The Role of Mitochondrial Dynamics in Doxorubicin-Induced Disease: Implications for Therapy
Huan Yue and Yousheng Chen contributed equally to this study
ABSTRACT
As an anthracycline chemotherapy drug, doxorubicin (Dox) is generally prescribed to treat a variety of malignant tumors. Nevertheless, Dox exhibited toxicity at a high dosage, which might eventually lead to injury of the body. Mitochondrial dynamics, including mitochondrial fission and fusion, regulates mitochondrial homeostasis and cellular function. Mounting evidence has demonstrated that imbalance in mitochondrial dynamics, manifested by increased mitochondrial fission or decreased mitochondrial fusion, is associated with the development of Dox-induced diseases. In this paper, we will elaborate the role of mitochondrial dynamics in Dox-induced diseases, and discuss the regulatory mechanism of mitochondrial dynamics in Dox-induced diseases, including apoptosis, fibrosis, myocardial atrophy and inflammation. Elucidating these issues may provide important value in the diagnosis and potential therapeutic strategies for Dox-induced diseases through regulation of mitochondria dynamics.
1 Introduction
In 2040, the number of cancer patients is expected to reach 28.4 million, a rise of 40% compared with 2020 (D'Angelo et al. 2022; DeSantis et al. 2014; Siegel et al. 2022). In recent years, antitumor drugs of anthracycline, widely used in clinical practice, have caused concerning issues such as cardiotoxicity, inflammation, and fibrosis (Anjos et al. 2021; Henriksen 2018; Jones and Dass 2022; Mattioli et al. 2023). Doxorubicin (Dox), a representative of anthracycline chemotherapeutic drugs, has been used to treat various malignant tumors by embedding into DNA and inhibiting nucleic acid synthesis (de Lima et al. 2024; Radford 2024). Long-term use of Dox causes cardiotoxicity, which may result in cardiac arrhythmia, angina pectoris and myocardial atrophy. Besides, Dox weakens the hematopoietic function of bone marrow and promotes the fibrosis of multiple tissues. A few patients may exhibit fever, hemorrhagic erythema and liver function damage after Dox treatment, seriously affecting the life quality. In addition, with the increased dose of Dox, cardiac injury, inflammation and fibrosis will worsen gradually, which are important causes of death besides tumor recurrence (Algieri et al. 2022; Kong et al. 2022; Rawat et al. 2021; Roca-Alonso et al. 2015; Sturgeon et al. 2019; Zhang et al. 2024). These adverse effects may lead to the development of Dox-induced diseases in patients, thereby imposing limitations on the clinical application of Dox (Li et al. 2024; Liu et al. 2024; Sawicki et al. 2021; Vuong et al. 2022; Wang et al. 2024; Yang et al. 2024; Zhang et al. 2024).
Mitochondria, organelles with double layered membranes, are responsible for energy production by oxidative phosphorylation. Live cell imaging shows that the shape, number, size, length and distribution of mitochondria within the cell can alter constantly. This delicate process is known as mitochondrial dynamics, which is an important pathway for mitochondrial quality control. Mitochondrial homeostasis and cellular function are maintained by mitochondrial dynamics, which includes mitochondrial fission and fusion (Adaniya et al. 2019).
In recent years, Dox-induced diseases have become the most widely studied forms of toxicity induced by drugs. Dox can disrupt the balance of mitochondrial dynamics, contributing to mitochondrial dysfunction. Altered mitochondrial quality control might lead to the occurrence and development of diseases caused by Dox. Over the last decade, potential strategies targeting mitochondrial homeostasis have been conceived for Dox-induced diseases treatment. In this review, we will elaborate the role of the mitochondrial dynamics in Dox-induced diseases, and discuss the molecular mechanisms and pathological significance of Dox-induced diseases. Besides, we will discuss the breakthrough in Dox-induced diseases treatment targeting mitochondrial dynamics. Elucidation of these issues might provide new insight into the function of mitochondrial dynamics in Dox-induced diseases, and potential targets for diseases prevention and treatment (Archer 2013; Cao et al. 2022; Huang et al. 2022; Maneechote et al. 2017; Schirone et al. 2022; Shi et al. 2020; Shi et al. 2021; Wu et al. 2022).
