SATB1, senescence and senescence-related diseases
Abstract
Aging leads to an accumulation of cellular mutations and damage, increasing the risk of senescence, apoptosis, and malignant transformation. Cellular senescence, which is pivotal in aging, acts as both a guard against cellular transformation and as a check against cancer progression. It is marked by stable cell cycle arrest, widespread macromolecular changes, a pro-inflammatory profile, and altered gene expression. However, it remains to be determined whether these differing subsets of senescent cells result from unique intrinsic programs or are influenced by their environmental contexts. Multiple transcription regulators and chromatin modifiers contribute to these alterations. Special AT-rich sequence-binding protein 1 (SATB1) stands out as a crucial regulator in this process, orchestrating gene expression by structuring chromatin into loop domains and anchoring DNA elements. This review provides an overview of cellular senescence and delves into the role of SATB1 in senescence-related diseases. It highlights SATB1's potential in developing antiaging and anticancer strategies, potentially contributing to improved quality of life and addressing aging-related diseases.
1 INTRODUCTION
The extension of the human lifespan has resulted in the aging population representing an increased proportion of the total population. This group faces higher risks of age-related diseases, including tumors, diminished success in immunotherapy, and higher recurrence rates posttreatment (Desdín-Micó et al., 2020; Fane & Weeraratna, 2020). Aging brings systemic chronic inflammation, organ dysfunction, and various diseases. Cellular senescence is central to aging and is marked by the accumulation of senescent cells, affecting numerous cellular aging processes. Senescence develops gradually, transitioning from a temporary state to a chronic, irreversible one (Hernandez-Segura et al., 2017; van Deursen, 2014). Recently, several findings have challenged the irreversibility of cellular senescence (Lee & Schmitt, 2019). For instance, reactivating human telomerase in oncogene-induced senescent cells can cause cell division to resume, and senescent cells that regain stem-like properties show increased proliferation and tumorigenesis potential (Patel, 2016; Wang et al., 2022a). A number of small molecules have shown promise in countering cellular senescence. Notably, Rapamycin has shown efficacy in reducing the senescence-associated secretory phenotype (SASP) and enhancing antiviral gene expression in the elderly (Mannick et al., 2018). Intermittent oral administration of Dasatinib (D) and Quercetin (Q) resulted in the alleviation of physical dysfunction and a notable increase in posttreatment survival in mice and decreased the burden of senescent cells in patients with diabetic kidney disease (Hickson et al., 2019; Xu et al., 2018). However, their lack of specificity raises concerns about side effects, including inflammatory responses and tissue deterioration. Despite this caveat, there is an increasing drive to identify new markers of senescence, which could help in better understanding this process and could possess prognostic significance.
All the changes described in cellular senescence are the consequences of new gene expression profiles. Recent studies have shown diffuse chromatin remodeling, specifically in senescent proliferating cells, as well as in general during adult stem cell aging (Pollina & Brunet, 2011; Rando & Chang, 2012). Special AT-rich sequence-binding protein 1 (SATB1) has emerged as a pivotal player in the architectural organization of chromatin, where it orchestrates gene expression by facilitating chromatin loop structures through its interactions with the nuclear matrix. This dynamic role of SATB1 extends to various cellular processes, including differentiation, proliferation, tumorigenesis, apoptosis, and senescence, underscoring its significance in cellular physiology (Wang et al., 2023). Furthermore, current advancements in research have linked SATB1 not only with the elongation of lifespan but also with the progression of numerous age-associated disorders. Understanding the intricate workings of cellular senescence, propelled by factors like SATB1, could open new avenues for innovative and more effective therapeutic interventions. This is particularly crucial in the context of the escalating healthcare challenges posed globally by aging populations. In this review, we delve into the intricate role of SATB1 within the realm of cellular senescence and propose possible interventional strategies targeting SATB1 to improve immunotherapy and cancer treatment and potentially extend life expectancy.
2 OVERVIEW OF CELLULAR SENESCENCE
Cellular senescence not only offers a mechanism contributing to an organism's natural aging but also serves as a process to halt the progression of malignant phenomena, thus safeguarding the organism from tumors. This process has gained attention as a target for delaying aging and preventing the progression of malignancies. The advent of senolytic drugs, designed to selectively eliminate senescent cells, has invigorated aging research, leading to significant animal studies and a series of preclinical and clinical trials (Cohn et al., 2023; Kennedy et al., 2014). This emerging focus holds promise for innovative therapeutic strategies in age-related health management.
2.1 Causes of cellular senescence
Cellular senescence can be categorized into replicative senescence and premature senescence. Replicative senescence, initially identified in the replicative exhaustion of human primary fibroblasts, is characterized by reduced cellular proliferation after numerous divisions, leading to complete arrest (Hayflick & Moorhead, 1961). This phenomenon is mainly due to telomere shortening in non-transformed cells after repeated cell divisions (Sharpless & Sherr, 2015).
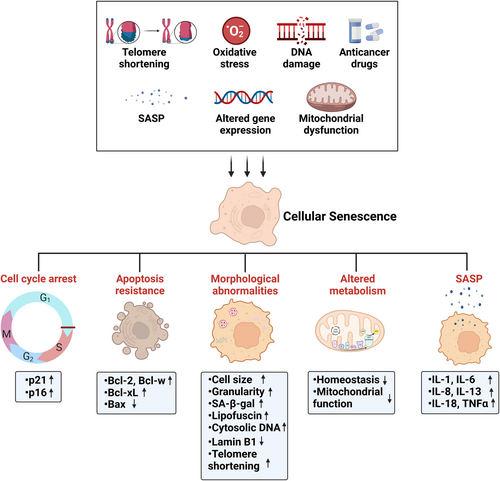
Premature senescence, on the other hand, can be triggered by various factors, including DNA damage, oxidative stress, chemotherapy, aberrant oncogene or tumor suppressor activity, mitochondrial dysfunction, epigenetic changes, and paracrine factors (Figure 1) (Hernandez-Segura et al., 2018). DNA damage accumulation is widely regarded as a key driver of aging, primarily through its role in triggering cell death, senescence, and tissue dysfunction. In vitro, various DNA-damaging agents can induce senescence, including radiation (both ionizing and ultraviolet and a range of drugs (Muñoz-Espín & Serrano, 2014). Oxidative-stress-induced senescence can be initiated by metabolic by-products or known oxidants, such as H2O2 (Hernandez-Segura et al., 2017). An increase in reactive oxygen species (ROS), exacerbated by impaired protein repair in mitochondria, leads to oxidative stress, proteasome activity decline, and extensive protein oxidation, setting the stage for inflammaging and senescence (Das et al., 2007; Ugarte et al., 2010). Various anticancer drugs, such as bleomycin and doxorubicin (causing DNA damage) and others like abemaciclib and palbociclib (acting via CDK inhibition), can also induce senescence (Petrova et al., 2016). Activation of oncogenes (e.g., Ras and BRAF) or inactivation of tumor suppressors (e.g., PTEN) can trigger oncogene-induced senescence (OIS) (Muñoz-Espín & Serrano, 2014; Sharpless & Sherr, 2015). Recent studies highlight mitochondrial dysfunction as a senescence inducer, particularly influencing the SASP (Wiley et al., 2016). Epigenetically induced senescence is triggered by inhibitors of DNA methylases (like 5-aza-2′-deoxycytidine) or histone deacetylases (such as suberoylanilide hydroxamic acid and sodium butyrate) (Petrova et al., 2016). Additionally, SASP factors secreted by senescent cells can induce senescence in neighboring cells (Acosta et al., 2013).
