Loss of muscle mass: current developments in cachexia and sarcopenia focused on biomarkers and treatment
Abstract
Loss of muscle mass arises from an imbalance of protein synthesis and protein degradation. Potential triggers of muscle wasting and function are immobilization, loss of appetite, dystrophies, and chronic diseases as well as aging. All these conditions lead to increased morbidity and mortality in patients, which makes it a timely matter to find new biomarkers to get a fast clinical diagnosis and to develop new therapies. This mini-review covers current developments in the field of biomarkers and drugs on cachexia and sarcopenia. Here, we reported about promising markers, e.g. tartate-resistant acid phosphatase 5a, and novel substances like epigallocatechin-3-gallate. In summary, the progress to combat muscle wasting is in full swing, and perhaps diagnosis of muscle atrophy and of course patient treatments could be soon support by improved and more helpful strategies.
Introduction
Loss of muscle mass is commonly observed in chronic diseases like cancer, chronic heart failure (HF), chronic obstructive pulmonary disease, chronic kidney disease (CKD), cystic fibrosis, liver cirrhosis, Crohn's disease, rheumatoid arthritis (RA), stroke, and many neurodegenerative diseases as well as in human immunodeficiency virus/acquired immune deficiency syndrome, malaria, and tuberculosis.1-3 A serious complication of these chronic illnesses is cachexia. Cachexia is defined as weight loss greater than 5% of body weight in 12 months or less in the presence of chronic illness or as a body mass index (BMI) lower than 20 kg/m2. In addition, usually three of the following five criteria are required: decreased muscle strength, fatigue, anorexia, low fat-free mass index, increase of inflammation markers such as C-reactive protein or interleukin (IL)-6 as well as anaemia or low serum albumin.4, 5 Loss of muscle mass and function, especially muscle strength and gait speed, associated with aging occurs in sarcopenia.6, 7 Indeed, sarcopenia, cachexia, and malnutrition are considered as the main causes of muscle wasting8 and affect millions of elderly people and patients.9 Moreover, muscle atrophy can develop independently from diseases and age through disuse of the muscles.10 For a better classification and common language in medical science for ‘muscle wasting disease’ there is a proposal to combine the concepts of muscle wasting, sarcopenia, frailty, and cachexia by disease aetiology and disease progression.8 Patients with muscle atrophy show decreased muscle strength and therefore reduced quality of life, which is caused by a lower activity and increased exercise intolerance.11 In sarcopenic patients, muscle wasting is frequently associated with loss of bone, which leads to a higher risk of hip and other fractures.12 Hip fracture also results in loss of musculature because of disuse atrophy.13 All these conditions lead to increased morbidity and mortality in patients,14 and therefore developments in biomarkers and treatment finding to improve patients' lives is necessary (for schematic representation of the process see Figure 1). The reason for muscle atrophy is an imbalance of protein synthesis and protein degradation. Three major protein degradation pathways play a role in development of muscle wasting: (1) activation of the ubiquitin–proteasome–system (UPS), (2) apoptosis through caspase signalling, and (3) autophagy.15
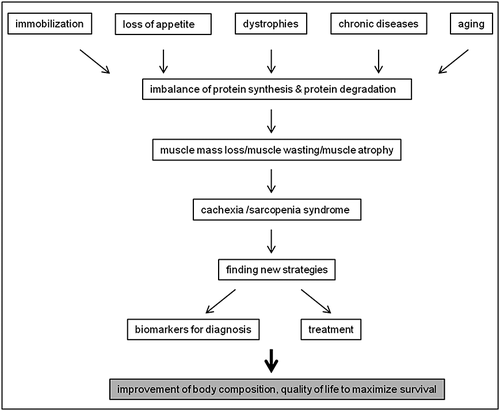
Current developments on muscle mass loss
The UPS pathway, which is conserved from yeast to mammals, plays a major role in degradation of most short-lived proteins. Most targets are cell cycle regulatory proteins as well as misfolded proteins. The target proteins undergo an ATP-dependent ubiquitination marking the protein for degradation. Polyubiquinated proteins are subsequently degraded by the proteasome16 while monoubiquitinated substrates are eliminated in lysosomes.17 At the beginning of the reaction, ubiquitin binds to an ubiquitin activating enzyme (E1) and forms a thio-ester bond. This reaction allows ubiquitin to transfer to an ubiquitin conjugating enzyme (E2) followed by the formation of an isopeptide bond which finally leads to the binding of E2 to an ubiquitin ligase (E3). The ligase specifically recognizes the substrate protein and transfers ubiquitin to the target protein.18 Subsequently, the