete variables, such as CHS_PCF categorization. Baseline characteristics were compared between two groups using t-test or Wilcoxon rank sum test for continuous variables, and chi-square test with Fisher's exact test when appropriate for categorical variables.
In our study, the outcomes of interest (e.g., frailty improvement) were measured at several time points (baseline, the 3rd month, the 6th month, and the 12th month). For estimating the repeated measurements of the intervention effect, the generalized estimating equations (GEE) model was used to compare the between-group frailty improvement, with adjustments for site, time, and treatment-by-time interactions. The GEE approach is an extension of the generalized linear model (GLM) and provides a semi-parametric approach to repeated categorical response. The intervention effect can be reasonably estimated by using GEE even if the covariance structure is not specified correctly. Longitudinal changes between groups and changes within a group for continuous variables (such as 5 m walking time) were analysed with the use of linear mixed models. For some variables — including exhaustion score and energy expenditure of walking — longitudinal changes between groups were analysed using Wilcoxon rank sum test, and changes within a group were analysed using Wilcoxon signed rank test. Under all circumstances, P < 0.05 was considered to be statistically significant.
Results
Participant flow
Overall 323 subjects received initial screening with the CCSHA_CFS and signed informed consent forms. Of those, 296 completed the first-stage baseline assessments and enrolled in the study. The enrollment periods were roughly 3 months. Before randomization, 7 dropped out of the study, and 289 were randomized into HLC (N = 143) and LLC (N = 146) groups. The major reasons for dropouts were lost contact (n = 2), and refusal (n = 2). At the end of intervention (6 months), 87% of HLC subjects and 90% of LLC subjects returned for assessments. At the end of the study (12 months), 87% of the HLC subjects and 92% of the LLC subjects completed the final assessments (Figure 1). The major reason for attrition was lost contact (n = 16 at 3 months; n = 22 at 6 months; n = 15 at 12 months). All 289 subjects were included in the ITT analyses. At all time points, the HLC group had a significantly higher proportion of subjects practicing at least 50% of the recommended home exercise sessions (63% vs 19% at 6 months, 60% vs 20% at 12 months, both p < 0.001) than the LLC group. In addition, 67% of HLC group subjects attended at least half of the hospital-based exercise sessions.
Baseline characteristics (Tables 1 and 2)
| Total (n = 289) | HLC (n = 143) | LLC (n = 146) | ||
|---|---|---|---|---|
| n (%) mean ± sda | n (%) mean ± sda | n (%) mean ± sda | p-value | |
| Demographics | ||||
| Age (y/o) | 71.6 ± 4.28 | 71.3 ± 4.54 | 71.8 ± 4.02 | 0.272b |
| Female sex | 152 (53) | 75 (52) | 77 (53) | 0.960c |
| Weight (kg) | 62.4 ± 9.92 | 61.7 ± 10.00 | 63.0 ± 9.84 | 0.270b |
| Height (cm) | 157.3 ± 7.94 | 156.8 ± 7.86 | 157.8 ± 8.02 | 0.299b |
| BMI (kg/m2) | 25.2 ± 3.52 | 25.1 ± 3.53 | 25.3 ± 3.53 | 0.582b |
| Health and healthcare-related characteristics | ||||
| Number of chronic conditionsd (n = 287) | 4.0 ± 2.01 | 3.9 ± 2.11 | 4.0 ± 1.90 | 0.537b |
| MMSE | 26.6 ± 3.80 | 26.5 ± 3.84 | 26.7 ± 3.76 | 0.663b |
| Barthel Index | 99.2 ± 3.60 | 99.1 ± 3.53 | 99.2 ± 3.68 | 0.776b |
| Healthcare-resources utilizatione | 1.8 ± 1.81 | 1.7 ± 1.54 | 1.9 ± 2.04 | 0.246b |
| EQ-5D | 0.9 ± 0.09 | 0.9 ± 0.10 | 0.9 ± 0.08 | 0.725b |
- a Categorical data: n (%); Continuous variables: mean ± standard deviation.