2 Mitochondrial Dynamics: Dynamic Processes of Mitochondrial Fission and Fusion
2.1 Mitochondrial Fission
Mitochondrial fission, namely one mitochondrion divides into two or more mitochondria, alters the amount and spatial distribution of mitochondria within the cell. Multiple proteins localized at the cytoplasm and outer mitochondrial membrane (OMM), including dynamin-related protein 1 (Drp1), fission factor-1 (Fis1), mitochondrial fission factor (MFF), mitochondrial dynamics proteins of 49 kDa (MiD49) and mitochondrial dynamics proteins of 51 kDa (MiD51), participate in mitochondrial fission (Figure 1). Drp1, mainly exists in the cytoplasm, binds to the receptor on the surface of the OMM and forms a ring around the mitochondria once activated. Drp1 contracts the ring via GTP hydrolysis and the mitochondria are divided into two or more, leading to mitochondrial fission. Drp1 has two phosphorylation modification sites including Ser616 and Ser637 sites. Phosphorylation of the Drp1ser616 site activates Drp1-mediated mitochondrial fission, while phosphorylation of the Drp1ser637 site reduces its translocation to mitochondria and inhibits mitochondrial fission (Wang et al. 2018; Yu et al. 2019). Fis1, MFF, MiD49 and MiD51, localized on the OMM, are receptors for Drp1 and enhance mitochondrial fission via Drp1 recruitment. Among these, Fis1 and MFF can function independently or together in the recruitment of Drp1 to promote mitochondrial division. Though Drp1 can be recruited by MiD49 and MiD51, it keeps inactive until cellular signals trigger mitochondrial fission. It has been shown that the phosphorylation of Erk1/2, p53 and Akt signaling pathway promote mitochondrial fission by activating p-Drp1 (Gharanei et al. 2013; Maneechote et al. 2022; Zhuang et al. 2021). Overexpression of MiD49 and MiD51 promotes mitochondrial fusion rather than mitochondrial fission, at which point Drp1 recruitment is enhanced, but the inhibitory phosphorylation of Drp1 on Ser637 is increased simultaneously (Losón et al. 2013).
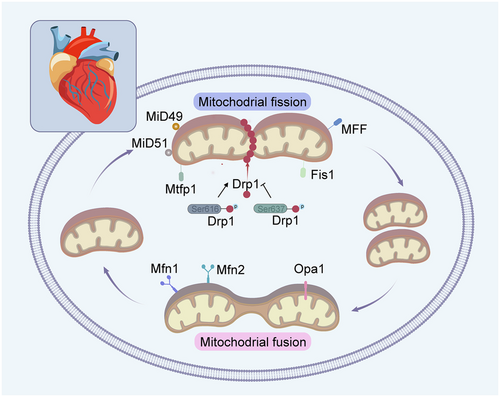
Mitochondrial fission maintains the energy supply of the cell by regulating the number of mitochondria. When cellular energy demands increase, mitochondrial fission promotes the number of mitochondria to provide more ATP (Beikoghli Kalkhoran and Kararigas 2022). The shortage of ATP maybe attributed to the impaired mitochondria. During the life cycle of mitochondria, mitochondrial fission enables both biogenesis of new mitochondria and clearance of dysfunctional mitochondria by mitophagy (Kleele et al. 2021). Mitochondrial fission ensures an even distribution of mitochondria within the cell, and avoids aggregation of mitochondria in a certain region leading to instability and dysfunction of the organelles (Quiles and Gustafsson 2022). Besides, mitochondrial fission promotes the activity of intracellular antioxidant enzymes and increases the surface area of mitochondria, which enhances the scavenging ability of oxidative stress. Moreover, mitochondrial fission regulates intracellular metabolite balance and ensures normal cellular metabolic processes (Toyama et al. 2016). However, the excessive mitochondrial fission can promote oxidative stress and the enrichment of Ca2+ in mitochondria, leading to mitochondrial swelling and eventually cell death.
2.2 Mitochondrial Fusion
Mitochondrial fusion regulators include mitochondrial fusion proteins 1/2 (mitofusin 1 and 2, Mfn1/2) and optic atrophy 1 (Opa1). Mfn1/2 mediate the fusion of neighboring mitochondrial OMMs and promote Ca2+ uptake of mitochondria. The GTPase activity of Mfn is modulated by Guanine nucleotide-binding protein β subunit 2 (Gβ2), and the GTPase activity of Mfn1 is higher than that of Mfn2. The binding of Gβ2 and Mfn1 enhances the pro-fusion effect of Mfn1 by promoting its aggregation at specific sites of the OMM. In addition to mediating mitochondrial fusion on the OMM, Mfn1/2 exert multiple roles including connecting the endoplasmic reticulum with mitochondria, mediating mitophagy, and promoting the stress-associated unfolded protein response (UPR) (Gottschalk et al. 2022).