Cellular senescence plays a key role during embryonic development, contributing to tissue remodeling and repair (Demaria et al., 2014; Jun & Lau, 2010; Muñoz-Espín et al., 2013; Storer et al., 2013). It also acts as a natural tumor suppressor, which is evident in various nonmalignant and premalignant tissues, including lung adenomas (Collado et al., 2005), benign melanocytic nevi (Michaloglou et al., 2005), benign prostatic hyperplasia (BPH) (Choi et al., 2000), colon adenoma (Bartkova et al., 2006; Fujita et al., 2009; Kuilman et al., 2008), precancerous urinary bladder (Bartkova et al., 2006), and intraepithelial prostatic neoplasia (PIN) as well as xenograft models (Majumder et al., 2008). However, senescent cells, being metabolically active and capable of secreting the SASP, can promote a protumorigenic environment (Coppé et al., 2010; Wang et al., 2022a). Cellular senescence stands as a prime example of antagonistic pleiotropy, where certain genetic traits that are beneficial early in life for tumor suppression and development become detrimental later in life (Calcinotto et al., 2019; Campisi, 2013; McHugh & Gil, 2018). Consequently, it is imperative to broaden our understanding of the mechanisms governing cellular senescence. Over the past decade, studies have revealed significant gene profile changes during cellular senescence that are influenced by transcription regulators and chromatin modifiers. However, the detailed impact of these factors on senescence requires further exploration.
2.2 Hallmarks of cellular senescence
Cellular senescence is a cellular state characterized by the following hallmarks: a durable and generally irreversible cell cycle arrest; morphological abnormalities; macromolecular damage; a SASP; altered metabolism; and a resistance to apoptosis (Figure 1) (Gorgoulis et al., 2019; Campisi & d'Adda di Fagagna, 2007).
Senescent cells display elevated expression of cell cycle regulators, notably Cdkn2a (p16) and Cdkn1a (p21), which play key roles in modulating cell cycle arrest. Morphologically, senescent cells exhibit increased cell size and granularity; positive staining for senescence-associated β-galactosidase (SA-β-gal); lipofuscin accumulation in lysosomes; and a number of nuclear changes including a loss of lamin B1, telomere shortening, and senescence-associated heterochromatin foci (Di Micco et al., 2021; Gasek et al., 2021). Macromolecular damage, manifested as cytosolic DNA and DNA damage in telomeres, is another hallmark of senescent cells. It is often induced by factors like ionizing radiation, chemotherapeutic drugs, oxidative stress, and telomere dysfunction (Richardson & Schadt, 2014). The SASP, first identified by Jean-Philippe Coppe and colleagues in 2008, secretes a range of soluble factors, such as IL-1, IL-6, IL-8, IL-13, IL-18, and tumor necrosis factor, along with their receptors (Bruunsgaard et al., 2003; Ershler & Keller, 2000; Ferrucci & Fabbri, 2018; Puzianowska-Kuźnicka et al., 2016). The SASP exerts diverse (patho-)physiological effects through pro-inflammatory cytokines, chemokines, growth hormones, angiogenic factors, and matrix metalloproteinases (Coppé et al., 2010). The variety of the biological activities triggered by SASP components suggests interactions with adjacent cells, impacting the microenvironment in either beneficial or harmful ways, contingent upon the factors secreted, cell types involved, and whether the stimuli are acute or chronic. Metabolic disturbances in senescent cells reflect a loss of homeostasis in molecules and proteins. These disturbances arise from factors such as the DNA damage response due to telomere attrition, reduced tricarboxylic acid cycle activity, and impaired mitochondrial function, which collectively lead to altered metabolic signaling and cellular metabolites. Furthermore, senescent cells commonly exhibit upregulated expression of antiapoptotic BCL proteins (e.g., Bcl-2, Bcl-w, and Bcl-xL) and epigenetic repression of proapoptotic factors like Bax (Childs et al., 2014; Yosef et al., 2016).
Currently, the most widely recognized marker of cellular senescence is senescence-associated beta-galactosidase (SA-β-gal), which is intricately linked to the lysosomal stress response, though it is not exclusively indicative of senescence (Lee et al., 2006). Additional markers, including p53, p21, p16, ATM/ATR, and RB protein activation, complement the identification of senescent cells, especially when considered alongside morphological changes, cell cycle arrest, secretory profile alterations, and chromatin remodeling (López-Otín et al., 2013). The presence of γ-H2AX foci and decreased lamin B1 levels are also increasingly acknowledged as indicators of senescence (Freund et al., 2012; Sharpless & Sherr, 2015). As no singular marker definitively identifies senescence, a combination of these indicators is necessary for the accurate identification of a senescent cell (Hernandez-Segura et al., 2018).
2.3 Mouse models and senolysis
Mouse models used in senescence studies encompass the senescence-accelerated mouse—prone, senescence pathway knockout mice (p53, p21, and p16), and the more recently developed senolysis mice (p16-3MR and INK-ATTAC). Investigations into cellular senescence using p21-null and p53-null mice have unveiled critical roles in embryonic development and the promotion of premature aging syndromes like Hutchinson-Gilford progeria syndrome and ataxia telangiectasia (Benson, 2009; Muñoz-Espín et al., 2013; Varela et al., 2005; Xu et al., 1998). The induction of cellular senescence mediated by p53 and p21 serves as a pivotal mechanism for tumor suppression; hence, the majority of p53-null and p21-null mice succumb to cancer (Donehower et al., 1992; Han et al., 2002; Herranz & Gil, 2018; Martín-Caballero et al., 2001; Takeuchi et al., 2010; Wu, 2007). Thus, p16 has emerged as a paramount in vivo marker for senescent cells, and its knockout has become a commonly employed model for studying cellular senescence (Liu, 2019; Sharpless et al., 2001). Studies with p16-null mice suggest that inhibiting cellular senescence in the kidney enhances recovery of renal function following ischemia-reperfusion injury and reduces interstitial fibrosis and tubular atrophy in renal transplant models (Braun et al., 2012; Docherty et al., 2019; Lee et al., 2012). Although these mice develop tumors later than p53 knockout mice, they are highly susceptible to carcinogen-induced tumors upon loss of p16 function (Sharpless et al., 2001, 2004). Selective elimination of cells expressing p16 in mouse models has been shown to increase both life- and health-span (Childs et al., 2018). Currently, two such models have been described: INK-ATTAC and p16-3MR mice (Baker et al., 2011; Demaria et al., 2014). Kirkland et al. utilized a promoter for the senescence biomarker p16INK4a and an inducible “suicide” gene to develop a novel transgene, INK-ATTAC, facilitating inducible elimination of p16INK4a-positive senescent cells upon administration of a drug (AP20187) (Baker et al., 2011; Pajvani et al., 2005). In these mice, eliminating a relatively small proportion (~30%) of senescent cells extends health span and prevents the development of multiple age-related morbidities in both progeroid and normal, chronologically aged mice (Baker et al., 2011; Farr et al., 2017; Ogrodnik et al., 2017; Roos et al., 2016; Schafer et al., 2017; Xu et al., 2018). The p16-3MR model, controlled by ganciclovir administration, effectively depletes senescent cells in the skin, lungs, muscle, and bone marrow and suppresses the SASP in either sub-lethally irradiated or normally aged mice (Chang et al., 2016; Demaria et al., 2014; Kim et al., 2019). Clearance of p16Ink4a-positive cells using INK-ATTAC delays tumorigenesis (Demaria et al., 2017), reduces overall hepatic steatosis (Ogrodnik et al., 2017), improves LCH disease (Bigenwald et al., 2021). prevents age-related bone loss (Farr et al., 2017), alleviates detrimental features of cardiac aging (Anderson et al., 2019), improves glucose metabolism and β-cell function (Aguayo-Mazzucato et al., 2019) and preserves cognitive function (Bussian et al., 2018; Ogrodnik et al., 2021). Additionally, Ogrodnik and colleagues found that clearance of senescent cells in obese mice alleviates anxiety and anxiety-related behavior (Ogrodnik et al., 2019).
