Cellular Prion protein moonlights vascular smooth muscle cell fate: Surveilled by trophoblast cells
Abstract
Uterine spiral artery remodeling (uSAR) is a hallmark of hemochorial placentation. Compromised uSAR leads to adverse pregnancy outcomes. Salient developmental events involved in uSAR are active areas of research and include (a) trophendothelial cell invasion into the spiral arteries, selected demise of endothelial cells; (b) de-differentiation of vascular smooth muscle cells (VSMC); and (c) migration and/or death of VSMCs surrounding spiral arteries. Here we demonstrated that cellular prion (PRNP) is expressed in the rat metrial gland, the entry point of spiral arteries with the highest expression on E16.5, the day at which trophoblast invasion peaks. PRNP is expressed in VSMCs that drift away from the arterial wall. RNA interference of Prnp functionally restricted migration and invasion of rat VSMCs. Furthermore, PRNP interacted with two migration-promoting factors, focal adhesion kinase (FAK) and platelet-derived growth factor receptor-β (PDGFR-β), forming a ter-molecular complex in both the metrial gland and A7r5 cells. The presence of multiple putative binding site of odd skipped related-1 (OSR1) transcription factor on the Prnp promoter was observed using in silico promoter analysis. Ectopic overexpression of OSR1 increased, and knockdown of OSR1 decreased expression of PRNP in VSMCs. Coculture of VSMCs with rat primary trophoblast cells decreased the levels of OSR1 and PRNP. Interestingly, PRNP knockdown led to apoptotic death in ~9% of VSMCs and activated extrinsic apoptotic pathways. PRNP interacts with TRAIL-receptor DR4 and protects VSMCs from TRAIL-mediated apoptosis. These results highlight the biological functions of PRNP in VSMC cell-fate determination during uteroplacental development, an important determinant of healthy pregnancy outcome.
Abbreviations
-
- Bax
-
- Bcl2 associated X-protein
-
- Bcl-XL
-
- B-cell lymphoma extra large
-
- DR4
-
- Death Receptor 4
-
- FAK
-
- focal Adhesion Kinase
-
- OSR1
-
- odd-skipped-related transcription factor 1
-
- PDGF A
-
- platelet-derived growth factor subunit A
-
- PDGF B
-
- platelet-derived growth factor subunit B
-
- PDGFRβ
-
- platelet-derived growth factor receptor beta
-
- TRAIL
-
- tumor necrosis factor-alpha-related apoptosis-inducing ligand
-
- uNK cells
-
- uterine natural killer cells
1 INTRODUCTION
Successful development of a fetus postimplantation is completely dependent on the quality and quantity of nutrients, gaseous exchange, and efficient waste disposal by the uterine vasculature. The uterine arteries give rise to arcuate arteries, which give rise to radial arteries (Epinzoa et al., 2006). The radial arteries further branch to basal arteries and “spiral arteries,” the latter supplying blood to the endometrium bed. They develop during the secretory phase of the menstrual cycle under the influence of progesterone and estrogen (Chen & Magness, 2021; Kim et al., 2013) and are eventually shed off after each cycle. However, following implantation, these vessels supply nutrients and respiratory gases to the growing fetus (Adamson et al., 2002). To ensure uninterrupted flow of essential materials to the fetus, the spiral arteries undergo several structural and functional changes, which include 10-fold increase in vessel diameter and three- to four-fold increase in the volume of the blood delivered to the inter-villous space at a surprisingly low pressure (Kim et al., 2013; Robertson & Warner, 1974). This pregnancy-dependent structural changes of the vessel wall are known as “spiral artery remodeling” (SAR), failure of which leads to developmental defects such as pre-eclampsia and intrauterine growth restriction (IUGR) (Pijnenborg et al., 2006). The initial stages of SAR are mediated by the uNK cells (Robson et al., 2012) and succeeded by the invasive trophoblast cells (Meekins et al., 1994; Nandy et al., 2020; Robson et al., 2012). The vascular smooth muscle cells (VSMCs) residing in the tunica media surrounding these arteries exhibit two phenotypic forms: (i) contractile or differentiated form, (ii) synthetic or de-differentiated form, characterized their specific array of markers (Chen & Magness, 2021; Nandy et al., 2020; Rensen et al., 2007). The two main characteristics acquired by the VSMC in synthetic phenotype are proliferation and migration. This form is predominant during embryonic angiogenesis but uncontrolled proliferation and migration in adult vessels lead to pathophysiological conditions like atherosclerosis and neointimal hyperplasia (Basatemur et al., 2019; Pahk et al., 2017). In rats, as the trophendothelial cells invade into uterine spiral arteries, alpha-smooth muscle actin disappears surrounding the spiral arteries (Nandy et al., 2020; Rosario et al., 2009). Lack of contractile VSMCs around uterine spiral arteries could be explained by trophoblast-mediated apoptosis (Harris et al., 2006; Whitley & Cartwright, 2009) and migration of VSMC (Bulmer et al., 2012).