- b Performed by t-test.
- c Performed by χ2 test.
- d From 27 diseases.
- e Emergency room, hospitalization, or clinic visits in the screen stage.
- EQ-5D, EuroQol Quality of Life Scale; HLC, High-Level Care; LLC, Low-Level Care; MMSE, Mini-Mental State Examination.
| Total (n = 289) | HLC (n = 143) | LLC (n = 146) | ||
|---|---|---|---|---|
| n (%) mean ± sd median (IQR)a | n (%) mean ± sd median (IQR)a | n (%) mean ± sd median (IQR)a | p-value | |
| Frailty and sarcopenia-related characteristics | ||||
| CHS_PCF categorization | ||||
| Pre-frail (1–2) | 229 (79) | 115 (80) | 114 (78) | 0.624b |
| Frail (3–5) | 60 (21) | 28 (20) | 32 (22) | |
| Original frailty indicators | ||||
| Weight loss (yes) | 63 (22) | 30 (21) | 33 (23) | 0.738b |
| Exhaustion (yes) | 54 (19) | 27 (19) | 27 (18) | 0.933b |
| Low level physical activity (yes) | 27 (9) | 13 (9) | 14 (10) | 0.884b |
| Slow walking speed (yes) | 112 (39) | 59 (41) | 53 (36) | 0.387b |
| Weak grip strength (yes) | 251 (87) | 126 (88) | 125 (86) | 0.530b |
| Modified frailty and sarcopenia indicators | ||||
| Exhaustion scorec | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0.948d |
| Energy expenditure of walking (MET-min/week) | 693 (297–1386) | 693 (297–1386) | 693 (297–1386) | 0.662d |
| 5 m walking time (s) | 6.6 ± 2.07 | 6.7 ± 2.10 | 6.5 ± 2.05 | 0.585e |
| Dominant hand grip strength (kg) | 19.4 ± 7.40 | 19.2 ± 7.20 | 19.5 ± 7.61 | 0.788e |
| Other sarcopenia indicators | ||||
| FFMI (kg/m2) (n = 287) | 17.4 ± 1.60 | 17.5 ± 1.55 | 17.4 ± 1.65 | 0.782e |
| Timed-up-and-go-test (s) | 12.0 ± 4.36 | 12.1 ± 4.05 | 12.0 ± 4.65 | 0.717e |
| One-leg-stand time (s) (n = 286) | 15.9 ± 19.31 | 14.6 ± 15.81 | 17.1 ± 22.21 | 0.264e |
- a Categorical data: n (%); continuous variables: mean ± standard deviation or median (interquartile range).
- b Performed by χ2 test.
- c Summation of levels of exhaustions from two questions (maximum 6).
- d Performed by Wilcoxon rank sum test.
- e Performed by t-test.
- CHS_PCF, Cardiovascular Health Study_Phenotypical Classification of Frailty; FFMI, Fat Free Mass Index; HLC, High-Level Care; LLC, Low-Level Care.
For the entire cohort (N = 289), mean age was 71.6 ± 4.3, 152 (53%) were female, mean weight was 62.4 ± 9.9 kg, mean height was 157.3 ± 7.9 cm, and mean BMI was 25.2 ± 3.5 kg/m2. Other important baseline health-related characteristics including physical and cognitive function are listed in Table 1.
With the CHS_PCF, 229 (79%) were classified as pre-frail and 60 (21%) as frail. For the five original frailty indicators, 87% of the subjects scored low on hand grip strength, while 39% had a slow walking speed, 22% had weight loss, 19% had exhaustion, and 9% had low energy expenditure.
In the modified frailty and sarcopenia index domain, the median exhaustion score was 0 (0–2), whereas the median weekly energy expenditure of walking was 693 (297–1386) kcal, the mean 5 m walking time was 6.6 ± 2.1 s, and the mean dominant hand grip strength was 19.4 ± 7.4 kg.