Opa1, a mutated protein in dominant optic atrophy, regulates the fusion of inner mitochondrial membrane (IMM). Opa1 is vital for mitochondrial cristae remodeling and enhances mitochondrial respiratory efficiency by promoting the assembly of supercomplexes in electron transport chain, which facilitates the oligomerization of ATP synthase (Meeusen et al. 2006). Opa1-mediated IMM fusion ensures the mitochondrial cristae formation, a key structure for mitochondrial oxidative phosphorylation (Meeusen et al. 2006). Recent studies have revealed the structural properties of mitochondrial membrane remodeled by Opa1, and elucidated the key molecular mechanisms of Opa1-dependent membrane remodeling and fusion (von der Malsburg et al. 2023). Opa1 is embedded in cardiolipin-containing membrane through a lipid-binding paddle domain, which further stabilizes its interaction with cardiolipin-rich membrane. Opa1 facilitates the helical assembly of a flexible Opa1 lattice on the membrane via dimerization of the paddle domain to promote inner mitochondrial membrane fusion.
2.3 The Role of Mitochondrial Dynamics in Apoptosis and Mitophagy
Mitochondrial dynamics plays a critical role in regulating apoptosis, the programmed cell death process. Apoptosis is generally initiated by mitochondrial outer membrane permeabilization (MOMP), which leads to the release of proapoptotic factors into the cytosol such as cytochrome c. In addition, mitochondrial dynamics influence the susceptibility of mitochondria to MOMP (Tábara et al. 2025). Excessive mitochondrial fission can promote MOMP by creating smaller and more fragile mitochondria that are prone to membrane rupture, which further accelerate the apoptosis of cells (Adebayo et al. 2021; Peña-Blanco and García-Sáez 2018).
It is known that mitophagy is a selective form of autophagy that specifically targets damaged or dysfunctional mitochondria for degradation. Mitophagy is a critical quality control mechanism that maintains mitochondrial health, ensures cellular energy balance, and prevents the accumulation of damaged mitochondria which might lead to oxidative stress, inflammation and cell death (Lu et al. 2023). Mitophagy is mainly regulated by molecular pathways such as the PINK1- Parkin system and some other proteins such as NIX/BNIP3L, BNIP3 and FUNDC1, which play a key role in maintaining mitochondrial quality control. By eliminating defective mitochondria, mitophagy prevents the overproduction of reactive oxygen species, and ensures proper energy metabolism, which is essential for normal cell development, differentiation and pathogens defense (Han et al. 2023; Lu et al. 2023).
3 Mitochondrial Dynamics in Dox-Induced Diseases and Potential Therapeutic Strategies
3.1 Dox-Induced Apoptosis
The disruption of mitochondrial dynamics is related to the occurrence of cardiovascular diseases, including ischemia-reperfusion injury, cardiomyocytes apoptosis, myocardial fibrosis, myocardial atrophy and inflammation (Aung et al. 2017; Dhingra et al. 2017; Marechal et al. 2011; Marques-Aleixo et al. 2018; Ong et al. 2010; Wallace et al. 2020; Yang et al. 2019; Zorzano et al. 2009). Dox could disrupt mitochondrial dynamics by increasing mitochondrial fission, followed by ROS production and apoptotic signaling activation, which in turn led to apoptosis (Catanzaro et al. 2019; Li et al. 2014; Marechal et al. 2011; Samant et al. 2014; Tang et al. 2017).