Various molecular mechanisms underlie the process of VSMC migration and proliferation. Platelet-derived factors, particularly PDGF via platelet-derived growth factor receptor beta (PDGFRβ) signaling, are associated with VSMC migration during disease (He et al., 2015) and vascular development (French et al., 2008). Complete loss of PDGF signaling in mice leads to mal-formed blood vessels (Leveen et al., 1994). Lamellipodia extension of any migrating cell requires breakage of focal points at the trailing edge and formation of new focal contacts between the cell membrane and extracellular matrix. Focal adhesion kinases (FAK), a renowned non-receptor tyrosine kinase, is one of the predominant regulators of cell migration and is upregulated during intimal hyperplasia (Owens et al., 2001). Endogenous FAK recruitment to the activated PDGFRβ enhances PDGF-BB-induced VSMC migration (Hauck et al., 2000). VSMC apoptosis through trophoblast-derived Fas ligand (Harris et al., 2006), or tumor necrosis factor α-related apoptosis ligand (TRAIL) (Keogh et al., 2007), also play critical roles in vascular reorganization.
The Prion protein (PRNP) is a conserved glycoprotein tethered to the outer cell membrane by a glycosylphosphatidylinositol (GPI) anchor (Puig et al., 2014; Zuegg & Gready, 2000), mostly known for causing a group of transmissible, chronic, progressive, and invariably fatal infections of the nervous system (Prusiner, 1998) by its scrapie isoform (PrpSc) (Alper et al., 1966). Apart from neurones, its (cellular prion) ubiquitous presence in other tissues and cell types (Bendheim et al., 1992), has made it an interesting, yet mysterious protein. The prion gene family consists of Prnp and its paralogs Sprn (encodes Shadoo) and Dpl (encodes Doppel) which are evolutionarily derived from an ancient zinc transporter ZIP LIV-1 (Ehsani et al., 2011; Schmitt-Ulms et al., 2009). Down the years, scientists have made endless endeavours to figure out the functional role of prion protein in the placenta and its associated disease (Alfaidy et al., 2013; Makzhami et al., 2014; Nishizawa et al., 2007). Direct evidence regarding functional importance of cellular Prion and its paralog Shadoo during murine development have been presented by Passet et al., where they found that embryos lacking both the prion paralogs show a reduced and disorganized ecto-placental cone and also fragmented invasive trophoblast layer, ultimately resulting in embryonic lethality past Day 7.5 (Passet et al., 2011). Early embryonic lethality impeded the understanding of Prion biology during uteroplacental development. We, therefore, sought to unveil the crucial role played by endogenously expressed cellular isoform of the Prion protein during mammalian development, by identifying its cellular site of synthesis in the utero-placental compartment of time-pregnant rats. PRNP has been previously demonstrated to be expressed in mouse decidua (Ding et al., 2018). We discovered its presence in VSMC at the site of uSAR in the rat. We, therefore, sought to analyze biological role of endogenous PRNP in VSMCs involved in the process of uSAR. Since uSAR is primarily governed by trophoblast cells, we utilized primary rat trophoblast cells and analyzed how trophoblast cells direct uSAR mediated by PRNP. Our findings have broad biological implications in VSMC cell-fate determination at the utero-placental interface and its consequence in healthy pregnancy.