Other sarcopenia indicators included mean FFMI (17.4 ± 1.6 kg/m2), mean timed-up-and-go-test time (12.0 ± 4.4 s), and mean one-leg-stand time (15.9 ± 19.3 s). (Table 2)
There were no between-group differences in baseline characteristics, indicating adequate randomization.
Primary outcomes (Figure 2)
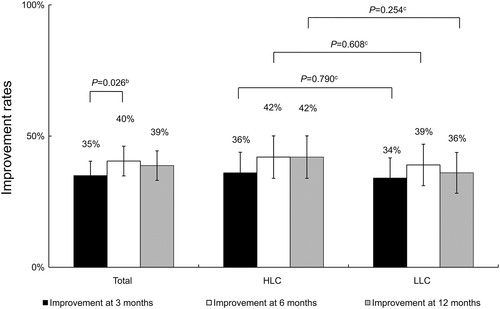
For the entire group, 35% had improvement of frailty status at 3 months, 40% at 6 months (end of intervention), and 39% at 12 months (Figure 2). The differences in improvement between 3 months and 6 months were statistically significant (p = 0.026). However, the HLC and LLC groups were similar at all assessment time-points.
Secondary outcomes (Tables 3-5)
| Total (n = 289) | HLC (n = 143) | LLC (n = 146) | ||
|---|---|---|---|---|
| n (%) | n (%) | n (%) | p-valuea | |
| Original frailty indicators | ||||
| Weight loss (yes) | ||||
| Improvementb at 3 months | 46 (16) | 22 (15) | 24 (16) | 0.828 |
| Improvementb at 6 months | 52 (18) | 24 (17) | 28 (19)† | 0.610 |
| Improvementb at 12 months | 56 (19) | 28 (20)† | 28 (19) | 0.909 |
| Exhaustion (yes) | ||||
| Improvementb at 3 months | 28 (10) | 10 (7) | 18 (12) | 0.128 |
| Improvementb at 6 months | 40 (14)†† | 22 (15)††† | 18 (12) | 0.438 |
| Improvementb at 12 months | 44 (15) | 22 (15) | 22 (15)† | 0.941 |
| Low level physical activity (yes) | ||||
| Improvementb at 3 months | 18 (6) | 11 (8) | 7 (5) | 0.313 |
| Improvementb at 6 months | 20 (7) | 13 (9) | 7 (5) | 0.157 |
| Improvementb at 12 months | 17 (6) | 10 (7) | 7 (5) | 0.431 |
| Slow walking speed (yes) | ||||
| Improvementb at 3 months | 82 (28) | 44 (31) | 38 (26) | 0.371 |
| Improvementb at 6 months | 90 (31)† | 48 (34) | 42 (29) | 0.378 |
| Improvementb at 12 months | 82 (28)† | 44 (31) | 38 (26) | 0.373 |
| Weak grip strength (yes) | ||||
| Improvementb at 3 months | 51 (18) | 30 (21) | 21 (14) | 0.142 |
| Improvementb at 6 months | 55 (19) | 33 (23) | 22 (15) | 0.084 |
| Improvementb at 12 months | 55 (19) | 34 (24) | 21 (14) | 0.042 |
- † p < 0.05,
- †† p < 0.01,
- ††† p < 0.001 for the comparison of improvement at 6 months vs 3 months, and 12 months vs 6 months within the group, using generalized estimating equations (GEE) model.
- a Intervention effect, after adjusting for site, time, and treatment-by-time interactions, by GEE model.
- b After intervention 3, 6, and 12 months ‘Dr. Fried frailty characteristics’ has progressed from ‘yes’ to ‘no’.