Treatment of primary cardiomyocytes with Dox ranging from 0.86 to 1.72 µmol/L contributed to a dramatic decreased expression of Mfn1, Mfn2, and Opa1, and an elevation of Drp1ser616 phosphorylation (Li et al. 2014; Tang et al. 2017). Similar results were obtained in H9c2 cells (Figure 2) (Ding et al. 2023). These studies demonstrated that Dox inhibits mitochondrial fusion and promotes mitochondrial fission. To explore the function of mitochondrial dynamics in Dox-induced cardiomyocytes apoptosis, H9c2 and rat adult cardiomyocytes were incubated with gradient concentration of Dox for different times (Gharanei et al. 2013; Xu et al. 2020; Yin et al. 2018; Zhuang et al. 2021). The study found that Dox upregulates the level of Drp1, p-Drp1ser616, Fis1 and MFF, and downregulates the expression of Drp1ser637, contributing to a significant increase in mitochondrial fission. Besides, Dox decreased the protein expression of Mfn1/2 and Opa1, leading to decreased mitochondrial fusion (Christidi and Brunham 2021; Maneechote et al. 2019; Xia et al. 2017). Additionally, neonatal rat ventricular myocytes (NRVMs) treated with Dox showed activation of Drp1 and decreased level of Mfn1/2 and Opa1 (Ding et al. 2022; Du et al. 2019; Liang et al. 2020; Tang et al. 2017). Likewise, after treated with 250 nM or 1 µM Dox for 24 h, excessive Drp1-mediated mitochondrial fission could be examined in AC16 human cardiomyocyte cell line (Shi et al. 2021; Yin et al. 2018). When treating adult mouse cardiomyocytes with 1 µM Dox for 24 h, a decrease in Drp1ser616 was found to be associated with mitochondrial elongation (Shi et al. 2021).
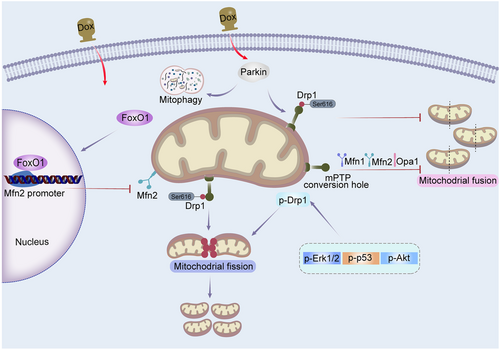
It has been reported that MiD49 is targeted and suppressed by Forkhead box O3 (Foxo3a), a transcription factor that belongs to the Forkhead family (Table 1) (Zhou et al. 2017). Cardiac specific Foxo3a transgenic mice exhibited reduced mitochondrial fission, apoptosis and cardiotoxicity by suppressing MiD49 upon Dox administration. Besides, it has been found that blockage of Mfn2-regulated mitochondrial fusion is a key phenotype in cardiotoxicity caused by Dox (Ding et al. 2022). In cardiomyocytes treated with Dox, the recovery of mitochondrial fusion mediated by Mfn2 promoted mitochondrial oxidative metabolism, and suppressed apoptosis and mitochondrial oxidative stress. Compared with control group, cardiac-specific Mfn2 transgenic mice injected with Dox exhibited attenuated myocardial injury. The increased expression of FoxO1 induced by Dox repressed Mfn2 transcription by binding to its promoter, thereby inhibited Mfn2-mediated mitochondrial fusion. Besides, transfection of adenovirus encoding Mfn2 (Ad-Mfn2) restored the level of Mfn2 in primary cardiomyocytes upon Dox treatment, and enhanced cell viability and reduced apoptosis in cardiomyocytes treated with Dox (Ding et al. 2022).