2 MATERIALS AND METHODS
2.1 Animals and tissue collection
To obtain timed utero-placental tissues, sexually mature Sprague Dawley female rats were caged overnight with fertile males. Day 0.5 of pregnancy was designated by the presence of sperm in the vaginal smear. Utero-placental tissues were collected from pregnant females on different days of pregnancy. Tissues were snap-frozen in liquid nitrogen for RNA and protein isolation and in dry ice-cooled hexane for cryo-sectioning. The IICB Animal Care and Use Committee approved all procedures for handling and experimentation with rodents in accordance with the regulations set forward by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India (http://cpcsea.nic.in).
2.2 Culture of A7r5 and HEK293T cell lines
Rat smooth muscle cell line, A7r5, was obtained from ATCC (USA). A7r5 cells were cultured in DMEM high glucose basal media (D7777, Sigma) supplemented with 10% FBS and 1% penicillin–streptomycin (Invitrogen). HEK293T were obtained from ATCC (USA). HEK293T cells were cultured in DMEM (D7777, Sigma) supplemented with 10% FBS, 2 mM l-glutamine and 1% penicillin–streptomycin (Invitrogen). All the cells were cultured in the presence of 5% CO2 at 37°C in a humidified incubator.
2.3 Isolation of rat primary trophoblast cells and coculture with VSMC
Junctional zones, isolated from rat placentas on E16.5, were thoroughly washed with sterile DPBS and minced into fine pieces. They were further minced in the presence of 0.05% Trypsin-EDTA (0.5 mL) for 2 min. Then 4.5 mL 0.05% Trypsin-EDTA was added and the suspension was incubated in the presence of 5% CO2 at 37°C for 15 min. Following incubation, the cell suspension was pipetted vigorously until a slurry-like appearance was obtained. Trypsin neutralization was then performed using 5 mL trophoblast media (RPMI-1640 (Sigma-Aldrich) supplemented with 20% FBS (Invitrogen), 1% penicillin–streptomycin (Invitrogen), 1% Glutamax (Invitrogen), 1 mM sodium pyruvate (Sigma-Aldrich), and 100 µM β-mercaptoethanol (Sigma-Aldrich)). Cells were then washed three times with DPBS and the pellet was resuspended in 5 mL RBC lysis buffer (1.5 M NH4Cl, 140 mM NaHCO3, 10 mM EDTA, pH 7.3), incubated for 5 min at room temperature. Ten milliliters of DPBS were added to the cell suspension and centrifuged at 1000 rpm for 5 min. The cell pellet was resuspended in fresh TS media and strained using 70 µm cell strainer (cc, USA) to obtain single-cell suspension. 2 × 105 cells were plated on each culture insert (BD Falcon), which were placed on companion plates (BD Falcon) containing 24 h culture of A7r5 cells. After 48 h of co-culture RNA and protein was isolated from the A7r5 cells. Protein from the primary trophoblast cells was extracted on the day of isolation.
2.4 RNA isolation, reverse transcription, and real-time PCR analysis
Total RNA from tissues or cells was isolated using TRIzol reagent (Invitrogen), as per the manufacturer's protocol. Tissue samples were homogenized and cultured cells were scraped in ice-cold Trizol. Phase separation was done by adding chloroform. RNA was precipitated from the aqueous phase using isopropanol, followed by 70% ethanol wash. The RNA pellet was dissolved in nuclease-free DEPC-treated water. First-strand cDNA was synthesized with 2.5 µg of total RNA using M-MLV Reverse Transcriptase enzyme using the standard protocol (Invitrogen).
Ten-fold dilution of cDNAs and Power SYBR GREEN PCR Master Mix (Applied Biosystems) was used in the real-time PCR reaction. Reactions were run using a 7500 Real-Time PCR System (Applied Biosystems). Conditions included an initial holding stage (95°C for 10 min), then 40 cycles (95°C for 15 s and 60°C for 1 min) followed by a dissociation stage (95°C for 15 s, 60°C for 1 min, and then 95°C for 30 s). Primers used for real-time PCR are listed in Table 1. The amount of a specific mRNA was normalized relative to the amount of rpL7 (∆Ct = Ctgene − CtrpL7). Fold change of gene expression was measured by using f, where ∆∆Ct denoted the change in ∆Ct values between samples and reference sample. At least three different biological replicates were used. Error bars represent the standard error of the mean from three different biological replicates.
