Homologous chromosome pairing: The linchpin of accurate segregation in meiosis
Abstract
Meiosis is a specialized cell division that occurs in sexually reproducing organisms, generating haploid gametes containing half the chromosome number through two rounds of cell division. Homologous chromosomes pair and prepare for their proper segregation in subsequent divisions. How homologous chromosomes recognize each other and achieve pairing is an important question. Early studies showed that in most organisms, homologous pairing relies on homologous recombination. However, pairing mechanisms differ across species. Evidence indicates that chromosomes are dynamic and move during early meiotic stages, facilitating pairing. Recent studies in various model organisms suggest conserved mechanisms and key regulators of homologous chromosome pairing. This review summarizes these findings and compare similarities and differences in homologous chromosome pairing mechanisms across species.
1 MEIOSIS OVERVIEW
Meiosis involves the replication of genetic material and two successive rounds of cell division to generate haploid gametes, that is, sperms and eggs (Jordan, 2006; Richardson & Lehmann, 2010; Zickler & Kleckner, 2015). Before entering meiosis, genomic DNA is replicated and proteins required for meiosis are synthesized during the premeiotic interphase. Meiosis consists of two cell divisions - Meiosis I and Meiosis II (Figure 1a), and each division can be further divided into four phases: prophase, metaphase, anaphase, and telophase. Homologous chromosomes undergo segregation during Meiosis I, and sister chromatids separate in Meiosis II. Ultimately, each daughter cell inherits a haploid set of chromatids (Loidl, 2016). The faithful segregation of homologs in Meiosis I relies on the initial pairing of homologous chromosomes, which is stabilized by the formation of the synaptonemal complex (SC) (Fraune et al., 2012; Page & Hawley, 2004; Yang & Wang, 2009). The SC is a highly conserved meiosis-specific structure that links homolog axes along their lengths (Zickler & Kleckner, 2015). Recombination is initiated by the programmed formation of double-strand break (DSB) and employs the homologous chromosome as a template for repair. This process results in the reciprocal exchange of genetic material between maternal and paternal chromosomes (Bhalla & Dernburg, 2008; Nagaoka et al., 2012). These crossovers (COs) establish physical linkages known as chiasmata that keep homologs together after SC disassembly (Page & Hawley, 2003). Paired homologs then orient towards opposite poles within the spindle apparatus.
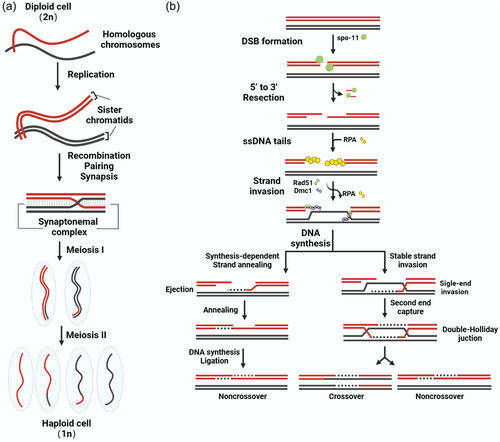
Among the two successive divisions in meiosis, prophase I marks the longest and most complex stage, accompanied by dramatic chromosomal events (Gao & Colaiácovo, 2018). The faithful progression of meiotic prophase I is essential for the entire meiotic program (Zickler & Kleckner, 2015). During prophase I, chromosomes undergo a series of distinctive modifications. These include the pairing of homologous chromosomes and a process known as homologous recombination (HR). HR enables the exchange of genetic material between homologs, ensuring their accurate segregation later, thus preserving the diploid state. Based on morphological transformations, prophase I can be further divided into substages - leptotene, zygotene, pachytene, diplotene and diakinesis. In leptotene, chromatin condenses into elongated, fiber-like structures. Zygotene involves pairing and synapsis between homologs. In pachytene, chromosomes further condense and undergo partial recombination between different DNA segments. During diplotene, homologs remain connected by chiasmata while desynapsis takes place. In diakinesis, chromosomes extensively condense into short rod shapes, the nuclear envelope disintegrates, and nucleoli disappear, approaching the end of meiotic prophase I (Figure 1a). After prophase I, cells enter metaphase I, where the nuclear membrane breaks down, the spindle forms, and bivalents align at the equator and orient on the metaphase plate. In anaphase I, homologous chromosomes separate and migrate to opposite poles, with each chromosome consisting of two sister chromatids. This separation results in a halving of the chromosome number. Conversely, during Meiosis II, sister chromatids separate, and the chromosome numbers remain unchanged before and after division, mirroring the process in mitosis (Figure 1a).
The “two-stage” meiotic progression is not confined to a specific order where homologous chromosomes separate in Meiosis I followed by the separation of sister chromatids in meiosis II or vice versa, commonly referred to as “inverted meiosis” or “reverse meiosis.” While it is indeed common for homologous chromosomes to separate first in monocentric organisms, it's important to recognize that many holocentric organisms, particularly in the realm of plants and insects, complete meiosis in an “inverted” or “reversed” fashion. In-depth analyzes of meiotic processes in plants, such as Luzula elegans and various Rhynchospora species, including R. pubera and R. tenuis, provide insights into this phenomenon (Cabral et al., 2014; Heckmann et al., 2014). During Meiosis I, sister chromatids exhibit a bi-oriented configuration, attaching to spindle fibers before subsequently parting ways. Remarkably, homologous non-sister chromatids remain interconnected by chromatin threads enriched with satellite DNA well beyond metaphase I, separating only during metaphase II. This results in a distinct sequence of meiotic sister chromatid segregation (Cabral et al., 2014; Heckmann et al., 2014). Significantly, it's essential to note that even in mammals, including humans, a certain proportion of gametogenesis often displays an “inverted” pattern (Ottolini et al., 2015). These variations can have implications for gamete formation and genetic diversity, underscoring the intricate nature of meiosis, replete with diverse regulatory mechanisms capable of deviating from the classical sequence, ultimately contributing to genetic diversity within populations.
Meiosis is an extremely precise process where any errors can lead to aberrant chromosome segregation, resulting in numerical abnormalities of single or multiple chromosomes in gametes and the generation of aneuploid reproductive cells (Bloomer & Bonkovsky, 1989). Some women of optimal reproductive age without reproductive pathologies may encounter infertility or recurrent miscarriage, possibly stemming from errors arising at specific stages of meiosis (Hassold et al., 2007). Moreover, studies indicate a significant age-related increase in the incidence of meiotic nondisjunction and resultant chromosomal abnormalities in oocytes, with 20% of oocytes from 35-year-old women being aneuploid, rising to nearly 60% in women over 43 years old (Kuliev et al., 2011). This underscores the paramount importance of gamete genomic integrity, with a normal chromosome number, for a successful pregnancy and proper development of the offspring. Therefore, elucidating the intricate mechanisms ensuring accurate chromosome segregation and genomic fidelity during meiosis holds great significance for human reproductive health.