Recent studies have highlighted senolytic drugs for the selective clearance of senescent cells from “aged” tissues, resulting in delayed onset of age-related pathologies (Tchkonia & Kirkland, 2018). Intermittent oral administration of these senolytics, namely Dasatinib (D) and Quercetin (Q), to both young mice transplanted with senescent cells and naturally aged mice, resulted in the alleviation of physical dysfunction and a notable increase in posttreatment survival, along with a reduction in mortality hazard (Xu et al., 2018). In a Phase-1 clinical trial conducted 4 years after their identification as potential senolytics, the combination of Dasatinib + Quercetin was found to decrease the burden of senescent cells in patients with diabetic kidney disease (Hickson et al., 2019). Additionally, Fisetin (F), another naturally occurring flavonoid, has shown senolytic properties (Yousefzadeh et al., 2018; Zhu et al., 2017). Both Dasatinib + Quercetin and fisetin have been demonstrated to reduce senescent cell abundance in vivo without harming nonsenescent cells (Xu et al., 2018; Yousefzadeh et al., 2018). However, Navitoclax (N) has been found to decrease bone senescent cells in old mice but does not alleviate age-related osteoporosis due to its direct cytotoxicity to bone-forming osteoblasts (Kirkland & Tchkonia, 2020; Sharma et al., 2020). Despite selectively targeting senescent cells, senolytic drugs may inadvertently harm neighboring organisms, leading to common side effects like transient thrombocytopenia and neutropenia among patients (Rudin et al., 2012; von Kobbe, 2019; Wissler Gerdes et al., 2020). Nonetheless, these senolytics have demonstrated promise in preliminary clinical trials in humans (Hickson et al., 2019; Justice et al., 2019). Understanding senescence's role in diseases is essential for translating anti-senescence therapies in humans.
3 SATB1: STRUCTURE AND FUNCTIONAL DYNAMICS
Beyond traditional epigenetic modifications, the 3D architecture of chromatin is also believed to play a crucial role in gene regulation. Increasing studies have focused on the dynamic alterations in chromatin's 3D structure, aiming to understand how these changes influence transcriptional regulation. The polymer-like structure of chromatin fibers, characterized by long, repeating territories and compartments, is essential for comprehending their dynamic interactions and behavior at the genomic level (Papadogkonas et al., 2022). In eukaryotic cell nuclei, proximity between two genomic loci increases the probability of their physical interaction, especially with the aid of proteins. Due to the low likelihood of random genomic loci interacting, the genome undergoes dynamic conformational changes to reshape gene expression patterns (Dekker & Mirny, 2016). In mammalian cells, genomes are intricately packed and organized within the nucleus to enable precise gene expression regulation (Dekker et al., 2017). Different regions of the genome are segregated into self-interacting domains known as topologically associating domains (TADs), which significantly influence local gene transcription regulation (Dixon et al., 2012). These TADs are grouped into A and B compartments based on their chromatin conformation status, with A compartments being more open and located near the nucleus's interior and B compartments being denser and situated closer to the nuclear lamina (Oudelaar & Higgs, 2021). The CCCTC-binding factor (CTCF), a global chromatin remodeler, marks the boundaries of many TADs (Dowen et al., 2014; Hanssen et al., 2017; Narendra et al., 2015; Phillips & Corces, 2009). Recent studies have revealed that SATB1 extensively co-localizes with CTCF across the genome. SATB1 is instrumental in 3D genome organization, influencing chromatin topology around CTCF-binding sites and playing a key role in gene regulation (Figure 2) (Wang et al., 2023).
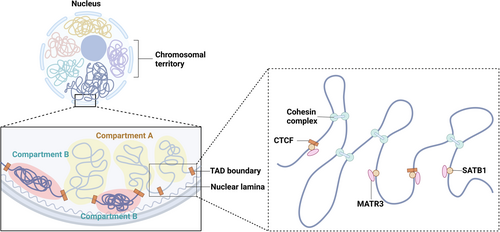
SATB1 is a chromatin remodeler that orchestrates the spatial and temporal actions of transcription factors by tethering specialized AT-rich genomic regions, leading to the formation of chromatin loops (Cai et al., 2006; de Belle et al., 1998; Galande et al., 2007). This loop extrusion is essential in facilitating the interaction between enhancers and their respective promoters while isolating them from other regulatory elements, thus preventing unwanted regulatory interactions (Dowen et al., 2014; Hnisz et al., 2016). Studies have revealed that SATB1 mediates chromatin loops at various IL and MHC genomic loci in Th2 and Jurkat cells (Cai et al., 2006; Kumar et al., 2007). SATB1 was first recorded in the thymus by Dickinson et al. in 1992 (Dickinson, 1992). They observed it binding to matrix attachment regions (MARs/SARs) in the cell nucleus. The Satb1 gene is located on chromosome 17 in mice and on chromosome 3 in humans (Zelenka & Spilianakis, 2020). Murine SATB1, sharing more than 98.3% identity with its human homolog, can multimerize through a ubiquitin-like domain (Wang et al., 2012). There are various transcript isoforms of the Satb1 gene, which is regulated by NF-κB signaling via multiple promoters (Khare et al., 2019).
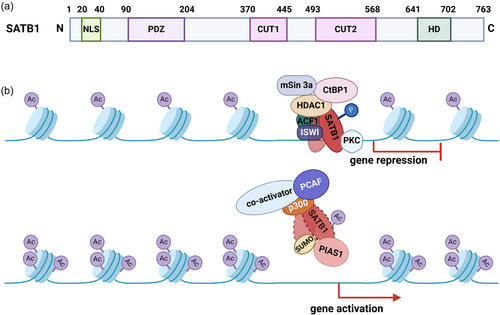
The N-terminus of SATB1 contains a nuclear localization signal (residues 20–40), followed by an oligomerization domain similar to the PDZ domain (Galande et al., 2001; Nakayama et al., 2005), which structurally resembles ubiquitin and is hence termed a ULD (ubiquitin-like domain) (Wang et al., 2012). The dimerization of this N-terminal domain is essential for DNA binding (Purbey et al., 2008), with further tetramerization of these dimers proposed as a mechanism for mediating chromatin loops (Wang et al., 2012, 2014). Other key DNA binding domains in SATB1 include CUT1, CUT2, and a C-terminal homeodomain (HD). The CUT1 domain is critical for efficient chromatin binding, contributing significantly to SATB1's high-affinity binding. The HD domain, on the other hand, ensures binding specificity, interacting with DNA features like negative propeller twist and high AT contents (Figure 3) (Ghosh et al., 2019). These domains enable efficient DNA binding and maintain structural and functional integrity under the torsional stress of chromatin changes in the nucleus. Characterized by rapid dissociation from binding sites, SATB1 adapts to various loci or form complexes in response to stimuli (Alvarez et al., 2000; Cai et al., 2003; Yasui et al., 2002).