| cDNA | Primer sequence (5′ to 3′) | Gene bank accession no. | |
|---|---|---|---|
| rPrnp | Forward | AGTGTACTACAGGCCGGTGGAT | NM_012631.3 |
| Reverse | ACCACACGCTCCATCATCTTC | ||
| rOsr1 | Forward | TGCGGTGTCCAGCCTAATG | NM_001106716.3 |
| Reverse | AAGGCCAGGTTAGCGAAATCA | ||
| rrpL7 | Forward | GCCCTCAAGACACTGCGAAA | NM_001100534.1 |
| Reverse | TGGTTCTGCGGGCACATAG | ||
2.5 Protein isolation, estimation, and western blot analysis
Tissue samples were homogenized and cells were scraped in ice-cold RIPA buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 0.2 mM PMSF, and 1 mM sodium orthovanadate) supplemented with protease inhibitor cocktail (Cell Signalling Technology). The homogenate was then centrifuged at 14,000 rcf for 10 min at 4°C and the supernatants were collected, aliquoted, and stored at −80°C until further use. Protein samples were quantified using the Bradford protein assay kit (Bio-Rad) as per the manufacturer's instructions.
The protein samples were denatured by boiling in a water bath for 5 min with SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, 2.5% SDS, 0.002% Bromophenol blue, 5% β-Mercaptoethanol, 10% glycerol) and then centrifuged briefly. Forty micrograms of protein samples were fractionated on SDS-polyacrylamide gel and transferred onto PVDF membrane. Membranes were blocked with either 2% or 5% skimmed milk for 1 h, then incubated overnight at 4°C with primary antibodies (diluted to the required concentration) on an orbital shaker. Membranes were rinsed and incubated with HRP-conjugated secondary antibodies for 1.5 h at room temperature on an orbital shaker. An ECL kit, Clarity MaxTM Western ECL Substrate (Bio-Rad) was used to develop chemiluminescence signals associated with protein bands. Images were acquired with the Chemidoc Imaging System (UVP).
2.6 Immuno-precipitation
Immuno-precipitation of proteins was done using Pure Proteome protein A/G magnetic beads (Millipore). Two hundred and fifty micrograms of protein were incubated with primary antibodies at required dilution overnight at 4°C. Twenty-five microliters of washed magnetic beads were then incubated with the protein-antibody solution for 2 h at room temperature. The beads were then washed three times for 30 s each with 500 µL of binding buffer. Following the last wash 40 µL of 1× SDS sample buffer was used to resuspend the beads and was heated at 70°C for 8 min. The beads were then captured with the magnetic stand and the supernatant was used for western blot analysis.
2.7 Immuno-histochemistry
Immuno-histochemical analyses were performed on 10-μm tissue sections prepared with the aid of a cryostat (Leica). Tissue sections were fixed in ice-cold acetone for 5 min, and incubated with 0.3% ice-chilled H2O2 in PBS for 5 min. Nonspecific binding was blocked by incubation with 1.5% normal goat serum in phosphate-buffered saline (PBS) for 1 h, and then exposed for 1 h to anti-PRNP antibodies in 1:600 dilution in PBS. Unbound antibodies were washed off with three changes of PBS, followed by incubation with biotinylated anti-rabbit secondary antibodies for 30 min (Vector laboratories) diluted in PBS. Samples were then washed three times with PBS for 5 min each and were incubated with ABC reagents (Vector Laboratories) for 30 min. Samples were washed again three times with PBS for 5 min each and were developed with AEC reagents (Vector Laboratories) in the dark and were monitored continuously under a microscope until specific brown color developed. Samples were washed with tap water and then counterstained with hematoxylin (Sigma). Stained tissue sections were examined and images were recorded with a Leica microscope equipped with a CCD camera and Leica Application Suite X (LAS X).