2 MOLECULAR MECHANISM OF HOMOLOGOUS PAIRING
Homologous pairing is an essential prerequisite for accurate chromosome segregation during meiosis, whereby homologous chromosomes from maternal and paternal genomes gradually approach each other spatially, mutually recognize, and intimately associate (Sybenga, 2020). Homologous pairing underlies pivotal events, including synapsis and crossing over, playing a crucial role throughout meiosis. It is initiated with homolog recognition and subsequently stabilized by SC formation. COs enable paired homologs to remain associated upon SC disassembly, orienting toward opposite poles during meiosis I metaphase. Thus, the initial pairing of homologs is indispensable for their proper segregation in anaphase I (Zickler & Kleckner, 1999, 2015). Although homologous chromosome pairing has been extensively studied, distinctions exist among species and the intricate underlying mechanisms remain incompletely understood. Elucidating the fundamental mechanisms governing homologous chromosome pairing will provide critical insights into meiosis and serve as an important reference for preventing and ameliorating human reproductive disorders. In the following sections, we review homologous pairing mechanisms across diverse meiotic systems (Table 1).
| Species | S. cerevisiae | S. Pombe | D. melanogaster | C. elegans | M. Musculus |
|---|---|---|---|---|---|
| Chromosome number (2n) | 32 | 6 | 8 | 12 | 40 |
| Meiotic DSB dependent | Yes | No | No | No | Yes |
| Chromosome movement stage | Prophase | Prophase | 8-cell cyst | Leptotene-zygotene | Leptotene-zygotene |
| Type of movement | Coordinated-autonomous | Horsetail movement | Nuclear rotation | Autonomous | Nuclear Rotation Autonomous |
| Type of bouquet | Telomeres | Telomeres | Centromere cluster | Half-moon shape | Telomeres Centromere |
| Movement by | Actin | Microtubules | Microtubules | Microtubules | Microtubules |
| Homologous pairing Primary mediator | DSB repair | Horsetail Movement | ♂: X-Y pairing uses rDNA repeats, SNM and MNM ♀: heterochromatin | Pairing centers interact via ZIM1/2/3 (autosomes) and HIM-8 (X) | DSB repair |
| LINC | Mps3p/Mps2p/Csm4 | Sad1/kms1/kms2 | Klarsicht/klariod | SUN-1/Zyg-12 | SUN1/SUN2/KASH5 |
2.1 The canonical homolog pairing—Recombination-mediated homolog pairing
In most organisms, including budding yeast, Sordaria, fungi, plants, and mammals, homolog pairing depends on recombination, which enables the homologous chromosomes to find each other (Bhalla & Dernburg, 2008; Zickler & Kleckner, 2015). Deficiencies in recombination often result in meiotic arrest or improper chromosome segregation, which severely compromises fertility (Nagaoka et al., 2012; Page & Hawley, 2003). While the major steps in the HR pathway have been best elucidated in the budding yeast Saccharomyces cerevisiae, the conservation of the key proteins involved suggests that this process proceeds in a similar fashion across species (de Massy, 2013; Keeney et al., 2014). Recombination commences with the formation of meiotic DSBs. This process is programmed and induced by the conserved enzymatic activity of the topoisomerase-like protein Spo11, in conjunction with a group of partner subunits (Keeney, 2008; Lam & Keeney, 2015) (Figure 1b). Endonucleolytic (MRX (Mre11, Rad50 and Xrs2) and Sae2 in budding yeast) cleavage releases Spo11-oligonucleotide complexes, freeing the DSB ends so that the 5′ strand termini can be exonucleolytically resected to generate 3′ single-stranded overhangs (Exo1 in budding yeast), which are coated by the single-stranded DNA binding protein RPA (Garcia et al., 2011; Mimitou et al., 2017; Neale et al., 2005; Schiller et al., 2014; Symington, 2016; Yadav & Claeys, 2021). RPA is then displaced by the recombinases Rad51 and Dmc1, which assemble into a nucleoprotein filament. This filament searches for homologous sequences preferentially located on the homologous chromosome, leading to the formation of displacement loop (d-loop) structures (Brown & Bishop, 2015; Hong et al., 2001; San Filippo et al., 2008). Following DNA synthesis based on the homologous sequence as a repair template, the recombination intermediates proceed along one of two major pathways (Allers & Lichten, 2001; Bishop & Zickler, 2004; De Muyt et al., 2012; Ding et al., 2019; Yonkos et al., 2000). One pathway involves stable single-end invasion intermediates, followed by capture of the second end and formation of double Holliday junctions, which connect bivalents into COs (Gray & Cohen, 2016; Pyatnitskaya et al., 2019; Schwacha & Kleckner, 1995). This process ultimately yields recombinant DNA products (Figure 1b). The other pathway involves the invading strand being displaced from the donor by helicase action, providing an opportunity for the DNA ends to re-anneal. This process, known as synthesis-dependent strand annealing, generates noncrossover products (NCOs). Unlike crossover products involving the exchange of chromosomal fragments, NCOs are not formed through fragment exchange (Martini et al., 2011; Palmer, 2003). Rather, NCOs represent the local transfer of genetic information from the intact repair template strand to the broken DNA molecule. Specifically, sequence information is copied locally from the template sister chromatid containing the correct sequence into the break via polymerase-driven DNA synthesis, thereby achieving local repair of the damage. Repair of any given DSB can result in either the reciprocal exchange of the flanking chromosome arms (CO) or no exchange of the flanking arms (NCO). COs help link homologous chromosomes together on the metaphase I spindle during meiosis (Gray & Cohen, 2016). However, all recombination events between homologs (including those leading to NCOs) promote homolog pairing in organisms that rely on recombination to complete this process (Keeney et al., 2014).
In all the organisms studied to date, DSBs are catalyzed by Spo11. The Spo11 protein itself is not strictly required. In Sordaria, radiation-induced DSBs can rescue pairing and the formation of the SC in the absence of Spo11 (Storlazzi et al., 2003). However, both of these processes are not as robust as in normal meiosis, partially due to the lower frequency of breaks in certain nuclei and also because programmed DSB formation mediated by Spo11/Ski8 is tightly regulated in terms of site localization (Zickler & Espagne, 2016). The number of Spo11-induced DSBs per genome is regulated on a species-specific basis (de Massy, 2013; Zickler & Kleckner, 2015). As a general rule, in organisms with the canonical meiotic program, DSB numbers tend to be higher in organisms with longer chromosomes, which aligns with the role of DSBs in homolog pairing. Moreover, DSBs occur less frequently in organisms where they are not required for pairing. For instance, there are approximately 200 ~ 300 DSBs per meiotic nucleus in mouse, compared to only around 12 and 14 DSBs in Drosophila Melanogaster and C. elegans, respectively (de Massy, 2013; Lichten & de Massy, 2011; Zickler & Kleckner, 2015). In contrast, the number of COs or chiasmata is relatively low in nearly all organisms, with just one to several per homolog pair, even in species with large genome sizes and long chromosomes (de Massy, 2013). In summary, DSB numbers correlate with chromosome length and pairing requirements between species, while CO numbers remain consistently low, illustrating the intricate regulation of these two interconnected meiotic processes.