Researchers have identified four alternative promoters (P1, P2, P3, and P4) for the Satb1 gene, with their interchanging usage determining the observed alternate Satb1 transcript expression. The preference for promoter usage in Satb1 gene expression is dynamic, varying with developmental stage and relying on TCR signaling. In CD4+CD8+ double positive thymocytes, P1, P3, and P4 transcript variants are predominant, with P3 being the most integral. Conversely, in CD4+CD8− thymocytes, the P2 transcript variant is highly expressed along with P3, whereas this pattern is absent in CD4−CD8+ thymocytes (Papadogkonas et al., 2022; Patta et al., 2020). Thus, SATB1 expression regulation is context-dependent. Mir et al. recently elucidated the mechanism of SATB1 regulation through Wnt/β-catenin signaling. They showed that TCF7L2 binds to the Satb1 promoter, promoting H3K4 trimethylation, and directly regulating SATB1 expression. Hyperactivation of Wnt signaling induces TCF7L2/β-catenin complex occupancy on the Satb1 promoter, leading to SATB1 induction. Knockdown of TCF7L2 and β-catenin, and their loss of occupancy on the Satb1 promoter, downregulate SATB1 and known downstream targets of Wnt signaling (Mir et al., 2016). In regulatory T cells, SATB1 is negatively regulated by FOXP3 to prevent conventional T cell polarization and maintain stable Treg cell identity (Trujillo-Ochoa et al., 2023). Release of SATB1 repression leads to loss of regulatory T-cell identity and induction of an effector T-cell program (Beyer et al., 2011). Furthermore, SATB1 expression in epidermal basal cells is regulated by p63, which binds to a proximal regulatory region of the Satb1 gene and controls its promoter activity. Ablation of p63 results in reduced SATB1 expression levels and abnormal epidermal morphogenesis (Fessing et al., 2011). Additionally, SATB1 is regulated by various microRNAs, including miR-191, miR-155, miR-448, miR-7, miR-302a-3p, and miR-21-5p (Coppé et al., 2006; Demaria et al., 2017; Faget et al., 2019; Guccini et al., 2021; Krtolica, 2001; Morton, 2010). These findings indicate that the regulation of SATB1 expression involves multiple signaling pathways and transcription factors and varies in different cell type.
SATB1 has been shown to associate with ACF, ISWI, and HDAC1, leading to deacetylation at specific binding sites (Galande et al., 2007). Studies performed on the conformation and structure of SATB1 showed that it is phosphorylated by protein kinase C (PKC), which binds to its PDZ-like domain. This phosphorylation determines SATB1's interaction with specific protein complexes (HDAC1 or PCAF) (Pavan Kumar et al., 2006). Specifically, the phosphorylated SATB1–HDAC1 interaction leads to gene repression in resting cells, while in activated cells, the dephosphorylated SATB1–PCAF interaction results in the de-repression of target genes. Additionally, phosphorylation regulates SATB1's SUMOylation, which in turn promotes its cleavage by caspase 6 in PML bodies (Galande et al., 2001; Tan et al., 2008, 2010). SATB1 also interacts with other remodeling complexes like NURD, serving as a “docking platform” (Figure 3) (Frömberg et al., 2018; Pavan Kumar et al., 2006; Stephen et al., 2017; Yasui et al., 2002). SATB1 is also deacetylated by HDAC5 at lysine 411, repressing SDHA-induced epigenetic remodeling and antiproliferative transcriptional programs (Sharma et al., 2023). More recently, studies have shown that SATB1 uses its prion-like domains to transition between liquid-like states and aggregated structures. This behavior is influenced by protein concentration, phosphorylation, and interactions with nuclear RNA (Zelenka et al., 2023).
Although not universally expressed, SATB1's importance in various yet under-recognized tissues is increasingly acknowledged. Initially known for binding to AT-rich genome regions, SATB1 associates with diverse genomic areas, each influencing gene expression in different cell types and processes (Yamasaki & Yamasaki, 2016). Its regulatory scope extends to epidermal differentiation (Fessing et al., 2011), cancer metastasis (Naik & Galande, 2019), thymocyte development, Th2 cell activation and cytokine production, brain and pituitary development (Balamotis et al., 2012; Skowronska-Krawczyk et al., 2014), X-chromosome inactivation (Agrelo et al., 2009), and embryonic stem cell differentiation (Ghosh et al., 2019; He et al., 2015; Zhang, 2019a). Recently, SATB1 has been linked to increased lifespan, with reductions in its expression observed with age and in age-related pathologies in mice (Zhang et al., 2009). This suggests that SATB1 has a broad role in combating senescence and aging pathways.
4 MECHANISMS OF SATB1 IN SENESCENCE AND SENESCENCE-RELATED DISEASES
4.1 SATB1 and senescence
Senescent cells exhibit strong expression of the p16INK4a and p53/p21 pathways, leading to irreversible cell cycle arrest. Accompanying this arrest is a distinct SASP, which generates pro-inflammatory cytokines, chemokines, growth factors, and metalloproteinases involved in inflammatory processes (Acosta et al., 2008; Hayflick & Moorhead, 1961; Kuilman et al., 2008; Wissler Gerdes et al., 2020; d'Adda di Fagagna, 2008). The SASP has been implicated in promoting aging and the progression of age-related diseases, including neurodegenerative, cardiovascular, metabolic, and immune system disorders. While acute senescence contributes to normal biological processes such as embryonic development and tissue repair, its induction in damaged cells serves as a crucial barrier to carcinogenesis (Lu et al., 2023). Persistent senescent cells, whether tumor or nontumor, can fuel tumor recurrence through SASP (Coppé et al., 2010; Demaria et al., 2017; Lee & Schmitt, 2019). Moreover, some evidence suggests that tumor cells may evade or suppress cellular senescence induction, enabling them to re-enter the cell cycle (Beausejour, 2003; Fujita et al., 2009; Milanovic et al., 2018).
Studies have demonstrated that SATB1 plays critical roles in cellular senescence. SATB1 knockdown delays DNA repair post H2O2 exposure, leading to an increase in OGG1-sensitive oxidized bases within genomic DNA and a decrease in 8-oxoG cleavage activity. Conversely, ectopic expression of SATB1 accelerates DNA repair, reducing levels of oxidized bases in genomic DNA. SATB1 directly interacts with OGG1, enhancing its binding to 8-oxoG-containing DNA and promoting base excision repair, thus inhibiting H2O2-induced cell senescence (Kaur et al., 2016). Genetic deletion of SATB1 in human embryonic-stem-cell-derived dopaminergic neurons induces a senescence phenotype by repressing CDKN1A (encoding p21), triggering senescence and the SASP (Riessland et al., 2019). Additionally, reduced SATB1 expression leads to de-repression of miR-22-3p, decreasing GBA expression and resulting in GluCer accumulation, which induces cellular senescence dependent on S100A9 and stress factors (Russo, 2024). Further studies have revealed that miR-191 binds directly to the 3′ UTRs of SATB1, reducing its expression and inducing cell cycle arrest in the G1 phase, leading to replicative senescence in keratinocytes (Lena et al., 2012). Similarly, miR-21 negatively regulates SATB1 by binding to its 3′ UTRs in keratinocytes, contributing to the onset of senescence and various skin diseases (Ahmed et al., 2019). SATB1 expression in epidermal basal cells is regulated by p63. Ablation of p63 results in reduced SATB1 expression levels, abnormal epidermal morphogenesis and cell senescence (Fessing et al., 2011). Recent findings indicate that SATB1 also plays a crucial role in preventing premature T cell exhaustion by repressing programmed cell death protein 1 (Pdcd1) expression, which encodes the inhibitory receptor PD-1 (Nüssing et al., 2019; Stephen et al., 2017). Collectively, SATB1 is assumed to play an important role in nontransformed cells in maintaining proliferation and is a negative regulator in cellular senescence.
On the contrary, Agrelo et al. discovered that SATB1 expression in mouse embryonic fibroblasts induces cell cycle arrest and senescence, accompanied by elevated p16 protein levels. This result suggests that SATB1 could induce cellular senescence. Deletion of p16 overcomes SATB1-induced senescence. Moreover, in the absence of p16, SATB1 promotes anchorage-independent growth and an invasive phenotype in fibroblasts by interacting with the retinoblastoma (RB)/E2F pathway downstream of p16 (Agrelo et al., 2013). Furthermore, increasing evidence showed that SATB1 is highly expressed in many human tumors, including breast cancer (Han et al., 2008), rectal cancer, and cutaneous malignant melanoma (Chen et al., 2011; Cheng et al., 2010; Lu et al., 2010; Meng et al., 2012; Zhao et al., 2010). These suggest that SATB1 is a promoter for cell proliferation in progenitor and tumors leading to a highly metastatic phenotype. However, whether SATB1's oncogenic role is related to its pivotal function in cellular senescence remains unclear. This suggests that there is a dynamic balance between SATB1 expression levels in regulating normal development and cancer metastasis. A comprehensive understanding of the intricate mechanisms governed by SATB1 is warranted. Here, we focus on recent findings regarding the mechanisms of SATB1 in senescence. We spotlight SATB1's significant roles in various human aging-related diseases and discuss promising interventions and treatments.