2.8 Immunofluorescence staining
Tissue sections were fixed in ice-cold acetone for 5–10 min, washed with PBS, then blocked with blocking buffer containing 5% goat serum and 0.3% Triton-X-100 in PBS for 1 h. Sections were then incubated with either H-caldesmon (1:200) or PRNP (1:200) for 2 h at room temperature and overnight at 4°, respectively. Samples were then washed three times with PBS, 5 min each, followed by incubation with FITC-conjugated anti-rabbit IgG (1:200 dilution) (Sigma) and TRITC-conjugated anti-rabbit IgG (1:1000 dilution) (Sigma) for 1.5 h at room temperature. Sections were again washed three times, followed by nuclear staining with Hoechst at a concentration of 2 µg/mL. Sections were again washed five times with PBS before mounting with Fluoroshield solution (Sigma). Images were captured with a Leica microscope equipped with a CCD camera and Leica Application Suite X (LAS X).
2.9 Designing, cloning of shRNA oligos in pLKO.1 vector, generation of lentiviral particles harboring shRNA, and transduction in VSMC
Broad institute portal https://portals.broadinstitute.org/gpp/public/seq/search was used to design shRNA oligos targeting coding exons. The oligos having higher intrinsic scores were selected and purchased from IDT. The sequence of the oligos is given in Table 2. Individual shRNAs were cloned in separate pLKO.1 vector (Addgene). Forward and reverse oligos were annealed by boiling for 4 min followed by slow cooling at room temperature using 20 µM of each of the oligos in 50 µL of NEB buffer2. The pLKO.1 vector was digested with EcoR1 and Age1 enzymes and ligated with shRNA oligos having compatible sticky ends in a 1:10 molar ratio using T4 DNA ligase (NEB) following standard ligation protocol. One Shot® Mach1™T1™ chemically competent Escherichia coli (Invitrogen) cells were transformed with the ligated mix. The transformants were then plated on LB agar plates containing ampicillin as a selection marker. Correctness of clones was confirmed by restriction digestion and sequencing.
| Sequence (5′ to 3′) | ||
|---|---|---|
| Prnp-shRNA1 (targets exon 3) | Forward oligo | CCGGTCGTGCACGACTGTGTCAATACTCGAGTATTGACACAGTCGTGCACGATTTTTG |
| Reverse oligo | AATTCAAAAATCGTGCACGACTGTGTCAATACTCGAGTATTGACACAGTCGTGCACGA | |
| Prnp-shRNA2 (targets exon 2) | Forward oligo | CCGGGTGGCAGGACTCCTGAATATACTCGAGTATATTCAGGAGTCCTGCCACTTTTTG |
| Reverse oligo | AATTCAAAAAGTGGCAGGACTCCTGAATATACTCGAGTATATTCAGGAGTCCTGCCAC | |
| Prnp-shRNA3 (targets between exon1 and 2) | Forward oligo | CCGGTTTCAACCCAACTGAAGTATTCTCGAGAATACTTCAGTTGGGTTGAAATTTTTG |
| Reverse oligo | AATTCAAAAATTTCAACCCAACTGAAGTATTCTCGAGAATACTTCAGTTGGGTTGAAA | |
| Osr1-shRNA (targets between exon1 and 2) | Forward oligo | CCGGCTACGGGACCACAGATATATTCTCGAGAATATATCTGTGGTCCCGTAGTTTTT |
| Reverse oligo | AATTCAAAAACTACGGGACCACAGATATATTCTCGAGAATATATCTGTGGTCCCGTAG | |
For producing lentiviral particles, HEK293T cells (7 × 105 cells) were plated on 6 cm tissue culture plates with antibiotic-free media and incubated at 37°C, 5% CO2 overnight. Next day, before transfection, transfection cocktail was prepared using 1 µg pLKO.1 shRNA plasmid (Sigma), 750 ng psPAX2 packaging plasmid, 250 ng pMD2.G envelope plasmid (Addgene) to a final volume of 100 µL serum free OPTI-MEM. In a separate tube 100 µL Master Mix was prepared with 6 µL Fugene and 94 µL OPTI-MEM and incubated for 5 min. The master mix was added to transfection cocktail to make a final volume of 200 µL and allowed to stand for 20–30 min. Following the incubation period DNA-Fugene mix was added drop wise on cells and spread by gentle swirling. Next day, transfection reagents containing media were removed and replaced with fresh complete growth media. Media containing viral particles were harvested after every 48 h in two rounds. Two batches of media containing viral particles were pooled, centrifuged, and stored in small aliquots at −80° C until used.