Meiosis has a fundamental characteristic where interactions and repair mediated by DSBs occur distinctly between homologous nonsister chromatids rather than between sister chromatids as seen in mitotic DSB repair. This bias towards homologs, often referred to as “homolog bias,” plays a pivotal role in various aspects of meiotic recombination (Zickler & Kleckner, 2015). It is crucial for activities such as recombination-mediated homolog pairing, ensuring the proper genetic outcomes like interhomolog CO, and establishing the interhomolog connections known as chiasmata, which are essential for the regular separation of homologous chromosomes. This homolog bias is not a late-stage phenomenon but is established early in the recombination process as DSBs initially identify their homologous partners. Furthermore, this bias needs to be actively maintained during the later stages of recombination when the lagging end of the DSB is involved in forming a double Holliday junction intermediate necessary for CO recombination (Hong et al., 2013; Kim et al., 2010).
Efficient homolog pairing requires sustained rapid chromosome movements from leptotene through pachytene stages, which rely on telomere bouquet formation. In budding yeast, telomere clustering is observed concomitant with meiotic homologous chromosome pairing and recombination (Hiraoka & Dernburg, 2009; Kim et al., 2022). The meiosis-specific protein Ndj1 tethers telomeres to the nuclear envelope by interacting with the SUN domain protein Mps3 of the Linker of nucleoskeleton and cytoskeleton (LINC) complex, thereby attaching telomeres to the nuclear membrane (Chua & Roeder, 1997; Conrad et al., 1997, 2007). Csm4 and Mps2 are KASH-like proteins, with Csm4 expression being meiosis-specific. Both paralogs mediate rapid chromosome motility by interacting with myosin II, which travels along actin filaments (Fan et al., 2020; Lee et al., 2020; Wanat et al., 2008). Loss of Ndj1, Mps3 or Csm4 compromises telomere clustering and HR during meiosis (Chua & Roeder, 1997; Conrad et al., 1997, 2007; Wanat et al., 2008). Notably, unlike other organisms, meiotic chromosome movements in budding yeast involve actin instead of microtubules. Moreover, compared to other species, telomere tethering and clustering appear to play a relatively minor role in budding yeast. Disrupting telomere attachment or mobility results in subtle defects in recombination distribution and low chromosome nondisjunction, while the progression of meiosis and the ability for homolog pairing and synapsis remain largely unaffected (Chua & Roeder, 1997; Conrad et al., 2007; Conrad et al., 2008; Lee et al., 2012; Trelles-Sticken et al., 2000). This could be attributed to the sufficiency of homology search mediated by recombination within the compact yeast nucleus for efficient homolog pairing. Researchers developed an agent-based model to simulate homologous chromosome pairing, derived from the dynamics of naturally occurring chromosome ensembles. This model accurately reproduces the efficiency and kinetics of homologous chromosome pairing observed in wild-type and mutant budding yeast during meiosis (Chriss et al., 2023). The study supports that nuclear crowding and nonhomologous chromosome collisions promote homologous chromosome pairing. By identifying thresholds for chromosome velocity, number, and repulsive forces, it is suggested that collisions between nonhomologous chromosomes may facilitate pairing by crowding homologous chromosomes into a limited nuclear area, thereby creating conditions required for close-range homologous chromosome pairing. Additionally, by simulating chromosome movements and interactions, the model also further reveals the complex dynamics of homologous chromosome pairing during meiosis.
The DSB-induced repair model, as a classical model for HR, seems plausible to explain homologous pairing. However, with increasing research, the limitations of this model have become apparent. First, compared to other higher eukaryotes, the nuclear volume of yeast is relatively small. If DNA still forms a nucleoprotein filament during the search process in higher eukaryotes, the difficulty of finding homologous sequences would greatly increase. Secondly, the model is not applicable to organisms that can complete homologous pairing without DSB formation. For example, in fission yeast (Schizosaccharomyces pombe), recombination-independent and recombination-mediated pairing occurs but SC is absent (Bähler et al., 1993; Egel-Mitani et al., 2008). In Drosophila, males do not induce DSB formation or form a SC (Tsai & McKee, 2011), but can still pair and segregate their chromosomes. In female Drosophila and Caenorhabditis elegans, pairing and synapsis take place independent of recombination, which occurs later in the context of the SC (Lake & Hawley, 2012; Lui & Colaiácovo, 2013; Rog & Dernburg, 2013). Next, we will review the pairing mechanisms in these different species separately (Table 1).
2.2 Schizosaccharomyces pombe
The fission yeast Schizosaccharomyces pombe, as a unicellular eukaryote, is highly similar to higher organisms in aspects such as RNA processing, chromatin modification and genetic behavior. It is an ideal model organism for studying meiosis. The S. pombe has three pairs of chromosomes, but does not form SC. During the transition from mitosis to meiosis, the nucleus of S. pombe cells exhibits dramatic changes in chromosome organization. During mitotic interphase, centromeres are clustered near the nuclear spindle pole body (SPB) through the SUN/KASH protein complex (composed of the SUN-domain protein Sad1, and the KASH-domain proteins Kms1 and Kms2) (Miki et al., 2003; Wälde & King, 2014) (Figure 2a left), while telomeres are clustered at the SPB before entering meiosis (Figure 2a Right). Upon entering meiosis, telomeres remain clustered at the SPB, with the SPB located at the leading edge of the moving nucleus (Ding et al., 1998) (Figure 2a Right). The entire nucleus is elongated and moves back and forth in the cell under the action of motor proteins, resembling a horse's tail, so this period is also called the horsetail stage (Ding et al., 1998, 2004). Mutants lacking efficient telomere clustering, like taz1, rap1, kms1, and bqt mutants, show a reduced occurrence of meiotic recombination and homologous loci pairing. Likewise, mutants with compromised nuclear movement, such as dhc1 and dlc1 mutants, exhibit similar phenotypic traits. Observations of live cells in a series of mutants showed that telomere clustering and nuclear movement during the horsetail stage are necessary to increase contact between homologous regions, and promote homologous chromosome pairing during meiosis (Ding et al., 2004; Hiraoka, 2020).