4.2 SATB1 in neurodegeneration
Senescence has been identified in different cell types of the nervous system, including neural stem cells, neurons, astrocytes, oligodendrocytes, and microglia (Al-Mashhadi et al., 2015; Chinta et al., 2018; Flanary & Streit, 2004; Jurk et al., 2012; Kujuro et al., 2010; Salminen et al., 2011; Sedelnikova et al., 2004). Neurodegenerative diseases, including Alzheimer's, Parkinson's, and multiple sclerosis, are increasingly associated with cellular senescence (Hoehn & Yahr, 1967; Martin-Ruiz et al., 2020; Oost et al., 2018; Zhang, 2019b). The prevalence of Alzheimer's and Parkinson's diseases, which are characterized by cognitive decline and neuron loss, rises with age (Rocca et al., 2011). Cellular senescence is hypothesized to play a role in either initiating or exacerbating these neurodegenerative diseases, potentially by promoting chronic inflammation, diminishing regenerative capabilities, and exacerbating the age-related decline in the blood-brain barrier and the microvasculature (Han et al., 2020).
Recent studies have increasingly highlighted the potential role of SATB1 in neuronal development within the brain. SATB1 is notably expressed in various brain regions, including the neocortex, the nucleus of the diagonal bundle, the amygdala, the hippocampus, and the spinal cord. Interestingly, SATB1-positive neurons are sparsely present in the substantia nigra of the midbrain, predominantly among dopaminergic neurons (Huang et al., 2011; Turovsky et al., 2021, 2023). Variations in SATB1 expression levels have also been observed across different neuronal subtypes (Denaxa et al., 2012; Fogarty et al., 2007). Additionally, SATB1 has been identified as a key facilitator of neuronal plasticity in cortical neurons, with its absence leading to hindered neuronal maturation (Balamotis et al., 2012; Turovsky et al., 2021). These findings indicate that SATB1 is a critical regulator in neurons. However, as for its various expression in the brain, the potential role of SATB1 in neurodevelopment and neurodegenerative diseases is little known.
4.2.1 Alzheimer's disease (AD)
AD is a prevalent age-related neurodegenerative disorder affecting approximately 30 million people worldwide, with no effective treatments currently available (Hou et al., 2019; Saez-Atienzar & Masliah, 2020; Winblad et al., 2016). Recent research has established a close connection between cellular senescence and AD (Baker et al., 2011; Baker et al., 2016; Childs et al., 2015; Gerenu et al., 2017; Schafer et al., 2017; Sturmlechner et al., 2017; Tacutu et al., 2011; Xu et al., 2018). In both AD patient brains and mouse models, senescent cells like astrocytes, microglia, endothelial cells, and neurons have been identified with high levels of senescence markers such as p16, p53, p21, and SA-β-gal (Baker & Petersen, 2018; Bhat et al., 2012; Gaikwad et al., 2021; Garwood et al., 2014; Han et al., 2020; Holtzman & Ulrich, 2019; Hu et al., 2021; Musi et al., 2018; Naderi et al., 2006; Saez-Atienzar & Masliah, 2020; Streit et al., 2009; Turnquist et al., 2016; Zhang, 2019b). Studies have shown that removing these senescent cells in AD mouse models reduces the neuropathology associated with β-amyloid (Aβ) peptides and tau proteins (Bussian et al., 2018; Liu, 2022; Musi et al., 2018; Wei et al., 2016; Zhang, 2019b).
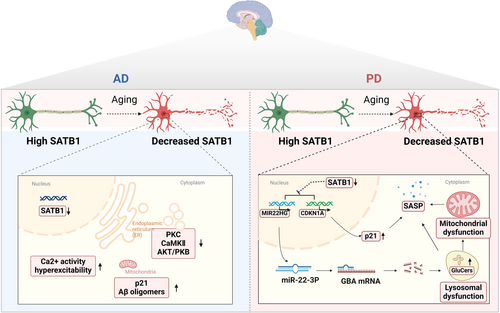
Rossi et al. found that the expression of SATB1 decreases with age (Rossi, 2005). In addition, inhibiting the C. elegans ortholog of SATB1, dve-1, reduces lifespan extension via bacterial dilution dietary restriction (bDR). Furthermore, in a transgenic Aβ42 model of AD, RNA interference targeting dve-1 counteracted the protective effects of bDR and accelerated Aβ42-related pathology (Zhang et al., 2009). Neuronal hyperexcitability is a significant symptom in AD. Research involving SATB1 mutant mice demonstrated that SATB1 deletion in neocortical projection neurons leads to the downregulation of protein kinases like PKC, CaMKII, and AKT/PKB. Partial deletion of SATB1 does not dramatically disrupt kinome and Ca2+ signaling, but full deletion results in increased spontaneous Ca2+ activity and hyperexcitability, particularly during simulated epileptiform activities (Figure 4). Additionally, the absence of SATB1 disrupts preconditioning mechanisms in neurons under hypoxic conditions (Turovsky et al., 2023). Thus, SATB1 might maintain the normal neuronal function and the decreased SATB1 expression with age is a vital contributor to AD development.
4.2.2 Parkinson's disease (PD)
PD ranks as the second most prevalent neurodegenerative disease, following AD. It impacts an estimated 7–10 million individuals globally, predominantly those over 65 years of age. PD is a chronic and progressively worsening neurodegenerative disorder. It presents as dyskinesia, including impaired movement range and speed, stiffness, or resting tremors, primarily due to a deficit in striatal dopamine. PD's hallmark feature is the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta of the midbrain, which exhibit a substantial accumulation of α-synuclein (α-syn) (Zhong et al., 2022). Around 20 genetic mutations are linked to PD, including missense mutations in SNCA (α-syn), PARK7, and LRRK2 as well as missense or loss-of-function mutations in PINK1, PRKN, PLOG, and GBA (Blauwendraat et al., 2020; Goedert et al., 2013; Polymeropoulos, 2019). Research has shown increased levels of senescence markers like p16 and p21 and inflammatory markers such as IL-6 in PD patients compared to healthy individuals, and these markers are correlated with a faster cognitive decline (Martin-Ruiz et al., 2020).
SATB1 has recently been identified as a risk factor for PD (Chang et al., 2017; Zhang, 2023). A decrease in SATB1 activity has been noted in the brain regions most vulnerable to PD (Brichta et al., 2015). Studies suggest that SATB1 protects dopaminergic neurons from senescence in vivo (Figure 4) (Riessland et al., 2019). Genetic deletion of SATB1 in human embryonic-stem-cell-derived dopaminergic neurons induces a senescence phenotype, with SATB1 directly repressing p21 expression. Blocking p21 in SATB1 knockout cells reduces this senescence effect (Riessland et al., 2019). SATB1 represses CDKN1A (encoding p21) in these neurons, triggering senescence and the SASP. Elevated p21 levels are found in the substantia nigra's dopaminergic neurons and neural stem cells in the Parkin-deficient (Prkn−/−) PD mouse model (Park et al., 2017).