A7r5 cells were plated on 35 mm dishes and allowed to reach ~70% confluency overnight. Next day morning, the media was removed and fresh media supplemented with either lentiviral particles containing empty vector or a cocktail of shRNA 1,2,3 were added in separate dishes. After 24 h the lentiviral containing media was discarded and replenished with fresh medium and cells were harvested for further experiments.
2.10 Cloning and characterization of full-length rat Osr1 cDNA
Rat Osr1 full-length cDNA was cloned in pCAG-DsRed vector (Addgene) using the directional cloning strategy. RNA isolated from the heart was reverse-transcribed using MMLV-RT (Invitrogen) and full-length rat Osr1 (NM_001106716.3) cDNA was amplified from using LA-Taq DNA polymerase (TaKaRa, Japan). Primers used were Forward: 5′-TATAGGTACCAATGGGCA GCAAAACCT-3′ and Reverse: 5′-TATAGCGGCCGCTTAGCATTTGATCTTG-3′ having KpnI and Not1 restriction sites, respectively. The amplified product was cloned in-frame into pCAG-dsRed vector (Addgene) by deleting the dsRed using the standard protocol. Correctness of the clone was verified using restriction digestion and sequencing. Expression of OSR1 was assessed using the A7r5 cell line.
2.11 Scratch wound assay
2 × 105 A7r5 cells were seeded onto a 35 mm dish and after 24 h cells were treated with either control vector or a cocktail of shRNAs to downregulate Prnp. Next day, the virus-containing media was replaced by fresh media and a scratch was made using sterile cell combs (cell comb scratch assay kit, Merck Millipore) to form a cell-free area of approximately 700 µm of width. Images of the scratch wound were recorded immediately after scratch (t = 0 h) and 24 h after scratch (t = 24 h) using a Leica DMi8 microscope.
2.12 Cell invasion assay
2 × 105 A7r5 cells were seeded onto a 35 mm dish and after 24 h cells were treated with either control vector or a cocktail of shRNAs and were cultured for 24 h after transduction. Cells were trypsinized and seeded on ECMatrixTM inserts (Cell Invasion Assay kit, ECM550, Merk Millipore) at a density of 1 × 105 cells per insert in 300 µL of serum-free media. Media with 10% FBS was added to the lower chamber. After 24 h of migration, the residual media as well as non-invaded cells were removed from the inner surface of the inserts using cotton swabs. The inserts were dipped in staining solution for 25 min and the background was destained with several changes of distilled water. Images of invaded cells were taken using a Leica DMi8 microscope. Invaded cells were counted from five to six different microscopic fields from each biological replicates. Invaded cells were also quantified by dissolving the stain at the lower surface in 10% acetic acid and measuring the absorbance at 560 nm using a multi-mode plate reader (PerkinElmer).
2.13 Flow cytometric analysis to detect early apoptosis using Annexin-V-PI staining
A7r5 cells transduced with either control or shRNA lentivirus cocktail were trypsinized, washed, and resuspended at a concentration of 3 × 105/mL. The cell suspension was again centrifuged, and the pellet was dissolved in 96 µL of Annexin-V binding buffer followed by Annexin-V and PI staining as per the manufacturer's protocol (Cell Signaling Technology). The percentage of cells undergoing early apoptosis was analyzed using a Flow cytometer (LSR, Fortessa, BD).
To detect TRAIL-induced apoptosis, A7r5 cells were transduced for 24 h with either control or sh-Prnp lenti-particles. Both control and Prnp knockdown cells were then treated with increasing doses (10, 50, and 100 ng/mL) of recombinant murine TRAIL (Preprotech) and incubated for 24 h at 37°C. Apoptosis was then analyzed by Annexin-V and PI staining.