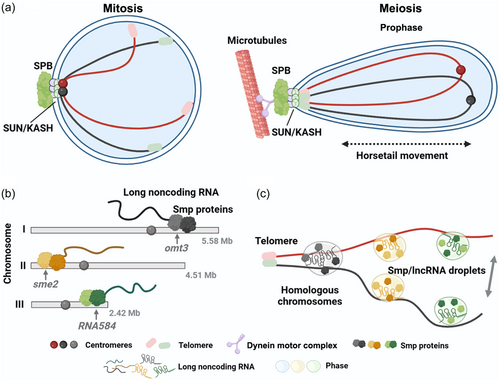
Chromosome-associated RNA plays a crucial role in the regulation of meiosis in S. pombe. Studies have revealed that in rec12- mutant cells, which lack Rec12 (a homolog of Spo11) required for DSB formation, the pairing frequency of the sme2 loci is comparable to that in wild-type cells (Ding et al., 2012). Consequently, the sme2 gene locus exhibits stable pairing during the horsetail stage, independent of DSB formation (Ding et al., 2012). Sme2 can be transcribed into a long noncoding RNA (lncRNA) specific to meiosis, and the accumulation of lncRNA at the sme2 locus is essential for stable pairing (Ding et al., 2012, 2013). To understand the mechanism by which chromosome-associated RNAs mediate homologous chromosome pairing, researchers identified lncRNA-associated protein factors at the sme2 locus, named Smp (sme2 RNA-associating proteins) (Ding et al., 2019) (Figure 2b). In addition to localizing to the sme2 locus on chromosome II, Smp also localizes to the omt3 locus on chromosome I and the lncRNA584 locus on chromosome III (Figure 2b). Smp can couple with RNA polymerase II (Pol II) to terminate its transcription and retain lncRNA at its transcription site. Loss of Smp binding sites does not eliminate pairing of the entire chromosome, the effect of sme2 RNA appears to be limited to about 200 kb around the sme2 locus, suggesting that RNA can mediate stable pairing of chromosomes, but this pairing is local. In addition, multiple Smp binding sites along the chromosome were detected by ChIP-sequencing analysis (Ding et al., 2019). These minor sites, together with the major sites (barcode model), provide an array of recognition sites (Ding et al., 2019; Hiraoka, 2020). Therefore, the combination of local pairing sites arranged along the chromosome in a directed configuration may facilitate the long-range recognition and pairing of homologous chromosomes (Ding et al., 2019; Hiraoka, 2020).
When meiotic cells are transiently treated with 1,6-hexanediol, the accumulation of lncRNA at Smp binding sites and the pairing of these sites are disrupted (Ding et al., 2019). After the removal of 1,6-hexanediol, pairing at these sites is restored (Figure 2c). The liquid droplet-like properties of Smp-lncRNA complexes suggest that Smp-lncRNA may mediate homologous pairing at this site through liquid-liquid phase separation (Figure 2c). When studies at the DNA level fail to explain the fundamental mechanism of homologous pairing, the theory that chromosomes promote homologous pairing through RNA seems feasible. The local and transient accumulation of protein complexes containing lncRNA at gene loci, probably through phase separation, may provide a dynamic and reversible process for chromosome recognition (Ding et al., 2019; Zhang et al., 2023). The hypothesis that multiple lncRNAs distributed along the chromosome jointly participate in homologous pairing may also optimize subsequent meiotic events and provide a new way of thinking for solving the problem of homologous recognition.
2.3 Drosophila Melanogaster
In somatic cells of Drosophila Melanogaster, chromosomes exist in pairs, a phenomenon observed in many Dipteran insects. Thus, for a long time, people considered the pairing of chromosomes during meiotic reductional division as a continuation of somatic pairing (Copenhaver et al., 2013). However, with advances in cytological techniques enabling direct observation of pairing events in female Drosophila Melanogaster gonads, we now know that meiotic pairing is not simply an extension of somatic pairing (Christophorou et al., 2013; Joyce et al., 2013). Rather, it is a poorly understood process occurring during premeiotic mitotic divisions. These events also differ markedly between males and females in Drosophila Melanogaster.
2.3.1 Female Drosophila Melanogaster
Drosophila Melanogaster is an ideal model organism for studying the early events of meiosis, owing to the developmental arrangement of female ovaries. In females, the steps of meiosis manifest as stage-wise progression along each ovariole comprising the ovaries (Hughes et al., 2018). Homologous chromosome pairing and assembly of the SC occur before and independently of recombination, with DSBs subsequently taking place within the pre-formed SC (Lake & Hawley, 2012; Zickler & Kleckner, 2015). Meiosis initiation occurs in the anterior germarium, which can be divided into four zones: regions 1, 2A, 2B, and 3 (Figure 3a). Meiosis begins in the germarium, where two to three germline stem cells (GSCs) surrounded by support cells reside at the tip (Huynh & St Johnston, 2004). After a GSC division, the resultant cystoblast daughters coupled with progenitors undergo four rounds of synchronous, incomplete cytokinesis to generate a 16-cell interconnected cyst, which eventually forms the oocyte (Mathieu et al., 2013). Intriguingly, events promoting homologous chromosome pairing seem to take place in region 1 while nuclei still undergo premeiotic mitotic divisions (Takeo et al., 2011; Tanneti et al., 2011).
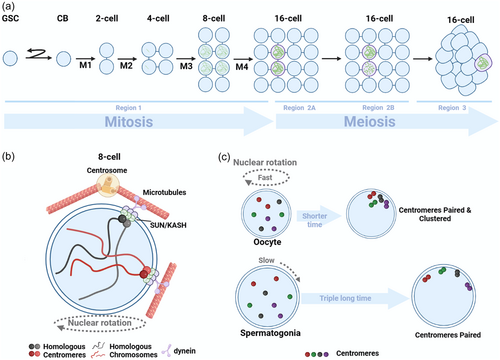
In region 1 of the germarium, homologous chromosomal centromeres start pairing, and complete pairing in 8 cells cysts (Christophorou et al., 2013; Joyce et al., 2013). After the final premeiotic mitosis, paired centromeres on average coalesce into two groups. These paired centromeres persist through prophase I of meiosis (Christophorou et al., 2013; Takeo et al., 2011; Tanneti et al., 2011). The first cytologically detectable landmark of meiosis appears in region 2 A of the germarium, where SCs can assemble along chromosome arms in up to 4 nuclei of 16-cell cysts (Hinnant et al., 2020) (Figure 3a). As the cysts diffuse through the germarium, two of the initial four nuclei with SC disassemble and exit the meiotic program, followed by the disassembly of SCs in another nucleus in region 2B (Figure 3a). This results in a single pro-oocyte maintaining full-length SCs along chromosome arms through region 3. The remaining 15 cells serve as nurse cells playing supportive roles (Huynh & St Johnston, 2004) (Figure 3a).