target proteins are unfolded and degraded by an ATP-dependent process.19 Two muscle-specific E3 ubiquitin ligases named muscle atrophy F-box (MAFbx, atrogin-1) and muscle RING finger-1 (MuRF-1) were first described in 2001 and are significantly up-regulated during muscle atrophy.20, 21 However, the loss of these E3-ligases only leads to partial protection against muscle wasting.20 MAFbx was shown to target regulatory factors for protein synthesis like MyoD22 and the eukaryotic initiation factor of protein synthesis elF3-f.23 MuRF-1 binds to titin24, 25 and is targeting to myofibrillar proteins like myosin heavy chain, myosin light chain, and myosin-binding C.26 Another muscle-specific ubiquitin ligase named tripartite motif 32 (TRIM32) was discovered in 2005.27 TRIM32 is thought to ubiquitinate the thick myofibrillar filament as well as actin and dysbindin.27 A muscle-specific F-box protein was foun in 2007,28 which induces the ubiquitination of insulin receptor substrate 1 thereby providing a negative feedback on the IGF1R/IRS1/PI3K/Akt pathway by early signal termination.29 In addition 2010, tumour necrosis factor (TNF) receptor-associated factor has been found to play a critical role in atrophy as an E3 ubiquitin ligase.30
Cancer cachexia animal models are showing significant wasting of the myocardium.31-33 In one study it was shown that the heart muscle weight is decreased by 20% on average.34 In another cancer cachexia study, cardiac wasting was associated with left-ventricular (LV)-dysfunction.35 Treatment with selected agents (bisoprolol, spirolactone, and imidapril) used in HF resulted in placebo-treated group of AH-130 rats in a loss of 21 ± 2% LVmass while the LVmass was stabilized by bisoprolol (+2 ± 8%, P < 0.0001) and increased by spirolactone (+9 ± 3%, P < 0.0001) whereas imidapril had no effect.35 Moreover, a decrease in the trypsin-like activity of the UPS was seen in bisoprolol and spirolactone-treated animals in contrast to imidapril which enhanced proteasome activity.35 However, under oxidative stress conditions an up-regulated expression level of the ubiquitin ligases MAFbx and MuRF-1 in cachectic hearts leads to the induction of the UPS.34 MAFbx and MuRF-1 are elevated as well at the mRNA level linked to the degradation of cardiac troponin I, α-actin-2, and MyoD which is leading to impaired contractility.36 Furthermore, another study showed a reduced heart rate and fractional shortening using echocardiography in the myocardium of cancer cachectic mice.37 Elevated levels of reactive oxygen species in cachectic skeletal muscle have been linked to an activation of the UPS.38 In general, cytokines including IL-1, IL-6, TNF-α, and interferon-γ have been shown to contribute a catabolism net in skeletal muscle and to form a state of oxidative stress.39 These cytokines lead to an activation of nuclear factor kappa-light-chain-enhancer of activated B-cells (NFκB) and forkhead transcription factors (FoxO) in muscle40 resulting in increased proteolysis by inducing the expression of MAFbx and MuRF-1.15 The involvement of the NFκB pathway was originally observed in a model of disuse atrophy41 where it binds directly to the MuRF-1.42 Furthermore, increased oxidative stress activates the NFκB pathway.43 Surprisingly, an inhibition of NFκB via the IkappaB kinase complex only partially rescues the phenotype of the cachectic gastrocnemius in a murine model of cancer cachexia.44 The FoxO family members consist of three isoforms as FoxO1, FoxO3, and FoxO4. It was shown that FoxO1 and FoxO3a are significantly up-regulated in cachectic muscles from Lewis Lung Carcinoma45 and Colon 26 tumour-bearing mice46; FoxO1 is also up-regulated in skeletal muscle in human cancer cachexia patients.47 Thus, these findings strongly support the involvement of NFκB and FoxO in the process of muscle atrophy. However, mitochondrial dysfunction and loss of mitochondria in skeletal muscle contribute to disrupted muscle function.48 Indeed, investigations with markers of mitochondrial function and activity like the mitochondrial enzymes pyruvate dehydrogenase (PDH) and the cytochrome c oxidase (COX) showed that the protein concentrations of PDH and COX in the skeletal muscle of colon cancer patients were decreased and a lower activity of PDH was observed as well.49