Further research revealed that reduced SATB1 expression leads to the de-repression of miR-22-3p microRNA, decreasing GBA expression and resulting in GluCer accumulation. This accumulation impairs lysosomal and mitochondrial function, inducing cellular senescence dependent on S100A9 and stress factors. Such dysregulation in the SATB1-MIR22-GBA pathway, observed in PD patients and under normal aging, culminates in lysosomal and mitochondrial dysfunction, promoting a senescence-like phenotype in dopaminergic neurons (Russo, 2024). These findings highlight the crucial role of SATB1 in cellular senescence during the development of PD and AD. While future research will be essential to unraveling the detailed mechanisms by which SATB1 contributes to neurodegenerative diseases in humans, the development of drugs specifically targeting SATB1 could herald a new direction in the treatment of these ailments.
4.3 SATB1 in skin senescence
During the aging process, skin cells exhibit distinct aging characteristics, such as dry skin (xerosis), a loss of elasticity, and functional senescence. These features are closely linked to the slowed proliferation and functional decline of epithelial and mesenchymal cells (Engelke et al., 1997; Zhang et al., 2009). The epidermis, a perpetually renewing epithelial layer, requires precise control over keratinocyte proliferation to orchestrate the differentiation stages in its upper strata. p63, a pivotal transcription factor, plays a key role in regulating basal keratinocyte turnover during development and in adulthood (Candi et al., 2006). Depletion of p63 leads to cellular senescence in actively proliferating keratinocytes (Rivetti di Val Cervo, 2012; Senoo et al., 2007; Shalom-Feuerstein et al., 2011) and precipitates accelerated aging in p63-heterozygous mice in vivo (Keyes et al., 2005; Paris et al., 2012). Intriguingly, the △Np63α variant of p63, lacking the N-terminal transactivation domain, has been found to counteract keratinocyte replicative senescence by inhibiting senescence-associated microRNAs (Rivetti di Val Cervo, 2012).
SATB1, expressed in basal epidermal keratinocytes, has been recognized as a new target gene of p63. p63 binds to a proximal regulatory region of the SATB1 gene, and p63 ablation results in a marked reduction in the SATB1 expression levels in the epidermis. SATB1 facilitates p63-driven cell differentiation, particularly by structuring the EDC (epidermal differentiation complex) locus. SATB1(−/−) mice show impaired epidermal morphology and cellular senescence in keratinocytes (Fessing et al., 2011). Further studies have shown that miR-191 binds directly to the 3′ UTRs of SATB1, reducing its expression and consequently inducing cell cycle arrest in the G1 phase, leading to replicative senescence in keratinocytes (Lena et al., 2012). Additionally, miR-21 negatively regulates SATB1 by binding to its 3′ UTRs in keratinocytes. The downregulation of SATB1, mediated by miR-21, contributes to the onset of senescence and various skin diseases (Ahmed et al., 2019).
These findings suggest a profound connection between SATB1 and the processes of proliferation and senescence in keratinocytes. This link sheds new light on the intricate role of SATB1 in skin aging and related pathologies, providing a foundation for developing strategies to counteract the impacts of aging on skin.
4.4 SATB1 in immunosenescence
Immunosenescence, characterized by inflammaging, is a significant aspect of aging. It includes the deterioration and remodeling of immune organ structures and a decline in innate and adaptive immune functions. Key changes observed in immunosenescence encompass thymic involution, hematopoietic stem cells (HSCs) dysfunction, altered ratios of naïve to memory T and B cells, an increase in senescent cells, diminished responses to new antigens, mitochondrial dysfunction, and genomic instability (Lanna et al., 2017; Nikolich-Žugich, 2018; Ucar et al., 2017). Chronic antigen stimulation and changes in T-cell pools can lead to early senescence in immune cells, which develop a pro-inflammatory secretory phenotype that intensifies inflammaging. Immunosenescence results in less effective vaccination outcomes, increased vulnerability to infections, a higher prevalence of age-related diseases, and an elevated risk of malignancies (Kennedy et al., 2014; McElhaney & Effros, 2009; Pawelec, 2007; Thomas et al., 2020).
The senescence of HSCs forms the foundation of immunosenescence. Aged HSCs differentiate into dysfunctional immune cells, contributing to immunosenescence (Liggett & Sankaran, 2020). Senescent HSCs exhibit reduced self-renewal, altered differentiation tendencies, and metabolic changes. They shift from anaerobic glycolysis to oxidative respiration under inflammatory conditions (Mistry et al., 2019, 2021). The accumulation of ROS can induce extensive DNA damage, leading to HSC senescence or even apoptosis (Shao et al., 2011). Inflammation negatively impacts the self-renewal ability of HSCs, accelerating their aging. Studies in mice show that early to mid-life exposure to inflammatory stimuli leads to peripheral blood hemocytopenia, bone marrow cytopenia, and increased BM adipocytes, mirroring typical aging-related hematopoiesis changes (Bogeska et al., 2022). SATB1, identified in microarray analyses, is significantly downregulated in aged HSCs (Chambers et al., 2007; Rossi, 2005). There is an approximately 80% reduction in SATB1 transcripts in aged HSCs compared to those from younger mice, indicating that SATB1 might be a crucial molecule in the context of HSC senescence. SATB1 expression is lower in aged HSCs with reduced lymphopoietic potential, and its forced expression partially restores this capacity. Overexpression of SATB1 also confers growth advantages to hematopoietic progenitors and decreases the expression of Bcl2 without compromising their viability, indicating SATB1 has an essential role in lymphoid lineage replenishment and a link to HSC senescence (Figure 5) (Satoh et al., 2013). The specific mechanisms of SATB1's role in HSC senescence are not fully understood, but its function in this process is clinically significant.
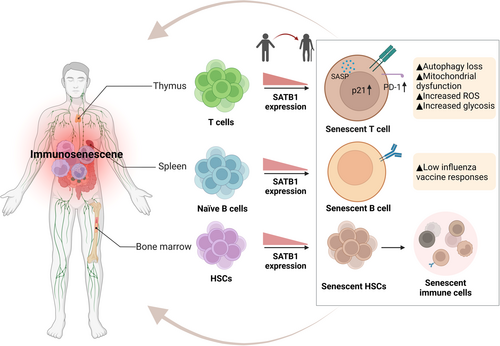
Age-related changes in B-cell composition significantly impact the antibody responses to vaccines and infections in older adults (Li et al., 2023). The aging process is linked to an increase in late-stage exhausted memory B cells (Colonna-Romano et al., 2009) and a notable decrease in memory B cells that correlate with influenza vaccine responses (Frasca et al., 2012; Qin et al., 2016). Research has revealed that SATB1 expression is initially low during B-cell differentiation in the bone marrow but significantly increases in naïve B cells in the spleen (Ozawa et al., 2022). Based on varying levels of SATB1 expression, splenic naïve B cells can be categorized into two types: SATB1high and SATB1−/low. SATB1high naïve B cells exhibit less susceptibility to death and a higher proliferative capacity than SATB1−/low cells when incubated with an anti-IgM antibody (Ozawa et al., 2022). This indicates that SATB1 is crucial for the survival and function of naïve B cells. Future research is needed to understand the molecular mechanisms through which SATB1 influences B-cell senescence.