Moreover, there is a high correlation between nuclear rotation and chromosome pairing in Drosophila Melanogaster (Christophorou et al., 2015). These nuclear rotations are mainly driven by microtubules nucleating from the nuclear envelope and centrosomes (Rubin et al., 2020) (Figure 3b). Although no telomere bouquets form in Drosophila Melanogaster, aggregation of centromeres does occur, which may serve a similar function to telomere bouquets (Carpenter, 1975; Takeo et al., 2011). The microtubule motor dynein is essential, as nuclear rotation is not observed in dynein mutant cells, homologous chromosomes fail to pair, and SC formation is severely impacted (Christophorou et al., 2015). The LINC complex, comprised of SUN domain protein Klaroid and KASH domain protein Klarsicht, transmits cytoskeletal forces to the nuclear envelope and chromosomes. Both Klaroid and Klarsicht are localized to the nuclear envelope and connected to centromeres (Figure 3b). The dynamic movements of centromeres, as well as centromere pairing and clustering, depend on microtubules, dynein, Klaroid and Klarsicht. In klarsicht and centrosome mutant cells, nuclear rotation speed is significantly slowed, leading to obvious defects in initial centromere pairing and SC formation (Christophorou et al., 2015). Thus, nuclear rotation plays an important role in homologous chromosome pairing and SC formation. Moreover, the pairing and synapsis of chromosomes can occur even in the absence of HR in Drosophila Melanogaster oocytes (McKim et al., 1998). Electron microscopy analysis of oocytes from females homozygous for two meiotic mutants (mei-W68 and mei-P22), which eliminate both meiotic CO and gene conversion, revealed normal formation of the SC (McKim et al., 1998). Additionally, centromere clustering, but not the pairing of homologous centromeres, necessitates the presence of components from the SC, which is the proteinaceous structure responsible for holding homologous chromosomes together (Christophorou et al., 2013; Takeo et al., 2011; Tanneti et al., 2011). C(3)G and Corona are transverse filament and central element components of the SC, localized at centromeres (Christophorou et al., 2013). SC polymerization initiates at centromeres, then extends to some euchromatic regions, and eventually spreads across chromosomes (Christophorou et al., 2013; Joyce et al., 2013; Takeo et al., 2011; Tanneti et al., 2011).
2.3.2 Male Drosophila Melanogaster
In contrast to female Drosophila Melanogaster, male do not form SCs, do not recombine, and have no CO (McKee et al., 2012). Yet, males are still able to properly segregate their chromosomes. Analysis of early steps in homolog pairing in males found that homologous centromeres are not paired in GSCs, but pair during mitotic divisions in the mitotic zone, similar to females (Joyce et al., 2013). Male spermatocyte nuclei in 2-, 4-, 8-, and 16-cell cysts are larger than in females. In each interphase, males have more time for growth than females. Cell cycle durations are similar in female and male GSCs. However, in female cysts, cell cycles shorten at each division, while in males, cyst cell cycles are longer than in GSCs (Hinnant et al., 2017). Thus, the developmental window for homolog pairing in males is approximately three times that in females (Rubin et al., 2022). Male centromeres also exhibit motion, but even at the most dynamic 8-cell stage, the relative nuclear volume occupied by centromeres is significantly less than in females, and complete nuclear rotations are not observed. This slower dynamics in males is compensated by the longer cell cycles (Rubin et al., 2022). Chromosome movement in males is also microtubule-dependent like in females, which is required for effective centromere pairing (Rubin et al., 2022). Male SUN/KASH complex components Klar and Koi co-localize with centromeres and microtubules. Mutation or deletion of these proteins demonstrates the LINC complex (Klar/Koi) is essential for centromere pairing in males. While males do not form SCs, SC proteins are expressed in the male germline and localize to centromeres, where they promote pairing (Christophorou et al., 2013). Male and female meiosis have significant differences, but sex-specific cell cycle dynamics integrate similar molecular mechanisms to achieve proper centromere pairing (Figure 3c).
Although male Drosophila Melanogaster do not undergo CO, bivalents still form during meiosis, suggesting an alternative homolog conjunction (AHC) system may be functioning (Weber et al., 2020). The sex chromosomes and autosomes in male Drosophila Melanogaster have different homologous pairing mechanisms. The X and Y chromosomes in male Drosophila Melanogaster have strong heteromorphism (McKee & Karpen, 1990). Pairing of the heteromorphic sex chromosomes in spermatocytes relies on rDNA repeat clusters on the X and Y chromosomes. More specifically, the 240 bp intergenic spacer (IGS) upstream of the 18S and 28S rDNA repeats is essential for X-Y chromosome pairing (Hylton et al., 2020). X chromosomes with rDNA defects cannot segregate properly in late meiosis I. When an rDNA transgene containing these 240 bp repeats is inserted into the rDNA-deficient X chromosome, chromosome segregation is restored (Hughes et al., 2018; McKee, 1996; Thomas & McKee, 2007). The pairing site in this gene shares some similarities with the pairing centers (PCs) in nematodes (to be discussed later). Unlike sex chromosomes, autosomal pairing in Drosophila Melanogaster does not seem to involve specific localized PCs (McKee et al., 2012). Instead, major autosomes utilize homology across the entire genome, rather than site-specific pairing, to establish homology and direct segregation patterns (McKee et al., 2012). Chromosomes 2 and 3 exhibit capacity for pairing in euchromatic regions but not heterochromatic regions, indicating their pairing may be restricted to specific sequences (McKee, 2009; Tsai & McKee, 2011). Cytogenetic analyzes demonstrate that only autosomes with homology in heterochromatic regions fail to segregate from each other at the end of meiosis I (Hilliker et al., 2009; McKee et al., 1993; Yamamoto, 1979), suggesting autosomes may lack the ability to pair within heterochromatin. So far, four genes - mnm (modifier of mdg4 in meiosis) (Sun et al., 2019; Thomas & McKee, 2007), snm (stromalin in meiosis/SA-2) (Sun et al., 2019; Thomas & McKee, 2007), tef (teflon) (Arya et al., 2006), and uno (univalents only) (Weber et al., 2020) - have been identified as specific essential components of the AHC system, as mutations in these genes affect chromosome segregation in male but not female Drosophila Melanogaster. During spermatocyte maturation, SNM and MNM proteins localize to rDNA repeats on the sex chromosomes. Mutations in either protein affect segregation of all four chromosomes (Sun et al., 2019; Thomas & McKee, 2007). Less is known about TEF and its localization is unclear, but segregation of autosomes is affected in tef mutant spermatocytes (Arya et al., 2006). UNO localizes to all four chromosomes, especially the sex chromosomes, and can be cleaved by separase to trigger bivalent separation in late meiosis I (Weber et al., 2020).