Despite a large number of studies, our understanding of the development of muscle wasting and the involved pathways remains very limited. For instance, in some diseases like RA muscle wasting is not well investigated yet,50-52 but better understanding is imperative for designing further studies and to develop new therapies. A recent study was aimed at an evaluation of muscle atrophy in skeletal muscle in a mouse model of RA and to establish a relation between disease score and muscle wasting.53 Findings implicated the existence of a progressive development of muscle wasting with an early onset, which was especially associated with increased serum levels of cytokines, e.g. IL-6.53 Another not well-studied muscle wasting disease is stroke, although it is known that stroke rapidly leads to an increase in muscle loss.54 Hence, it is difficult to treat muscle atrophy in stroke patients. However, a large prospective stroke study with the main objectives to study changes in body composition, and metabolic and functional changes of muscle tissue in patients with acute ischemic stroke is underway.55 This study unites the knowledge of neurologists, cardiologists, and endocrinologists, and their findings might improve rehabilitation after stroke. Generally, impaired feeding, reduced caloric intake, and loss of appetite lead to a negative nutritional and nitrogen balance,56, 57 and immobilization causes physical inactivity and muscle atrophy after stroke.58, 59 It has been shown that elevated volumes of TNF-α are responsible for muscle loss and that plasma concentrations of the enzyme visfatin were significantly elevated in patients after ischemic stroke.60, 61 For that, investigations to changes of inflammation parameters and its relation to body composition, insulin sensitivity, and patient's survival will be made as well.55
Current news on biomarker research
Exact quantification of skeletal muscle mass is challenging. To better determine skeletal muscle mass, many measurement methods were developed in the last two centuries (for a historical overview see 62). Since the early 1970s computed tomography, magnet resonance imaging, and dual-energy X-ray absorptiometry came into application.62 A problem of these methods is that they are all expensive and thus only available at larger institutions. Moreover, these methods are only able to detect tissue wasting, but they are incapable to show the risk of developing muscle atrophy.63, 64 But there is described a practical screening tool in a validated model to improve screening for low skeletal muscle mass in older adults.65 It has been suggested that the BMI is strongly associated with a low skeletal muscle mass index which could be helpful for primary care settings and treating elderly populations at risk of sarcopenia.65 However, it is imperative to find new robust biomarkers, which are cheap and easily available for diagnosis and therapy monitoring in clinics.64 Potential candidates are summarized in Table 1 and are described in more detail below. Serum creatinine may be such a reliable, cheap, and easily accessible biomarker of skeletal muscle mass in human subjects, for example in CKD patients.66 The adaption of the liquid chromatography–tandem mass spectrometry based on D3-creatine dilution method from an oral dose and detection of urinary creatinine enrichment by isotope ratio mass spectrometry79 could be an accurate tool to measure total body creatine skeletal muscle mass change.80 The drawback of this method is the high cost and limited availability of the necessary machinery. Furthermore, serological neoepitopes have been suggested as muscle wasting biomarkers to solve some of these problems mentioned before. Neoepitopes do not reflect a condition or state like creatinine reflecting muscle mass, but a process which allows an early detection of a muscle loss in disease. In fact, neoepitope biomarkers are parent proteins that are produced through post-translational modifications, i.e. glycosylation, phosphorylation, acetylation, nitrosylation, methylation, and ubiquitination of an existing molecule and are formed by protease cleavage or addition of chemical groups in tissues of interest.67 The most common parent proteins for muscle loss biomarkers are sarcomeric proteins (e.g. myosin, actin, troponin, and tropomyosin) and components of the extracellular matrix (e.g. laminins).67 That makes neoepitopes interesting to be biomarkers of muscle pathology.67 Other serological biomarker candidates for muscle