| Dox-induced diseases | Mitochondria dynamics | Alteration | Model | Dox Dosage | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Dox-induced cardiomyocyte apoptosis | Fission/Mitophagy/Autophagy | ↓ Mfn1 ↓ Mfn2 ↓ Opa1 ↑ Drp1 |
Rats | 14 mg/kg | Dox reduced the expression of Mfn1/2 and Opa1, increased the expression of Drp1, MIEF2 and Fis1, and produced mitophagy, resulting in augmentation of mitochondrial permeability transition pore (mPTP) and apoptotic signaling. | Marques-Aleixo et al. (2018) |
| Fission/Fragmented mitochondria (4 h)/Mitophagy | ↓ Mfn2 | NRVMs | 1.72 µM | Dox reduced mitochondrial fusion, leading to mitochondrial fragmentation and cardiomyocyte apoptosis. | Tang et al. (2017) | |
| Fission/Mitochondrial length/width/Mitophagy | ↑ p-Drp1ser616 | AMCM cells/AC16 cells | 1 µM (24 h) | Dox mediated mitochondrial fission and fragmentation, leading to mitochondrial dysfunction and cardiomyocyte apoptosis. | Shi et al. (2021); Xu et al. (2020) | |
| Fission | ↑ Fis1 ↑ hFis1 |
Rats/H9c2 cells | 12 mg/kg/0.75 µM (24 h) | Dox suppressed mitochondrial fusion and promoted fission, causing mitochondrial dysfunction. | Govender et al. (2018); Hu et al. (2022) | |
| Fission | ↑ MIEF2 | Mice cardiomyocytes | 3 µM (24 h) | Foxo3a was downregulated in the cardiomyocytes with Dox treatment, leading to increased mitochondrial fission. | Zhou et al. (2017) | |
| Dox-induced fibrosis | Fission/Mitochondrial length | ↑ p-Drp1ser616 | Mice | 12 mg/kg | DOX induced cardiotoxicity by promoting apoptosis and mitochondrial fission. | Zhuang et al. (2021) |
| Dox-induced myocardial atrophy | Fission/Fragmented mitochondria/Mitophagy | ↓ Mfn2 ↑ p-Drp1ser616 ↓ p-Drp1ser637 |
NRVMs/mice | 1.72 µM/12 mg/kg | Dox contributed to mitochondrial dysfunction and myocardial atrophy by decreasing fusion and promoting oxidative stress, apoptosis, thus resulting in cardiac dysfunction. | Tang et al. (2017); Zeng et al. (2020) |
| Dox-induced inflammation | Fission/Mitophagy | ↑ MEF ↑ Fis1 ↓ Mfn1 ↑ Opa1 |
H9c2 cells | 0.75 µM (24 h) | Dox induced mitochondrial fusion and promoted fission and mitophagy, thereby leading to mitochondrial dysfunction and inflammation. | Hu et al. (2022) |
Jenelle Govender et al. showed that mitochondrial fission was enhanced and mitochondrial fusion and mitophagy were inhibited upon Dox treatment, which in turn led to apoptosis and cardiac dysfunction (Govender et al. 2018). Increased mitochondrial fission and impaired fusion may be associated with the severity of Dox-induced cardiotoxicity through various molecular signaling pathways, including mitochondrial dysfunction, mitophagy, and oxidative stress, ultimately causing decreased cell viability and apoptosis (Abdullah et al. 2019; Ajoolabady et al. 2020; Deus et al. 2015; Dhingra et al. 2014; Hoshino et al. 2013; Li et al. 2022; Yin et al. 2018).
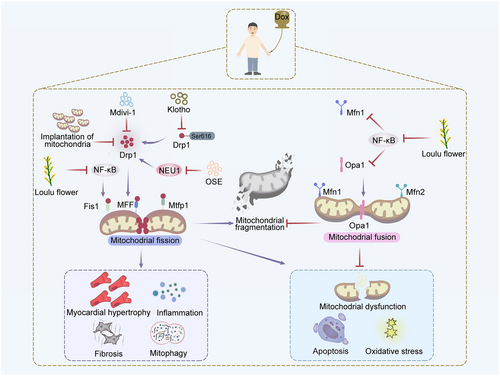
Taken together, studies have indicated that Dox causes the imbalance of mitochondrial dynamics, thus leading to the cardiomyocyte apoptosis (Aung et al. 2021; Catanzaro et al. 2019; Govender et al. 2018; Hu et al. 2022; Qin et al. 2021; Shi et al. 2021; Zeng et al. 2020). The exact mechanism by which Dox regulates mitochondrial dynamic proteins remains elusive. It has been proved that Drp1 translocation is regulated by mitochondrial fission process protein 1 (Mtfp1) (Aung et al. 2017). HL-1 cardiomyocytes transfected with Mtfp1 shRNA showed reduced mitochondrial fission and apoptosis. Intriguingly, recent evidence has demonstrated that deacetylase Sirtuin-3 (SIRT3) regulates mitochondrial dynamics by modulating the acetylation level of Opa1 (Samant et al. 2014). NRCMs transfected with Ad-Sirt3 demonstrated reduced level of Opa1 acetylation and apoptosis (Samant et al. 2014), suggesting that SIRT3 might play a protective function in cardiotoxicity models induced by Dox (Figure 3).