- HLC, High-Level Care; LLC, Low-Level Care.
| Total (n = 289) | HLC (n = 143) | LLC (n = 146) | ||
|---|---|---|---|---|
| mean ± sd median (IQR)a | mean ± sd median (IQR)a | mean ± sd median (IQR)a | p-valueb | |
| Modified frailty and sarcopenia indicators | ||||
| Exhaustion scorec | ||||
| Change at 3 months | 0 (−1–0)*d | 0 (−1–0)d | 0 (−1–0)d | 0.805e |
| Change at 6 months | 0 (−1–0)***†††d | 0 (−2–0)***†††d | 0 (−1–0)*d | 0.090e |
| Change at 12 months | 0 (−1–0)***d | 0 (−1–0)**††d | 0 (−1–0)**d | 0.604e |
| Energy expenditure of walking (MET-min/week) | ||||
| Change at 3 months | 0.0 (−462.0–346.5)d | 0.0 (−396.0–247.5)d | 0.0 (−462.0–363.0)d | 0.604e |
| Change at 6 months | 0.0 (−231.0–462.0)**††d | 165.0 (−33.0–693.0)***†††d | 0.0 (−462.0–346.5)d | <0.001e |
| Change at 12 months | 0.0 (−346.5–495.0)*d | 115.5 (−198.0–693.0)**d | 0.0 (−462.0–462.0)d | 0.032e |
| 5 m walking time (s) | ||||
| Change at 3 months | −1.29 ± 1.91***f | −1.50 ± 1.81***f | −1.09 ± 1.99***f | 0.067f |
| Change at 6 months | −1.55 ± 2.32***†f | −2.03 ± 1.79***††f | −1.08 ± 2.66***f | <0.001f |
| Change at 12 months | −1.32 ± 3.05***f | −1.71 ± 1.54***f | −0.94 ± 3.98***f | 0.033f |
| Dominant hand grip strength (kg) | ||||
| Change at 3 months | 1.48 ± 4.77***f | 1.87 ± 4.90***f | 1.09 ± 4.62**f | 0.160f |
| Change at 6 months | 1.70 ± 4.63***f | 2.51 ± 4.56***†f | 0.89 ± 4.58*f | 0.004f |
| Change at 12 months | 1.58 ± 5.05***f | 2.08 ± 5.04***f | 1.09 ± 5.03**f | 0.075f |
- † p < 0.05.
- †† p < 0.01.
- ††† p < 0.001 for the comparison of change at 6 months vs 3 months, and 12 months vs 6 months within the group.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001 for the comparison of the value at the follow-ups with the baseline value within the group.
- a Mean ± standard deviation if data have normal distribution. Median (interquartile range) if data do not have normal distribution.
- b Intervention effect, after adjusting for site, time, and treatment-by-time interactions.
- c Summation of levels of exhaustions from two questions (maximum 6).
- d Performed by Wilcoxon signed rank test.
- e Performed by Wilcoxon rank sum test.
- f Performed by linear mixed model.
- HLC, High-Level Care; LLC, Low-Level Care.
| Total (n = 289) | HLC (n = 143) | LLC (n = 146) | ||
|---|---|---|---|---|
| mean ± sd | mean ± sd | mean ± sd | p-valuea | |
| Other sarcopenia indicators | ||||
| FFMI (kg/m2) (n = 287) | ||||
| Change at 12 months | −0.01 ± 0.52 | 0.03 ± 0.54 | −0.04 ± 0.51 | 0.222 |
| Timed-up-and-go-test (s) | ||||
| Change at 3 months | −1.16 ± 2.91***b | −1.67 ± 2.99***b | −0.66 ± 2.75*b | 0.010 |
| Change at 6 months | −1.84 ± 3.51***†††b | −2.69 ± 3.13***†††b | −1.02 ± 3.67***b | <0.001 |
| Change at 12 months | −1.36 ± 3. 81***††b | −1.95 ± 3.29***††b | −0.79 ± 4.19**b | 0.003 |
| One-leg-stand time (s) (n = 286) | ||||
| Change at 3 months | 1.80 ± 17.42 | 3.72 ± 16.82*b | −0.09 ± 17.86 | 0.064 |
| Change at 6 months | 4.99 ± 21. 42***††b | 10.26 ± 25.87***†††b | −0.20 ± 14.15 | <0.001 |
| Change at 12 months | 6.75 ± 26.68***b | 11.88 ± 33.25***b | 1.69 ± 16.65 | 0.001 |
- † p < 0.05.