In Drosophila melanogaster, a hallmark of genome folding is somatic homologous pairing, where homologous chromosomes are intimately paired along their entire lengths. It is widely believed that pairing is driven by specific interaction “buttons” encoded along the chromosomes (Viets et al., 2019). A recent study formulated somatic homolog pairing into a precise biophysical model, which also proposes that chromosome-wide pairing can be established through random encounters between specific interaction buttons distributed at various possible densities along homologous chromosomes, using reasonable parameters for a set of protein-protein interactions (Child et al., 2021). Testing whether such a model applies to meiotic pairing during gametogenesis would be highly informative.
2.4 Caenorhabditis elegans
C. elegans has been at the forefront of understanding chromosome movements and pairing during meiosis in multicellular organisms (Lui & Colaiácovo, 2013). As a classic model for studying meiosis, its germ cells are arranged in a spatiotemporal gradient within the gonads, allowing observation of all stages of meiosis I prophase (Figure 4a). Germ cells are generated from mitotic stem cells at one end of the gonad, enter meiosis at the transition zone (TZ), corresponding temporally to leptotene and zygotene stages. Germ cells at the TZ have their chromosomes polarized towards one side of the nucleus in a characteristic crescent shape (Crittenden et al., 2006; Woglar & Jantsch, 2013). As nuclei move down the gonad, aligned homologous chromosomes become evident by pachytene (Crittenden et al., 2006; Mlynarczyk-Evans & Villeneuve, 2017; Woglar & Jantsch, 2013). Chromosome condensation is a hallmark of diplotene, when the six bivalents are clearly visible as individual chromatin masses, which continue to condense during diakinesis (Figure 4a). C. elegans has six pairs of holocentric chromosomes, meaning kinetochores are distributed along the entire lengths (Rubin et al., 2020). Pairing initiates upon entry into meiotic prophase I at the TZ.
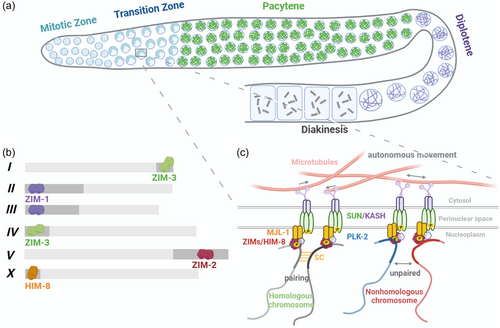
In C. elegans, alignment, pairing and synapsis of homologous chromosomes does not rely on formation of meiotic DSBs (Dernburg et al., 1998). This is evident as these processes still occur normally in the C. elegans spo11-null mutant (Dernburg et al., 1998). Chromosomes require cis-acting regions to pair their homologs (Lui & Colaiácovo, 2013; McKim et al., 1988; Rose et al., 1984; Villeneuve, 1994). These regions were initially defined as homolog recognition regions (HRRs) and later named “pairing centers” (PCs) (McKim et al., 1988; Villeneuve, 1994). PCs are roughly localized to the termini of C. elegans chromosomes but are not telomeres (Zetka & Rose, 1995) (Figure 4b). PCs contain highly repetitive DNA sequence motifs that recruit C2H2 zinc finger proteins ZIM-1, ZIM-2, ZIM-3 and HIM-8 (Phillips & Dernburg, 2006; Phillips et al., 2005, 2009; Sanford, 2001). HIM-8 is recruited to the PC of the X chromosome, ZIM-1 binds the PCs of chromosomes II and III, ZIM-2 binds the PC of chromosome V, and ZIM-3 binds the PCs of chromosomes I and IV (Phillips & Dernburg, 2006; Phillips et al., 2005) (Figure 4b). ZIMs/HIM-8 can each recognize a short ~12 bp repeat sequence within the PCs (Phillips et al., 2009), and deletion of a PC results in severe pairing and synapsis defects for the corresponding chromosome (Phillips et al., 2009). In the absence of the zinc finger protein ZIMs/HIM-8, SC does not form on the corresponding chromosome pair (MacQueen et al., 2005; Phillips & Dernburg, 2006; Phillips et al., 2005). However, failure of a single PC interaction does not affect SC on other chromosomes, but it does cause delayed release from the polarized configuration and DSB repair on other chromosomes (Phillips & Dernburg, 2006; Phillips et al., 2005). Although the phenomenon of different chromosomes recruiting the same pairing proteins exists, nonhomologous chromosomes do not pair, which may indicate that the pairing proteins recruited by PCs are not the only factors determining chromosome specificity, and the fundamental reason still lies in the PCs themselves. HIM-8 located on the X chromosome and other pairing proteins located on autosomes differ somewhat in their timing, with HIM-8 present from leptotene to late pachytene, while other autosomal pairing proteins are present from leptotene to early pachytene (Phillips et al., 2005).
During prophase I of meiosis, chromosome movement is highly dynamic. The motion of meiotic chromosomes promotes timely pairing and relies on microtubules (Sato et al., 2009). Depletion of motor proteins, the microtubule motor, results in delayed pairing and failed synapsis of chromosomes (Sato et al., 2009). This is analogous to the dynein-mediated horsetail motion of telomere-led chromosomes observed in fission yeast, which facilitates chromosome pairing (Ding et al., 2004; Hiraoka et al., 2000; Miki et al., 2002). PCs associate with the SUN/KASH complex comprising SUN-1 and KASH protein ZYG-12, interacting with SUN-1 across the inner nuclear membrane (Figure 4c) (Mlynarczyk-Evans & Villeneuve, 2017; Woglar & Jantsch, 2013). The KASH protein ZYG-12 interacts with cytoplasmic motors and microtubule motors to drive progressive chromosome motion, facilitating pairing and synapsis (Figure 4c). Meanwhile, ZYG-12 interacts with SUN-1 in the perinuclear space (Minn et al., 2009) (Figure 4c). Nocodazole treatment to depolymerize microtubules leads to nonhomologous synapsis in C. elegans (Sato et al., 2009). Thus, chromosome movement during meiosis is thought to not only facilitate homologous pairing but also disrupt interactions between nonhomologous chromosomes (Koszul & Kleckner, 2009). Recent studies have identified a previously uncharacterized meiosis-specific nuclear envelope protein MJL-1 (MAJIN-Like-1) that is crucial for the interaction between PCs and the SUN/KASH complex in C. elegans (Kim et al., 2023). MJL-1 tethers PC proteins and SUN-1 (Kim et al., 2023) (Figure 4c). Mutation of MJL-1 eliminates the active chromosome movement during meiosis, leading to impaired nonhomologous synapsis and homologous pairing (Kim et al., 2023). MJL-1 may interact with SUN-1 through their transmembrane domains or perinuclear regions (Kim et al., 2023). As in other organisms, the transmembrane SUN/KASH protein complex bridges chromosomes and the cytoskeleton, enabling dynein-powered force transfer across the nuclear envelope to the chromosomes (Sato et al., 2009). This drives highly dynamic yet random movements of PCs, while movement of other chromosomal regions appears more restrained (Li et al., 2010). Although PCs can aggregate, they never coalesce to form meiotic bouquet structures (Wynne et al., 2012). The random interactions between homologous PCs induced by their movements are believed to stabilize homolog pairings until the polymerization of the central region of the SC further stabilizes the connection between homologous axes (Woglar & Jantsch, 2013). The pairing of homologous non-PC regions of autosomes is mediated by chromosome domain protein MRG-1, which blocks nonhomologous synapsis along regions away from PCs (Dombecki et al., 2011).