2.14 Antibodies
Anti-PRNP (14025), anti-GAPDH (5174), anti-FAK (3285), anti-PDGFR-β (3169), anti-Caspase 3 (9665), anti-cleaved caspase-3 (9664), anti-Caspase-8 (4790), anti-Bcl-xL (2764), anti-caldesmon (12503 S) rabbit monoclonal antibody, anti-Bax (2772) rabbit polyclonal antibody were purchased from Cell Signaling Technology, anti-Caspase-8 (cleaved, AHP967) antibody was purchased from Bio-Rad laboratories, anti-OSR1 mouse monoclonal antibody (sc-376545) was purchased from Santa Cruz Biotechnology. Anti-DR4 rabbit polyclonal antibody (BML-SA225-0100) was purchased from Enzo Life Sciences. HRP-conjugated anti-rabbit (A120-101P) and anti-mouse (A90-134P) secondary antibodies were purchased from Bethyl Laboratories and used at a dilution of 1:10,000 for western blot. FITC-conjugated anti-rabbit IgG (F0382) and TRITC-conjugated anti-rabbit IgG (T6778) were purchased from Sigma.
2.15 Statistical analysis
All experiments were performed at least three times with three different biological replicates. The standard error of the mean was calculated using Graph Pad Prism software. Statistical significance among experiment groups was calculated by unpaired Student's t-test and Mann–Whitney U test for non-parametric variables.
3 RESULTS
3.1 Identification and spatiotemporal distribution of cellular prion in the metrial gland
The metrial gland (MG) marks the entry point of maternal blood vessels and the site for SAR in the rat. We carefully isolated rat metrial glands on embryonic days 11.5, 13.5, 16.5, and 19.5 without contaminating the surrounding smooth muscle layer. Quantitative real-time PCR was performed to evaluate the temporal expression pattern of Prnp transcripts with the progression of gestation. Prnp mRNA levels gradually increased from E11.5 to E 16.5, followed by a sharp decline in E19.5 (Figure 1a). These data were further affirmed by analyzing the PRNP protein levels in these tissue lysates using western blot analysis (Figure 1b). NIH ImageJ software was used to quantify the protein bands using GAPDH as endogenous control (Figure 1c).
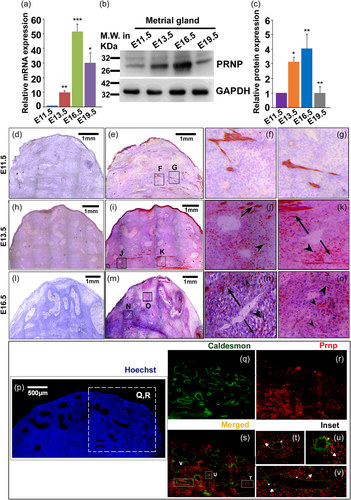
Owing to the heterogeneous nature of the metrial gland, we sought to decipher the specific cellular source of PRNP within this tissue by immuno-histological methods. Immuno-staining of PRNP protein on 10 µm cryosections revealed that the primary site of PRNP synthesis is the smooth muscle cells in the metrial gland on E11.5 (Figure 1e) and much less in the VSMCs (Figure 1f, see inset). On gestation day 13.5, specific signals were found in the smooth muscle layer (Figure 1h) as well as in the VSMCs that seem to migrate away from the arterial wall (Figure 1i,j, see inset). By E16.5, the maximum population of the VSMCs expresses prion and more migration was observed (Figure 1l–n). Staining the E13.5 metrial gland with either a smooth muscle marker, caldesmon (green, Figure 1q), or PRNP (red, Figure 1r) revealed that VSMCs surrounding almost all the arteries express caldesmon (Figure 1q). Upon merging two fluorescent images of the boxed region shown in Figure 1p, some cells were also found to express PRNP (Figure 1s), thus verifying these cells to be indeed VSMCs. On closer examination of individual arteries, it was observed that only the cells that are still strongly attached to the tunica of the arteries happen to express both caldesmon and PRNP, as the color of the cells is yellow denoting co-localization of both markers (Figure 1t–v, white asterisk). On the other hand, the VSMCs, which are in the process of migration and invasion, around their respective arteries, exclusively express prion and not caldesmon (Figure 1t–v, white arrowheads).
3.2 PRNP potentiates migration and invasion of VSMCs
The compelling presence of PRNP in migrating VSMC in the metrial gland, especially following trophoblast invasion into the metrial gland led to subsequent experimentation to understand the function of PRNP in VSMC. We therefore tested the A7r5 VSMC cell line for the presence of PRNP. As expected, PRNP was found to be abundantly expressed in the A7r5 cell line and the level of expression was comparable to that in the E16.5 metrial gland (Figure 2a). Furthermore, the immunocytochemical analysis showed that PRNP is expressed in the membrane of the A7r5 cells (Figure 2b).