T-cell senescence significantly impacts the immune function in aging populations and cancer patients. It involves a decline in both innate and adaptive immunity, primarily through the depletion of naïve and effector T cells with age (Chou et al., 2013). T-cell senescence is driven by two main mechanisms: replicative senescence, related to age and telomere shortening after many cell divisions, and premature senescence, which is telomere-independent and caused by external factors like cellular stress (Campisi, 1997; Dock & Effros, 2011). In replicative senescence, T cells typically lose co-stimulatory molecules like CD27 and CD28 while expressing markers such as KLRG-1 and CD57. Proteins regulating the G1 phase of the cell cycle, including p15, p16, and p21, are upregulated in replicative senescence (Erickson et al., 1998; Lanna et al., 2013; Liu et al., 2009, 2018; Qian & Chen, 2010; Xu & Larbi, 2017). In individuals over 65 years of age, there is an observable shift towards senescence and an increase in highly differentiated CD28− T cells (Mou et al., 2014). Additionally, accumulations of senescent CD8+CD28− T cells have been noted in several solid tumors, suggesting they have a suppressive role in immune evasion strategies (Shang et al., 2015; Zhang, 2015a, 2015b). Senescent T cells in patients can adversely impact the efficacy of T-cell-based immunotherapies. Finding therapeutic strategies to reverse T-cell senescence and restore T-cell homeostasis holds great promise for delaying aging, improving age-related diseases, and enhancing cancer treatments.
SATB1 is predominantly expressed in T cells. It orchestrates transcriptional programs essential for different T-cell subsets like CD4+ T cells, CD8+ T cells, Foxp3+ regulatory T cells, and T follicular helper cells (Alvarez et al., 2000; Cai et al., 2003; Chaurio et al., 2022; Kakugawa et al., 2017; Kitagawa et al., 2017; Kondo et al., 2016; Yasui et al., 2002). Disruption of Satb1 in mice leads to a critical impasse in T-cell development, particularly in the CD4+ CD8+ double-positive stage. This interruption leads to significant reductions in both CD4+ and CD8+ single-positive thymocytes and results in early lethality (Feng et al., 2022; Zelenka, 2022). Recent studies show that SATB1 also plays a key role in preventing the premature exhaustion of T cells by regulating programmed cell death protein 1 (Pdcd1) expression, which encodes the inhibitory receptor PD-1. In T cells lacking SATB1, PD-1 expression increases 40-fold, impairing their effector response to tumor cells (Nüssing et al., 2019; Stephen et al., 2017). Senescent T cells with elevated PD-1 levels disrupt the phosphorylation of zeta-chain-associated protein kinase 70 (ZAP70), initiated by lymphocyte-specific protein tyrosine kinase (LCK), directly counteracting TCR signals. This leads to the activation of the P38 pathway and the inhibition of PI3K-AKT-mTOR signaling, resulting in autophagy loss (Sheppard et al., 2004). Autophagy failure causes an accumulation of T cells with the SASP, triggering mitochondrial dysfunction, myeloid-derived suppressor cell production, and increased ROS levels and intensifying inflammaging (Figure 5) (Henson et al., 2014; Salminen et al., 2018).
The number of human effector memory (EM) CD8+ T cells increases with age, primarily comprising two distinct types: IL-7Rαhigh EM CD8+ T cells and IL-7Rαlow EM CD8+ T cells. IL-7Rαlow EM CD8+ T cells are highly cytotoxic and pro-inflammatory, and they show signs of replicative senescence with impaired T-cell-receptor-mediated proliferation and elevated senescence markers like CD57. Indeed, older adults have increased numbers of IL-7Rαlow cells compared to young adults (Kim et al., 2006, 2007; Shin et al., 2015). SATB1 expression is lower in human IL-7Rαlow EM CD8+ T cells than in IL-7Rαhigh EM CD8+ T cells. This reduced expression of SATB1 is associated with increased cytotoxicity, inflammatory responses, and cellular senescence. Additionally, SATB1 expression decreases with age in human peripheral blood mononuclear cells (Park et al., 2019), consistent with the reduced SATB1 levels in whole blood and CD8+ T cells in older individuals (Peters et al., 2015; Tserel et al., 2015). The diminished expression of the SATB1 gene in IL-7Rαlow EM CD8+ T cells may be linked to decreased chromatin accessibility and heightened DNA methylation. These mechanisms are thought to regulate SATB1 expression in CD8+ T cells, particularly in the context of aging (Ucar et al., 2017).
These findings highlight SATB1's critical role in maintaining T-cell functionality and the broad implications of its dysregulation in immune responses. Elucidating SATB1's mechanisms in T-cell senescence could lead to effective interventions for aging- and cancer-related immune dysfunctions.
4.5 SATB1 in cancer
Physiologically, elevated SATB1 levels are observed in embryonic stem cells and numerous adult progenitor cells (Alvarez et al., 2000; Kohwi-Shigematsu et al., 2012; Savarese et al., 2009). SATB1 plays crucial roles in embryonic development, thymocyte maturation, skin epithelial cell differentiation, and other processes requiring rapid changes in cell phenotype (Kohwi-Shigematsu et al., 2012). Its expression is indispensable for T-cell maturation and proper lung development during embryogenesis (Alvarez et al., 2000; Baguma-Nibasheka et al., 2007). However, SATB1 is overexpressed in various malignancies, including lymphomas, breast, colorectal, prostate, liver, bladder, ovarian cancers, osteosarcoma, and glioma (Chu et al., 2012; Frömberg et al., 2014; Han et al., 2008; Mao et al., 2013; Nodin et al., 2012; Nüssing et al., 2019; Tu, 2012; Zhang, 2013a). It has been implicated in promoting the epithelial-mesenchymal transition (EMT) process and cancer metastasis (Lv et al., 2016; Qi et al., 2017; Tu, 2012; Xiang et al., 2012). Elevated SATB1 expression, facilitated by miR-448 suppression, initiates signaling pathways leading to EMT and tumor aggressiveness (Li et al., 2011). Notably, induction of SATB1 expression is sufficient to transform noninvasive cells into aggressive, tumorigenic ones (Han et al., 2008). SATB1 knockdown in aggressive cancer cells restores normal morphology and reduces migration and invasion abilities (Han et al., 2008; Huang et al., 2016; Zhang, 2013b). Thus, SATB1 overexpression is associated with worse prognosis in many cancers, including breast (Pan et al., 2016), colorectal (Zhao et al., 2018), gastric (Yuan et al., 2016), pancreatic (Elebro et al., 2014; Guo et al., 2017), ovarian (Nodin et al., 2012; Xiang et al., 2012), endometrial (Zhang, 2015c) and cervical cancers (Wang et al., 2015). Mir et al. recently uncovered the regulatory mechanism of SATB1 via Wnt/β-catenin signaling. Hyperactivation of Wnt signaling prompts the TCF7L2/β-catenin complex to bind to the Satb1 promoter, leading to SATB1 expression induction (Mir et al., 2016). However, in certain subtypes of lung cancer, elevated SATB1 levels are associated with a favorable prognosis, whereas loss of SATB1 expression correlates with shorter overall survival (Glatzel-Plucinska et al., 2018; Selinger et al., 2011). Similarly, in clear cell renal cell carcinoma, elevated SATB1 expression's positive impact is mediated by miR-21-5p regulation (Kowalczyk et al., 2016). This variability underscores SATB1's diverse functions and prognostic significance across different cancer subtypes due to its heterogeneity. Moreover, the role of sumoylation-deficient SATB1 has recently emerged in cellular migration (Rao et al., 2018), suggesting that posttranslational modifications such as phosphorylation, acetylation, and SUMOylation linked to SATB1 may contribute to reprogramming gene expression patterns during tumor metastasis. Therefore, the cell type-specific expression of SATB1, in conjunction with the tumor microenvironment, may serve as primary determinants of tumor progression and inflammation.