The phosphorylation state of SUN-1 is critical for regulating chromosome morphology (Penkner et al., 2009). Specifically, CHK-2 phosphorylates PC-associated proteins to initiate their recruitment of Polo-like kinase PLK-2 (Harper et al., 2011; Labella et al., 2011). The phosphorylated PC-associated proteins can then recruit PLK-2 to the nuclear membrane, where PLK-2 carries out meiosis-specific phosphorylation of SUN-1 at serine 12 and induces relocalization of SUN/KASH complexs to PC-containing nuclear membrane regions (Harper et al., 2011; Labella et al., 2011) (Figure 4c). Additionally, the interaction between MJL-1 and PC proteins requires PLK-2 activity (Kim et al., 2023) (Figure 4c). Other proteins have also been reported to play important roles in homologous pairing. HAL-2/HAL-3 are regulatory factors of PLK-1/2, and their deletion leads to mislocalized PLK-2 and defects in homologous pairing and synapsis (Roelens et al., 2019; Zhang et al., 2012). FKB-6 belongs to a family of conserved cochaperones that are homologous to mammalian FKBP52, and localizes to the nuclear periphery (Alleva et al., 2017). FKB-6 is crucial for regulating dynein-driven chromosome movement by keeping excessive chromosome movement in check, thereby ensuring proper SC assembly and homologous chromosome pairing during meiosis (Alleva et al., 2017). Its absence leads to alterations in the microtubule network and fkb-6 mutants exhibit reduced chromosome pairing, increased nonhomologous synapsis, and meiotic DNA damage repair defects (Alleva et al., 2017). PPH-4.1, a highly conserved homolog of the serine/threonine phosphatase PP4, also plays a key role in chromosome homologous pairing (Sato-Carlton et al., 2014). When PP4 is absent, chromosomes tend to undergo incorrect pairing and synapsis with nonhomologous chromosomes, or self-synapsis by folding back on themselves (Sato-Carlton et al., 2014). Furthermore, loss of PP4 activity leads to independent reductions in the number of DSBs as well as CO recombination events (Sato-Carlton et al., 2014).
2.5 Mus musculus
In mice, homologous pairing relies on the classical DSB-mediated HR pathway (Zickler & Kleckner, 2015). DSBs precede and are required for efficient homolog pairing in mouse meiosis (Baudat et al., 2000; Kauppi et al., 2011; Romanienko & Camerini-Otero, 2000). In mutants lacking the DSB enzyme Spo11, pairing is absent (Baudat et al., 2000; Cole et al., 2010; Romanienko & Camerini-Otero, 2000), and the level of pairing decreases with declining DSB levels (Baudat, Manova et al., 2000).
Interestingly, homologous chromosome pairing was observed in mouse spermatocytes preceding programmed DSB formation. Transcriptomic studies have revealed that genes encoding meiotic proteins, including REC8 and SC proteins, are already expressed and translated in spermatogonial cells before meiotic initiation (Evans et al., 2014; Wang et al., 2001). This premeiotic expression pattern resembles that of the C(3)G gene in Drosophila Melanogaster. One study found transient pairing in late premeiotic S phase that depends on Spo11 but not its catalytic activity (Boateng et al., 2013). Two other studies supported that homolog recognition and association occur in early prophase I before axis formation and DSB production, and this initial connection transforms into close pairing during axis maturation. Even when telomere-led chromosome movement is disrupted in Sun1 knockout spermatocytes, or DSB-dependent homology search is absent in spo11 knockout spermatocytes, homolog recognition is retained (Ishiguro et al., 2014; Scherthan et al., 2014). However, deletion of the meiosis-specific cohesin RAD21L eliminates initial associations and subsequent pairing. Despite mechanistic differences, researchers widely agree these early pairings are DSB-independent, and later stabilized by recombination and SC formation. Furthermore, researchers employed a three-dimensional fluorescence in situ hybridization-based method to investigate homologous pairing in both premeiotic and meiotic Mus musculus cells (Enguita-Marruedo et al., 2018; Solé et al., 2022). They utilized DNA painting probes, enabling the analysis of the entire karyotype. Their findings unequivocally establish that 73.83% of homologous chromosomes are already paired during premeiotic stages. The percentage of paired homologous chromosomes gradually increases to 84.60% during the mid-preleptotene to zygotene stage, ultimately reaching 100% at the pachytene stage. Importantly, this pairing is not random, as the observed percentage is significantly higher than that seen in somatic cells (19.47%) and between nonhomologous chromosomes (41.1%) (Enguita-Marruedo et al., 2018; Solé et al., 2022). These findings have significantly enhanced our understanding of the dynamics of DSB-independent pairing. Although the role of these early homologous chromosome pairings remains to be thoroughly studied, they seem to play important roles in preparing for and initiating meiosis.
Observing chromosome movements and pairing is a formidable task in male spermatocytes, and it becomes even more arduous in oocytes due to the occurrence of early meiosis during fetal development. Nevertheless, it was also observed that the chromosomes move along the inner nuclear membrane (INM) to transiently cluster into a “meiotic bouquet” (Kim et al., 2022; Liebe et al., 2006; Rubin et al., 2020). In mouse spermatocytes, chromosomes exhibit oscillations from late leptotene to early pachytene, with the most rapid movements occurring during the zygotene stage, with speeds ranging between 24 and 130 nm/s (Lee et al., 2015). These rapid movements result from the combination of nuclear rotation and autonomous chromosome movements, persisting from leptotene/zygotene to pachytene (Lee et al., 2015). They decline in the diplotene stage, concomitant with the liberation of SUN1 from telomeres. Furthermore, during the bouquet stage, telomeres are constrained near the microtubule-organizing center (MTOC), resulting in the transient suppression of telomere mobility and nuclear rotation (Lee et al., 2015; Shibuya et al., 2014a). In early leptotene, centromeres also remain close to the nuclear periphery and cluster with the bouquet (Scherthan et al., 1996). These telomere movements rely on the microtubule cytoskeleton and microtubule-associated dynein (Lee et al., 2015). The research revealed that microtubule bundles are located along the nuclear surface, extending into a complex and interconnected cytoskeletal network (Lee et al., 2015). It's noteworthy that the speed of telomere movement changes as cells progress through meiotic prophase. These changes in chromosome behavior are accompanied by significant alterations in the organization of the microtubule network (Lee et al., 2015). Observations of trajectories have shown that several chromosomes can move along the same path, with individual chromosomes moving back and forth along specific trajectories, displaying frequent changes in direction (Lee et al., 2015). Similar to males, two types of movements were observed during early oocyte prophase: nuclear rotation and autonomous chromosome movements (Enguita-Marruedo et al., 2018). To observe meiotic chromosomes in oocytes, transgenic mice expressing N- or C-terminal fluorescent-tagged SYCP3 (lateral element of the SC) were generated, providing insights into SC dynamics. Real-time imaging in oocytes revealed that telomeres form a bouquet structure from zygotene to late stages, confirming previous observations in fixed samples (Enguita-Marruedo et al., 2018). This structure was highly dynamic, featuring rapid cycles of bouquet formation and dissolution (Enguita-Marruedo et al., 2018).