wasting are type VI collagen turnover-related peptides.68 In a study, blood was analysed for levels and their correlation of following biomarkers: a matrix metalloproteinase-generated degradation fragment of collagen 6 (C6M) and a type VI collagen N-terminal globular domain epitope (IC6).68 These fragments can only be considered biomarker candidates of muscle mass and change in young men but not in elderly men.68 However, circulating biomarkers like the N-terminal propeptide of type III procollagen (P3NP) and C-terminal agrin fragment (CAF) respond to resistance exercise training in older adults.69 Short-time resistance exercise training (6 weeks) improves leg extension muscle strength, measured on a knee lift, by 29% from 39.7 ± 16.5 to 51.1 ± 18.3 kg in the exercise group (P < 0.001) and muscle quality by 28% from 3.64 ± 0.85 to 4.67 ± 0.81 relative strength (leg extension strength in kg/lean quadriceps muscle mass in kg) in the exercise group (P < 0.001) in older adults and may result in changes P3NP and CAF.69 Indeed, CAF appears to increase in response to short-time resistance exercise training in older adults in contrast to P3NP where the results were less clear.69 P3NP showed a positive correlation to changes in lean body mass (r = 0.422, P = 0.045), and there was observed a positive correlation between change in circulating CAF and change in cross-sectional area of the vastus lateralis (r = 0.542, P = 0.008).69 However, P3NP is associated with subsequent changes in lean body mass and appendicular skeletal muscle mass and seems to be a useful early predictive biomarker of anabolic response to growth hormone and testosterone.81 3-Methylhistidine (3MH) has been proposed as a marker of myofibrillar proteolysis through post-translational methylation of specific histidine residues in actin and myosin.82-85 In a clinical scenario, 3MH has to be determined quantitatively in urine or plasma collections. A major disadvantage is that meat intake for 3 days prior to sample collection of patients can disturb the analysis of 3MH. A study from 2013 used 3MH, which was labelled with an isotope by using a non-radioactive isotope-based strategy.70 The labelled methyl-d3-3MH (D-3MH) was taken orally by healthy men, and urine and plasma samples were collected next day over 5–6 h, and were analysed for D-3MH enrichment by gas chromatography–mass spectrometry.70 The results suggest that it is possible to obtain an index of myofibrillar protein breakdown in urinary or plasma samples and that it is not necessary to quantify urine and plasma collections or to have an abstinence from meat for several days.70 Growth differentiating factor-15 (GDF-15) plays an important role in muscle wasting and cachexia.86 Results from studies suggest that GDF-15 induces weight and muscle loss, which makes GDF-15 a promising marker of cachexia and muscle atrophy.71 Myostatin is a known negative regulator of muscle growth and mass, which is associated with muscle wasting86 suggesting it as putative marker for muscle atrophy. However, this could not be confirmed in humans.87 Interestingly, follistatin (FST), an endogenous, strong inhibitor of myostatin-mediated muscle wasting, has been suggested as a potential biomarker in sarcopenia.72 FST binds to myostatin in the serum, thus, making myostatin often undetectable88 and moreover, FST-overexpressing transgenic mice have been shown a significant increase in muscle mass.89 Therefore, FST seems to be a positive regulator of muscle growth which makes it interesting to be a biomarker. Irisin, the extracellular cleaved product of fibronectin type III domain containing protein 5, seems to be a potential sarcopenia biomarker, because of its involvement in muscle physiology.72 Plasma levels and mRNA expression of irisin were found to be elevated in mice in response to exercise.90 Moreover, a positive correlation between circulating irisin and FST levels has been described in healthy men and obese persons.91 Other than inflammatory cytokines, like IL-1, IL-6, and TNF-α, which are associated with anorexia and weight loss,92, 93 hormonal factors have been postulated to play a role in development in muscle loss, especially in cachexia.94, 95 Such factors include for instance leptin,96 ghrelin,97 and obestatin98 which are all thought to play a major role in cancer cachexia. These emerging biomarkers were investigated in oncologic patients as