Silencing SATB1 expression with siRNA or shRNA has been shown to effectively inhibit cancer cell proliferation and invasion both in vitro and in vivo in mice xenograft models (Frömberg et al., 2014; Han et al., 2008; Mao et al., 2016; Shukla et al., 2013; Wang et al., 2017). These findings suggest that SATB1 could be a promising target for novel anticancer drugs. Baicalein and hydrophobic statins have demonstrated success in downregulating SATB1 levels in breast and colorectal cancer cells, leading to significant reductions in proliferation and invasion abilities (Gao et al., 2015; Lakshminarayana Reddy et al., 2010; Ma et al., 2016). Additionally, co-delivery of SATB1 shRNA and doxorubicin via immunoliposomes has exhibited an antitumor effect in gastric cancer cells (Peng et al., 2014). Therapy utilizing SATB1 shRNA to target gastric cancer stem cells is also actively under development (Yang et al., 2018). Moreover, SATB1 has been identified as a potential immune target for anticancer vaccines (Wang et al., 2013). Given its high prognostic significance across different tumors, it may also serve as a molecular marker for novel diagnostic tests. Furthermore, recent research has demonstrated that increased SATB1 expression upon T cell activation leads to inhibition of PD-1 expression, thus preventing premature T cell exhaustion (Stephen et al., 2017). SATB1 deregulation during aging results in immunosenescence, which is an elevated risk of malignancies. Forced expression of SATB1 might restore the immune protection against tumors.
Cellular senescence can act as a natural defense mechanism against tumorigenesis, making it a potential target in anticancer therapies. Cancer cell senescence is often triggered by prolonged exposure to mitogenic signals, DNA-damaging agents, and other therapeutic compounds, inducing senescence in vulnerable neoplastic cells and surrounding tissue elements. OIS results from the aberrant activation of oncogenes (e.g., Ras, SATB1, BRAF V600E, and c-Myc) or loss of tumor-suppressor genes (e.g., PTEN), temporarily halting tumor progression in a premalignant state (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Michaloglou et al., 2005). These mitogenic factors can overwhelm the growth control mechanisms in nonmalignant cells, activating abnormal signaling pathways and triggering unscheduled DNA replication. The cellular response to this unchecked pro-mitotic activity is a strict arrest of proliferation, highlighting the protective role of senescence in preventing tumor initiation and progression (Braig et al., 2005). Studies using mouse models and human tissue samples have confirmed the tumor-suppressive role of OIS (Braig et al., 2005; Collado et al., 2005; Lazzerini Denchi et al., 2005; Michaloglou et al., 2005; Rane et al., 2002). Senescent cells are often found in premalignant tumors but not in malignant ones (Collado et al., 2005), and they are present in human precancerous lesions, such as colon adenomas (Bartkova et al., 2006; Fujita et al., 2009; Kuilman et al., 2008), benign melanocytic tumors (nevi) (Michaloglou et al., 2005), and neurofibromas (Courtois-Cox et al., 2006). Research indicates that SATB1 expression in mouse embryonic fibroblasts induces cell cycle arrest and senescence, correlated with increased levels of p16 protein. Remarkably, removal of p16 reverses SATB1-induced senescence. In scenarios where p16 is absent, SATB1 promotes unregulated growth and an invasive phenotype in fibroblasts through interaction with the E2F/RB complex, exerting regulatory control over the cyclin E promoter in an E2F-dependent manner. These observations underscore the vital role of p16 in SATB1-triggered cell cycle arrest and indicate that p16 mutations collaborate with the oncogenic activity of SATB1 (Agrelo et al., 2013). Additionally, in certain subtypes of lung cancer and clear cell renal cell carcinoma samples, elevated SATB1 levels were associated with a positive prognosis, while loss of SATB1 expression correlated with shorter overall survival (Glatzel-Plucinska et al., 2018; Kowalczyk et al., 2016; Selinger et al., 2011). In this context, SATB1 is speculated to be involved in tumor-suppressive role by inducing cancer cell senescence, although mechanistic details remain unclear. Further investigation is warranted, particularly regarding the precise mechanism of SATB1 action in premalignant versus malignant tumors.
5 SUMMARY AND PROSPECT
The natural aging process is marked by the gradual accumulation of senescent cells, which varies considerably. These cells contribute to a range of age-related diseases in older organisms (Schmitt et al., 2022). Studies have shown that clearing senescent cells can enhance both the lifespan and the healthspan in mice (Amor et al., 2020; Baker et al., 2016; Xu et al., 2018), but certain senescent cells are crucial for organ integrity, and their forced elimination can lead to tissue damage and organ dysfunction (Grosse et al., 2020). The SASP, an extrinsic mechanism of senescence, can paradoxically foster tumor growth through factors that promote inflammation, stemness, and genotoxicity (Acosta et al., 2008; Wang et al., 2020). Considering the complex external effects that senescent cells exert on their environment, coupled with intrinsic cellular changes that drastically alter functionality and may lead to stem-like reprogramming, the implications of senescence are multifaceted and complex. In summary, senescent cells display diverse phenotypes capable of exhibiting both antitumor and tumor-promoting properties. It remains to be determined whether these differing subsets of senescent cells result from unique intrinsic programs or are influenced by their environmental contexts. The challenge lies in discerning the “bright” and “dark” sides of senescence, especially considering that tumor-suppressive and tumor-promoting effects can coexist within the same patient.
The chromatin profile undergoes significant changes during cellular senescence. Gaining a deeper understanding of how to preserve the epigenome and rejuvenate aging chromatin, thus reinstating its youthful structure and function, is crucial for advancing this field. SATB1, a protein with diverse capabilities, constructs a prospective nuclear scaffold, enlists chromatin-modifying enzymes, orchestrates long-range chromatin interactions, and significantly influences gene expression regulation across multiple levels. Various studies have consistently highlighted SATB1 as a key factor in different types of cellular senescence, underscoring its importance in cellular senescence processes. Several studies showed that SATB1 might be a target for antiaging therapy. Aging in HSCs is often marked by genetic alterations, including a decrease in SATB1 expression. Strategies aimed at preventing HSC dysfunction may involve genetic modulation, such as ablating genes associated with aging or overexpressing rejuvenative genes. Research has shown that forced expression of SATB1 restores the function of aging HSCs, leading to immune rejuvenation and improved organismal health (Li et al., 2020). In senescent cells (SCs), inhibiting the PD-1 could activate CD8+ T cells and improve senescence surveillance, further resulting in the reduced number of p16+ cells (Wang, 2022b). SATB1 has been shown to inhibit PD-1 expression, and its decrease leads to PD-1 upregulation (Stephen et al., 2017), suggesting that upregulating SATB1 expression could effectively decrease T cell senescence. Accumulating evidence suggests that cellular senescence contributes AD pathophysiology through the accumulation of β-amyloid (Aβ) (Liu, 2022). Treatment with senolytic drugs Dasatinib and Quercetin has been shown to reduce Aβ-related oligodendrocyte senescence, improve cognitive impairments, and decrease Aβ load and neuroinflammation in AD mice (Zhang, 2019b). Decreased SATB1 expression in SCs contributes to Aβ upregulation, suggesting that restoring SATB1 expression might alleviate AD symptoms by downregulating Aβ. However, considering that SATB1 overexpression could also promote cancer development, precise control of SATB1 expression in these therapies is crucial.
As there are multiple variants of SATB1, each likely possessing distinct molecular functions and operating within complex cellular contexts, further investigation is necessary to elucidate the specific mechanisms by which SATB1 contributes to various cellular senescence processes and tumors. To comprehensively understand its role, reliable tools for quantification and analysis, appropriate disease models, and novel therapeutic agents capable of targeting SATB1 in a tissue-specific manner are indispensable. Given the considerable therapeutic potential of targeting senescent cells, uncovering these mechanisms will illuminate a path towards addressing severe pathological conditions such as neurodegenerative diseases, immune dysfunctions, and tumors, where SATB1 may serve as a promising therapeutic target.
AUTHOR CONTRIBUTIONS
Lihui Zhang had the idea for the article; Wenjing Qi wrote the original draft. Jinping Bai drew the figures; Xianlu Zeng and Ruoxi Wang discussed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This work was funded by the Science and Technology Development Plan Project of Jilin Province (YDZJ202201ZYTS661). Images were created with BioRender.com.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