3.2 Dox-Induced Fibrosis
Dox-induced myocardial fibrosis is an important trigger of diastolic dysfunction of the heart. The proliferation and activation of fibroblasts are typical features of cardiac fibrosis, characterized by the excessive accumulation of extracellular matrix due to the secretion of collagen in myocardial tissues. Early fibrosis is a self-protection mechanism, but as fibrosis continues to develop, the structure and function of the heart become abnormal, resulting in impaired cardiac contractility. Thus, the defected function of early diastolic and late systolic in the heart will ultimately result in heart failure. The main cell type involved in myocardial fibrosis is fibroblasts, which account for 60% ~ 70% of the total amount of cells in the heart. Once stimulated, myofibroblasts transformed from cardiac fibroblasts secrete a large amount of extracellular matrix, leading to decreased cardiac diastolic function (Leask 2015; Pan et al. 2021).
Dox treatment can disrupt mitochondrial dynamics and mitochondrial function of cardiomyocytes, and contribute to the apoptosis of cardiomyocytes and activation of fibroblasts, ultimately resulting in the replacement of normal myocardial tissue with fibrous tissue. Zhuang et al. (Zhuang et al. 2021) injected Dox intraperitoneally into adult male C57 mice and showed that Dox-induced cardiotoxicity by promoting apoptosis and mitochondrial fission, as evidenced by the elevated expression of p-Drp1ser616 and increased length of mitochondria in myocardial fibrosis. Consistently, adult male SD rats injected with Dox intraperitoneally for 3 times showed that Dox-induced elevation of Neuraminidase 1 (NEU1), a lysosomal enzyme involved in fibrosis formation. NEU1 increased mitochondrial fission and mitophagy, resulting in myocardial fibrosis in response to cardiomyocytes apoptosis and cardiac dysfunction (Qin et al. 2021).
Klotho, an antiaging protein, exerts a variety of biological effects including anti-oxidation, anti-inflammation, antiapoptosis, anti-fibrosis and anti-vascular calcification. Zhuang et al. found that administrating Klotho to mice dramatically alleviated myocardial fibrosis induced by Dox via downregulating Drp1 expression and reducing mitochondrial fission and apoptosis (Table 2) (Zhuang et al. 2021). In addition, treatment of oseltamivir, an inhibitor of NEU, contributed to the reduced expression of apoptotic markers such as c-Caspase3 and Bax, and inhibited cardiac fibrosis in rats, suggesting that NEU inhibitor attenuated Dox-induced myocardial fibrosis via restraining Drp1-dependent mitochondrial fission, mitophagy and apoptosis (Qin et al. 2021). In conclusion, the Dox-induced myocardial fibrosis can be reversed by modulating mitochondrial dynamics.
| Intervention | Targeting gene | Mitochondrial dynamics | Alteration | Model | Dox Dosage | Disease | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| Ad-Mfn2 (48 h) | Mfn2 | Fusion/Mitochondrial size/Mitochondrial number | ↑ Mfn2 | NRVMs | 3 µM (24 h) | ↓ Cardiomyocyte apoptosis | Recovery of mitochondrial fusion mediated by Mfn2 promoted mitochondrial oxidative metabolism, decreased cell damage and apoptosis in cardiomyocytes treated with Dox. | Ding et al. (2022) |
| Klotho (0.01 mg/kg) | Drp1 | Fusion/Mitochondrial length | ↓ p-Drp1ser616 | Mice | 12 mg/kg | ↓ Fibrosis | Klotho alleviated cardiotoxicity induced by Dox via reducing apoptosis and mitochondrial fission through decreasing Drp1. | Zhuang et al. (2021) |
| Oseltamivir (20 mg/kg) | Drp1 | Fusion/Mitophagy | ↓ Drp1 | Rats | 15 mg/kg | ↓ Fibrosis | The NEU1 inhibitor efficiently inhibited Drp1-dependent mitochondrial fission, autophagy and apoptosis, resulting in attenuated cardiac dysfunction and remodeling. | Qin et al. (2021) |
| Mitochondria implantation (500 µg/rat) | Drp1 Mfn2 |
Fusion/Mitophagy | ↓ Drp1 ↑ Mfn2 |
Rats | 12.5 mg/kg | ↓ Myocardial atrophy | Mitochondria implantation efficiently suppressed mitochondria fission and enhanced fusion, resulting in preservation of LVEF and cardiac remodeling. | Yip et al. (2020) |