diagnostic and/or predictive markers, as well as their impact on patient survival.73 The study showed that ghrelin and leptin may be promising biomarkers for the identification of cachexia related to cancer and mark survival in cancer patients.73 Ghrelin serum levels were significantly higher in cancer patients in comparison to healthy subjects (573.31 ± 130 vs. 320.20 ± 66.48 ng/ml, P < 0.0001) and levels of leptin were significantly lower in cancer patients than in healthy controls (38.4 ± 21.2 vs. 76.28 ± 17.48 ng/ml, P < 0.0001).73 Ghrelin correlated negatively with leptin (r = −0.75; P < 0.0001) and inverse as well.73 By Kaplan Meier analysis, the survival prediction was tested, and it was shown that patients with the best profile were those with low levels of ghrelin associated with high levels of leptin and on the contrary patients with high ghrelin levels and low levels of leptin had a minor survival probability (log-rank (χ2) 8.02; P = 0.004).73 Furthermore, in a recently published study, in which a large number of putative biomarker candidates were tested, with a cohort of upper gastrointestinal cancer patients, β-dystroglycan was identified as a potential biomarker for weight-loss and myosin heavy chain or dystrophin as survival biomarkers.74 As mentioned before, inflammatory cytokines are used for prognosis of cancer cachexia. A new promising chronic inflammatory marker was found recently and is suggested to play a prognostic role in cancer cachexia: the tartrate-resistant acid phosphatase 5a (TRACP5a).75 Moreover, it is already known that serum TRACP5a is elevated in patients with RA76 and that the protein level of TRACP5a is reflected in cardiovascular diseases and sarcoidosis.77, 78
| Emerging biomarkers for cachexia and sarcopenia |
|---|
| Creatinine66 |
| Neoepitope67 |
| MMP-generated degradation fragment of collagen 6 (C6M)68 |
| Type VI collagen N-terminal globular domain epitope (IC6)68 |
| N-terminal propeptide of type III procollagen (P3NP)69 |
| C-terminal agrin fragment (CAF)69 |
| Methyl-d3-Methylhistidine (D-3MH)70 |
| Growth differentiating factor-15 (GDF-15)71 |
| Follistatin (FST)72 |
| Irisin72 |
| Ghrelin73 |
| Leptin73 |
| β-Dystroglycan74 |
| Dystrophin74 |
| Tartrate-resistant acid phosphatase 5a (TRACP5a)75-78 |
- MMP, matrix metalloproteinase.
Current news on treatment
In 2013 and 2014, many new biomarkers, as described before, were investigated in different diseases and models. But although many researchers and pharmaceutical companies tried to find therapies for muscle atrophy, including cachexia and sarcopenia, no solution has been established until now.71, 99, 100 Interestingly, Morley et al. discussed if we are closer to having drugs for treatment muscle wasting disease and therefore drugs were highlighted, which showed current advances in therapy for sarcopenia and cachexia (e.g. ghrelin agonists, selective androgen receptor molecules, megestrol acetate, activin receptor antagonists, espindolol, and fast skeletal muscle troponin inhibitors).101 Indeed, Morley et al. postulated that there is a remarkable increase in the knowledge of muscle wasting diseases because of new studies. However, a general strategy to avoid muscle mass loss and function is exercise.102, 103 Evidence of positive effects on fraility and sarcopenia through exercising are emerging.102, 104-108 Recently, an exercise investigation focused on muscle quality in men and women aged 50 years and older suggested that long-term exercise, especially resistance exercise, is beneficial for muscle quality.109 Interestingly, people over 60 years, who perform aerobic exercise once a week, also show positive association to muscle quality.109 In rat skeletal muscle, an example for a successful result of exercising was postulated for glycogen synthase kinase-3β (GSK-3), which has a big therapeutical potential when it is inhibited.110 An inhibition of the constitutively active kinase GSK-3β is considered to be beneficial, as it is involved in the regulatory inactivation of many anabolic pathways often leading to muscle wasting.111-116 In detail, it has been demonstrated that physical exercise significantly decreases GSK-3β activity in rat skeletal muscle within 10 min of exercise and remained depressed with 30 and 60 min of exercise.110 In the following part current treatment substances discoveries will be listed (see for detailed therapies117, 118).