Similar to other species, mouse chromosomes interact with microtubules through LINC complex during the process of meiosis. This complex resides within the nuclear envelope and comprises two main components: SUN1/SUN2 and KASH5 (Ding et al., 2007; Link et al., 2014; Morimoto et al., 2012). During meiosis in mice, SUN1 and SUN2 represent the exclusive SUN domain proteins expressed, and their localization coincides with the presence of the meiosis-specific KASH5 protein (Ding et al., 2007; Link et al., 2014). TERB1 is a highly conserved telomere-binding protein that plays a critical role during meiosis in multicellular organisms (da Cruz et al., 2020; Shibuya et al., 2014b). TERB1 localizes to telomeres by interacting with the shelterin protein TRF1 through its TRF1-binding (TRFB) domain. TERB1 further recruits the transmembrane adapters TERB2-MAJIN and SUN1-KASH5 to mediate telomere attachment and movement, respectively. Mutations in Terb1, Terb2, Majin, Sun1 or Kash5 lead to disrupted association between telomeres and LINC complexes, defective homologous chromosome pairing, and cell cycle arrest (Ding et al., 2007; Horn et al., 2013; Shibuya et al., 2014b, 2015; Wang et al., 2019). Recent studies have identified pathogenic mutations in TERB1, TERB2 and MAJIN genes in human patients with non-obstructive azoospermia, underscoring their significant roles in human reproduction (Alhathal et al., 2020; Salas-Huetos et al., 2020; Voigt et al., 2020). During male meiosis, cyclin-dependent kinase 2 (CDK2) has been proposed to regulate LINC complex assembly and function by phosphorylating TERB1 (Shibuya et al., 2015). Additionally, CDK2 may modulate LINC complexes through interactions with SUN1 (Mikolcevic et al., 2016). The atypical CDK2 activator Speedy/RINGO (rapid inducer of G2/M progression in oocytes) A (SPDTA) colocalizes with CDK2 at telomeres in mouse oocytes and spermatocytes (Chen et al., 2021), indicating the presence of a preassembled Speedy/RINGO-CDK2 complex at telomeres (Mikolcevic et al., 2016; Tu et al., 2016). Cdk2 mutations result in excessive nonhomologous associations and random chromosome pairing exchanges, implying that while Cdk2 is dispensable for initiating homologous pairing, its proper regulation is essential for faithful chromosome pairing (Mikolcevic et al., 2016; Viera et al., 2009).
3 SUMMARY AND PROSPECT
Through the investigation of homologous pairing in meiosis, we can summarize the following commonalities across pairing mechanisms in different species: First, chromosome movement (Alleva & Smolikove, 2017). After chromosomes are randomly distributed in the nucleus, they must move to search for their homologous chromosomes. Although different species adopt different modes of chromosome movement, such as individual movement, nuclear movement, or a combination, their common purpose is to bring chromosomes together to identify and pair with their homologous partners. Second, the connection between chromosomes and the cytoskeletal framework (Kim et al., 2022). Chromosome movement is driven by the cytoskeleton, usually microtubules, sometimes actin filaments. The connection is generally achieved through the LINC complex, composed of SUN proteins and KASH proteins spanning the inner and outer nuclear membranes, respectively. The SUN/KASH complex exhibits similar molecular structures across species, indicating its shared evolutionary origin and function during meiosis. During the pairing process, chromosomes undergo complex movements and interact with the cytoskeleton. Despite this, the nuclear envelope remains intact, serving as a barrier between the nucleus and cytoplasm. This ensures proper isolation between nuclear DNA and cytoplasmic contents, so that the pairing process can proceed in an orderly manner inside the nucleus. Third, chromosome clustering (Rubin et al., 2020). Chromosomes exhibit clustering during meiosis to facilitate homolog recognition and pairing. Major clustering patterns include telomere clustering and centromere coupling. In most eukaryotes, telomeres scattered throughout the nucleus gradually cluster at one end of the nuclear envelope during leptotene or zygotene, forming a telomere bouquet, bringing chromosomes together and reducing the search distance between homologs. Centromere coupling refers to interactions between centromeres to promote homolog search and arm pairing. Finally, most species effectively install the SC between homologs to stabilize pairing (Gao & Colaiácovo, 2018). Absent or misregulated assembly of the SC prevents the stabilization of pairing interactions that are essential for meiosis, leading to chromosome missegregation.
To intuitively and dynamically observe chromosome movements and pairing during HR, direct imaging of chromosome dynamics is required. However, such experiments face technical challenges, especially in multicellular organisms where meiosis takes place in inaccessible reproductive tissues that are difficult to immobilize and maintain in a physiologically functional state. Specifically, meiosis occurs deep within reproductive organs that are hard to access for in vivo imaging. It is also challenging to extract samples from these tissues for fixation while preserving physiological functions at both the tissue and cellular levels. Nevertheless, continual advances in genome editing, fluorescent labeling and microscopic techniques will enable more feasible and informative analyzes. Future application of quantitative live-cell imaging techniques, combined with molecular biology tools and quantitative modeling, may reveal unprecedented mechanistic details of the unique dynamic homologous chromosome pairing process during meiosis. We can see that the mechanisms of homologous chromosome pairing vary greatly across different species. Even in yeast, a relatively simple model organism, the factors mediating pairing are diverse and not yet fully elucidated. The process must be even more complex in higher eukaryotes than what humans currently understand. Revealing the precise molecular mechanisms of meiotic pairing remains a long journey that requires persistent exploration.
AUTHOR CONTRIBUTIONS
Yuqi Tian and Libo Liu wrote the original draft. Ruoxi Wang drew the figures. Jinmin Gao discussed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (31900557, 32370780 and 32022018), and China Postdoctoral Science Foundation Grant (grant number: 2019M662431). Images were created with BioRender. com.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.