| Loulu flowers (200 µg/mL) | Opa1 Mfn1 MFF Fis1 |
Fusion | ↑ Opa1 ↑ Mfn1 ↓ MFF ↓ Fis1 |
H9c2 cells | 0.75 µmol/mL (24 h) | ↓ Inflammation | LLF pretreatment alleviated Dox-induced ROS production, apoptosis, inflammation and aberrant expression of mitochondrial dynamics proteins. | Hu et al. (2022) |
| Mdivi-1 (1.2 mg/kg) | Drp1 Mfn1 Mfn2 Opa1 |
Fusion/Mitochondrial volume/Mitochondrial area | ↓ Drp1 ↓p-Drp1ser616 ↑ Mfn1 ↑ Mfn2 ↑ Opa1 |
Rats | 18 mg/kg | ↓ Inflammation | Mdivi-1 reduced mitochondrial dysfunction, oxidative stress, inflammation, and apoptosis, thus restoring heart function through regulation of mitochondrial dynamics associated proteins. | Maneechote et al. (2022) |
3.3 Dox-Induced Myocardial Atrophy
Myocardial atrophy is defined as a decrease in cardiac mass due to reduced cardiomyocyte size, loss of myocardial cells, and changes in interstitial components. Dox can cause inflammation, degeneration, necrosis, and interstitial edema in cardiomyocytes, which in turn lead to myocardial atrophy. In Dox-induced cardiomyopathy, the myocardial damage is characterized by loss of myofibrils, vacuolization of cytoplasm, or even cell necrosis. Besides, Dox can inhibit the oxidative phosphorylation in cardiomyocytes, leading to mitochondrial damage and affecting energy metabolism in cardiomyocytes. The electrophysiological properties of the myocardium can be affected upon Dox treatment, as evidenced by changes in myocardial depolarization and repolarization as well as increased myocardial stress, which may further contribute to the development of myocardial atrophy. Clinical symptoms of Dox-induced myocardial atrophy include arrhythmia, angina pectoris and cardiac enlargement. Severe patients may show more serious symptoms including cardiac infarction, heart failure or even sudden death (Forte et al. 2021).
It has been reported that mice treated with Dox (15 mg/kg) exhibited defected cardiac function with disordered mitochondria, reduced mitochondrial respiratory complex I activity, increased amount of TUNEL-positive cells, and elevated level of c-Caspase3, accompanied by severe myocardial atrophy (Xia et al. 2017). Another study showed that implantation of mitochondria into the left myocardium of the adult rats contributed to the downregulation of c-Caspase3, Bax, c-PARP and Cyt c (Yip et al. 2020), suggesting that the implanted mitochondria inhibit mitochondrial fission mediated by Drp1 and promote fusion mediated by Mfn2, reduce oxidative stress and apoptosis, thus effectively attenuating Dox-induced myocardial atrophy. These studies demonstrate that Dox-induced myocardial atrophy can be intervened by modulating mitochondrial dynamics.
3.4 Dox-Induced Inflammation
Dox can affect cell growth and division by inhibiting DNA synthesis. When this occurs in the lung, Dox can cause local inflammatory response and fibrosis, leading to lung damage. With prolonged or high dosage of Dox treatment, the persistent inflammation promotes fibroblast proliferation and fibrosis, impairing lung function. Prolonged and high-dose application of Dox may lead to the development of chronic interstitial pneumonitis, which usually occurs after administration for several months to years. Dox has high immunotoxicity, which can induce T-lymphocytes apoptosis, thereby triggering the immune response and the production of various cytokines. These cytokines, in turn, stimulate the activation of macrophage and release of inflammatory mediators, leading to the damage to the alveolar walls and pulmonary capillary endothelial cells, as well as exacerbation of lung inflammation (Kuerban et al. 2020; Wang et al. 2024). Besides, Dox may cause other types of inflammation, such as stomatitis and phlebitis. These inflammatory reactions are usually associated with the cytotoxic effects of Dox, which are manifested by renal injury, redness, swelling, pain, and ulceration of localized tissues (Li et al. 2023; Wu et al. 2021).