- †† p < 0.01.
- ††† p < 0.001 for the comparison of change at 6 months vs 3 months, and 12 months vs 6 months within the group.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001 for the comparison of the value at the follow-ups with the baseline value within the group.
- a Intervention effect, after adjusting for site, time, and treatment-by-time interactions, by linear mixed model.
- b Performed by linear mixed model.
- FFMI, Fat Free Mass Index; HLC, High-Level Care; LLC, Low-Level Care.
Original frailty indicators
When treated as a categorical variable, the indicator for physical activity indicator was the one least likely to improve (6–7%), whereas that for walking speed was the most likely to improve (28–31%). There were no appreciable between-group differences, except that HLC group subjects had a greater improvement in grip strength than LLC group subjects at 12 months (24% vs 14%, p = 0.042) (Table 3)
Modified frailty and sarcopenia indicators
With modified indicators from the CHS_PCF, significant improvements were found for exhaustion score (at 3, 6, and 12 months), energy expenditure of walking (at 6 and 12 months), 5 m walking time (at 3, 6, and 12 months), and dominant hand grip strength (at 3, 6, and 12 months) for the entire cohort. Improvements generally peaked at 6 months, when interventions were completed. HLC group subjects performed better at the following indices than LLC group subjects: energy expenditure of walking (at 6 and 12 months), 5 m walking time (at 6 and 12 months), and grip strength (at 6 months). At 6 months, the exhaustion score improved by 0 (−2–0) points in the HLC group, and by 0 (−1–0) points in the LLC group (p = 0.09) (Table 4).
Other sarcopenia indicators
Overall, there was no significant change in FFMI for the entire cohort over the 12 month period. The entire cohort had significant improvements on the timed-up-and-go-test (at 3, 6, and 12 months) and on one-leg-stand-time (at 6 and 12 months). The improvements for the timed-up-and-go-test peaked at 6 months, while the improvement for one-leg-stand-time peaked at 12 months. HLC group subjects had better performance on the timed-up-and-go-test (at 3, 6, and 12 months) and on one-leg-stand time (at 6 and 12 months) than LLC subjects (Table 5).
Harm
There were no serious adverse events related to study interventions. However, three participants expired and two had illness requiring hospitalization during the study period with even distribution between two groups.
Discussion
Our study demonstrates that both high-level and low-level integrated care improved frailty and sarcopenia outcomes for community-dwelling older adults. However, HLC group subjects had better improvements in several frailty and sarcopenia indices, especially at 6 month assessments when active interventions had just ended.
Compared with our prior pilot study,8 the current study had a longer intervention period (6 months vs 3 months). Therefore, the maximum effect was shown at 6 months in the current study. In the prior pilot study, the control group received only the education booklet for home exercise. In the current study, the LLC group received face-to-face training for 2 h and an educational booklet/CD-ROM to improve their adherence to the study's exercise programme. On the other hand, the HLC group received less frequent on-site exercise programmes, as in the prior pilot study. Because the training intensity gap between the HLC and LLC was smaller than what we have done in the prior pilot trial, we did not find significant between group differences in primary outcome as in the prior pilot study. Consistent with several previous studies, our prior pilot study found that on-site programmes are often superior than those that provide home exercise only.13-15 The findings of the current study suggest that when a home exercise programme is comprehensive and carefully implemented, many of its benefits could reach toward on-site training programmes. Nevertheless, the on-site programme was still superior in several frailty and sarcopenia indicators. Another encouraging finding was that at the 12 month assessment, most participants still performed better than their baseline status, with measures similar to those obtained at the 3 month assessment. This indicates a long-lasting effect, with the participants enjoying benefits even 6 months after the conclusion of the intervention. Although HLC group has better improvement in several frailty and sarcopenia indicators, these interventions were costlier than LLC group. These interventions need at least one exercise trainer and venue fees for on-site training programmes. Home-exercise programme has been shown to be cost-effective for mobility outcomes.16 Further economic evaluation are needed for our interventions.