Ghrelin, which was delineated as a good biomarker before,73 and its analogues BIM-28125 and BIM-28131 seem to have a beneficial effect after administration in a rat HF study.119 In that animal model it was shown that the expression of myostatin and the TNF-α concentration are significantly reduced in the gastrocnemius after treatment.119 Moreover in a pre-clinical study, treatment with a ghrelin receptor agonist, named anamorelin, showed a significant and dose-dependent increased food intake as well as body weight compared with the vehicle control in healthy rats.120 Thereby, with a treatment of anamorelin, a significant increase of growth hormone and insulin-like growth factor-1 plasma levels was observed in healthy female pigs in comparison to the placebos,120 which makes anamorelin a potential drug for treatment of cancer anorexia–cachexia syndrome. Espindolol, an anabolic catabolic transforming agent, was used in a sarcopenia rat study.121 Espindolol itself is a non-specific β-1 and β-2 adrenergic receptor blocker with intrinsic sympathomimetic activity on the β-2 adrenergic receptor which results in reduced catabolism and increased anabolism, and espindolol is a highly potent antagonist of 5-HT1A receptors which has an effect on food-intake as well as reduced fatigue and thermogenesis.122 A recent study, the ACT-ONE trial, which was a multicentre, randomized, double-blind, and placebo-controlled study for dose-finding of espindolol in cachectic patients with non-small cell lung cancer and colorectal cancer in stages III and IV was started.122 At the 7th Cachexia Conference, the first results of the ACT-ONE trial with 87 patients from 17 centers were presented.86 Patients were treated with two doses of espindolol twice daily for 16 weeks. The results showed that the higher dose improves lean and fat mass, and the that handgrip strength is significantly increased at both doses.86 Interestingly, in a rat sarcopenia model, espindolol treatment only had a small effect on overall body weight, but did significantly increase lean body mass, while at the same time reducing fat mass. This makes espindolol an attractive candidate for treating sarcopenic patients, as these patients are often obese.121 A highly potent β-2 adrenoceptor-selective agonist, formoterol, was used as a drug in a cancer cachexia rat model, and it significantly reduced muscle wasting but had no influence on heart weight and function as often described in literature.123 Epigallocatechin-3-gallate (EGCg), which is a component of green tea, was published to be an effective inhibitor of increased protein degradation and depressed protein synthesis in an in vitro study by using murine C2C12 myotubes.124 EGCg is not an approved drug but acts as a nutritional support and has been shown to attenuate skeletal muscle wasting in the Lewis lung carcinoma model of cancer cachexia.125
Conclusions
Muscle loss arises from a dysbalance of catabolism and anabolism, i.e. protein degradation and protein synthesis. Despite a large number of studies, knowledge of disease related muscle wasting remains unclear. But investigations in the last two years like studies focused on RA53 and stroke55 bring us one step ahead in understanding processes of muscle wasting because of those diseases. Cachectic and sarcopenic patients often suffer from quality of life including appetite loss and lower muscle strength, which makes finding appropriate biomarkers for diagnosis of muscle wasting associated diseases a timely matter. Although an ‘ideal’ marker has not yet been identified, the development of some emerging candidates (Table 1) promise much potential. Neoepitopes67 as biomarkers could be the solution for early diagnosis of a potential muscle mass loss allowing earlier detection and treatment to prevent morbidity and mortality in patients. In addition, finding new treatment strategies and drugs has to be developed to treat patient's symptoms. There are some very promising investigational drugs in studies related to cachexia and sarcopenia, but further research is necessary for a transition into the clinic. Maybe there is the need to combine existing treatment strategies with further novel approaches to treat muscle mass loss.
Disclosure
This paper is also published in parallel in the International Journal of Cardiology.
Acknowledgments
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7–8.)
Conflict of interest
Cathleen Drescher, Masaaki Konishi, Nicole Ebner, and Jochen Springer declare that they have no conflict of interest.