Dox treatment can interfere with mitochondrial dynamics, which in turn promotes the release of inflammatory factors and aggravates the inflammatory response in the myocardium. Zeng et al. injected mice intraperitoneally with Dox, and found that Dox treatment promotes mitochondrial fission, expression level of p-Drp1ser616 and over-activation of NLRP3 inflammasome, and decreases expression level of p-Drp1ser637, leading to eosinophilic cell death and inflammation (Zeng et al. 2020). Rats injected intraperitoneally with Dox (3 mg/kg) showed altered mitochondrial dynamics, characterized by elevated level of Drp1 and reduced level of Mfn1 and Mfn2, activation of autophagy, and increased oxidative stress (Prathumsap et al. 2022). The disrupted mitochondrial dynamics contributes to mitochondrial dysfunction and inflammation, ultimately resulting in apoptosis and cardiac dysfunction. Moreover, H9c2 cells or zebrafish treated with Dox showed increased level of MFF and Fis1, decreased expression of OPA1 and Mfn1, indicating that Dox contributes to mitochondrial dysfunction by regulating mitochondrial dynamics, inflammation, and apoptosis (Hu et al. 2022).
Loulu flowers (LLF), a member of the compositae family, has been commonly used for cardiovascular diseases treatment. Pre-treating H9c2 cells with LLF (200 µg/mL, 2 h) can alleviate cardiotoxicity induced by Dox via inhibiting nuclear factor kappa-B (NF-κB) signaling and regulating mitochondrial dynamics. Besides, LLF treatment regulated the integrity of mitochondrial membrane and increased cell viability by reducing ROS production and activating inflammation signaling in cells upon Dox treatment (Hu et al. 2022). Maneechote et al. showed that treatment of Mdivi-1, an inhibitor of Drp1, contributed to decreased expression of malondialdehyde (MDA) in serum and tissue, as well as reduced level of TNF-α, IL-6, Bax, c-Caspase3, and Cyt c, which suggested that Mdivi-1 reduces inflammation, oxidative stress and mitochondrial dysfunction, thereby improving cardiac function and reducing Dox-induced inflammation in rats via mitochondrial dynamics regulation (Maneechote et al. 2022).
4 Summary
Given that the disordered mitochondrial dynamics is a common mechanism involved in Dox-induced diseases, it is important to develop novel strategies for Dox-induced diseases treatment. Abnormal mitochondrial dynamics leads to impaired energy metabolism and insufficient supply of ATP, thus affecting cardiac contractile function. Besides, abnormal mitochondrial dynamics increases mitochondrial oxidative stress, activates the mitochondrial apoptotic pathway, and induces cardiomyocyte death. Furthermore, disordered mitochondrial dynamics might be associated with the progression of Dox-induced diseases through regulating calcium homeostasis and affecting cellular signaling pathways. Differences in etiology and severity of Dox-induced diseases are associated with different molecular mechanisms of mitochondrial dynamics. Inhibition of Drp1-regulated mitochondrial fission and restoration of Opa1- or Mfn1/2-regulated mitochondrial fusion can improve mitochondrial function and alleviate Dox-induced diseases.
Even though targeting mitochondrial dynamics is a potential therapeutic strategy for treating Dox-induced diseases, a number of issues remain to be addressed. It is worth noting that tumors affect organs such as kidneys, eyes and nervous system in addition to the heart. Thus, further study of the role of mitochondrial dynamics regulators in various organs is needed to deepen our understanding of their roles in Dox-induced diseases (Tahrir et al. 2019; Yang et al. 2020). Developing drugs targeting mitochondrial dynamics with high specificity and low side effects is urgent for Dox-induced diseases treatment (Cui et al. 2017; Khuanjing et al. 2021; Qin et al. 2021). Considering the involvement of multiple factors in the pathogenesis of Dox-induced diseases, it is possible to develop combined therapies to achieve better outcome. For instance, it might be more effective by administering drugs targeting mitochondrial dynamics combined with drugs that improve cardiac function, such as β-receptor antagonists, angiotensin converting enzyme inhibitors (Kobashigawa et al. 2014; Li et al. 2020; Liu et al. 2018; Najafi et al. 2020; Zhang et al. 2021; Zhang et al. 2023). Addressing these issues will help deepen our comprehension of the function of mitochondrial dynamics in Dox-induced diseases and develop drugs to restore mitochondrial dynamics balance, thus providing new therapeutic approaches for cancer patients (Mallick et al. 2023; Shi et al. 2022; Wang et al. 2023; Yao et al. 2024; Zeng et al. 2024).
Author Contributions
Huan Yue: writing original draft, investigation. Yousheng Chen: visualization, formal analysis, investigation. Junxiao Feng: visualization, investigation. Weiying Yue: investigation. Xingjuan Shi: writing – original draft, review and editing, supervision, conceptualization, funding acquisition.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (82170370, 81770381).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data will be made available on request.