Psychological intervention was also provided in our care models. In addition to being supplied with general knowledge about depression and coping strategies (in the educational booklet), those in the HLC group also received six sessions of PST. Our prior pilot study indicated that PST might have some positive effects on muscle strength.8 The current study showed that by adding PST to the exercise programme and nutrition consultation, there was a trend toward improving the exhaustion component of frailty, which was originally derived from the Center for Epidemiological Studies Depression (CES-D) scale.4
Several recent reviews found that structured exercise improved physical and psychological function, and frailty status, as well as prevented disability in frail older adults.5, 6, 17-20 Expert opinions and the results of clinical trials suggest nutritional consultation or supplementation as a component of frailty interventions.6, 21, 22 Exercise and nutritional interventions have long been proposed as major strategies for managing sarcopenia.6, 7, 23 Several recent trials have suggested that a protein-rich diet or supplements and/or structured exercise improved sarcopenia indicators.24-26 Because frailty and sarcopenia indicators are often inter-related,3, 4 exercise and nutritional interventions aimed at improving frailty may also have benefits on sarcopenia indicators, as demonstrated in two recent trials of obese frail adults22, 27 Our study adds new evidence that a combination of exercise, nutrition, and psychological interventions has a positive impact on frailty and sarcopenia.
Strengths and limitations of the current study
One major strength of the study is that both LLC and HLC interventions showed positive impacts on frailty and sarcopenia outcomes. Because home exercise programmes are less resource intensive than on-site ones, such low-level programmes can be promoted as basic interventions against frailty and sarcopenia. Our educational booklet and exercise CD were ready for dissemination. Healthcare organizations could host many 2-h education sessions to instruct community-dwelling older adults on home-based frailty and sarcopenia interventions. However, some telephone follow-ups are probably needed to increase adherence to home programmes. On the other hand, on-site sessions can also be opened to those with high motivation to gain maximum benefits, if resources permit.
The study also has several important limitations. First, compliance with the twice-weekly exercise sessions for the HLC group were still fair. If the study protocol is to be disseminated to many different sites, methods should be explored to ensure better adherence to the study protocol to gain better effects. Finally, we were not able to reach our target sample size of 300. However, based on our sample-size calculation, we needed 120 subjects retained at each group at final analysis. The attrition rate was lower than expected. We were able to get 125 in the HLC group and 134 in the LLC group for the final assessments. The study should still have enough power to detect statistically significant differences.
Conclusions
In summary, with 6 month interventions, both high- and low-level integrated care resulted in significant improvements in most frailty and sarcopenia indicators among community-dwelling older adults. However, the HLC had greater improvements on some indicators. Low-level care could be promoted as a basic intervention, while HLC could be reserved for those at high risk and with high motivation.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.28 This study was approved by the Research Ethics Committee of the National Health Research Institutes, Zhunan, Taiwan. All persons gave their informed consent prior to their inclusion in the study.
This work was supported by the National Health Research Institutes, Zhunan, Taiwan grant (HD-097-SP-08, PH-98-SP-10 and PH-99-SP-09 ‘Interventional Study of Geriatric Frailty, Osteoporosis and Depression in a Community Based Randomized Trial’). The funder support independent work of the research group without interfering with data analysis or interpretation.
Conflict of interests
None of the authors have conflict of interests.