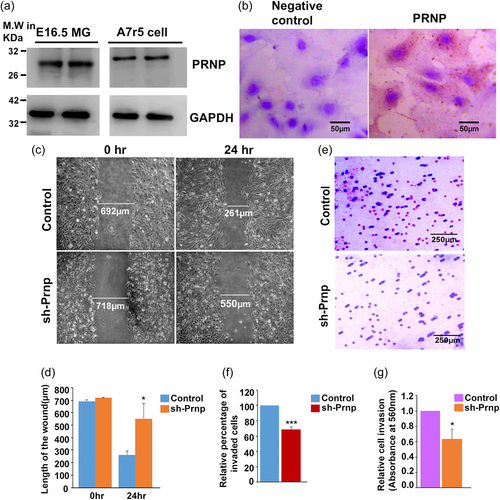
The presence of PRNP in migratory VSMCs in the metrial gland led us to investigate the influence of PRNP in VSMC migration and invasion. Prnp was knocked down in A7r5 with a cocktail of lentivirus containing three short hairpin oligos targeting different exons (Supporting Information: Figure S1B–E). Scratch wound assay was used to evaluate VSMC migration. Photo-micrographic images depicting the width of the wound at the beginning of the scratching and after 24 h is shown in Figure 2c. Cells transduced with empty vector showed around 62.2% of wound healing capacity, whereas PRNP knocked-down cells showed only 23.3% of wound closure after 24 h (Figure 2d).
A similar migration-promoting role of PRNP was observed in VSMC invasion toward the chemoattractant. VSMCs were plated on trans-well inserts pre-coated with extracellular matrix proteins, collagen, and elastin. The ability of cells to invade the matrix and attach to the underside of the trans-well membrane was scored as well as quantified. Representative photo-micrographic images of stained cells on the underside of the trans-well are shown in Figure 2e. At least five different fields per well were captured from three biological replicates and the cells in each field were counted. A significant decrease in the number of invaded cells was observed in cells with PRNP knockdown. The relative percentage of cell count between the two groups indicated that nearly 30%–35% fewer cells have invaded when PRNP was knocked down (Figure 2f). Colorimetric quantification of the stained invaded cells also showed that there was a 40% reduction in the absorbance in PRNP-knocked down cells as compared with the control (Figure 2g).
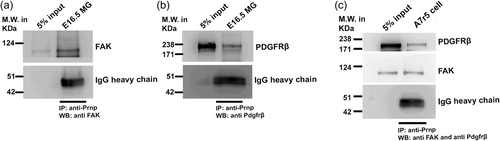
3.3 PRNP forms a termolecular complex with FAK and PDGF receptor β
We sought to investigate the plausible mechanism by which PRNP might influence VSMC migration. Activation of FAK through the PDGF–PDGFRβ signaling pathway, is known to play a key role in VSMC migration. PRNP is known to be attached to the outer leaflet of the plasma membrane through a GPI-anchor. This information led us to hypothesize that PRNP might act as a co-receptor to the PDGFRβ–FAK axis. In line with our hypothesis, both FAK (Figure 3a) and PDGFRβ (Figure 3b) were found to be co-immunoprecipitated with PRNP from the E16.5 metrial gland lysate. To further confirm this interaction, a co-immunoprecipitation assay was performed using VSMC lysate and it was found that PRNP form an immune complex with both FAK and PDGFRβ (Figure 3c).
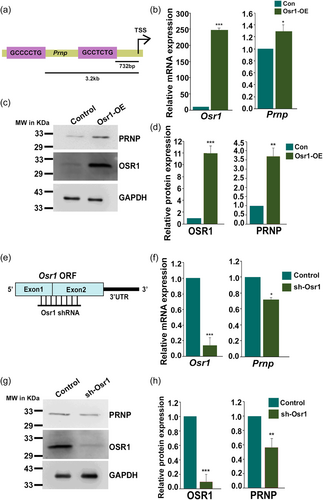
3.4 Odd-skipped-related 1 (Osr1) regulates Prnp in VSMCs
To understand regulation of Prnp expression promoter analysis of rat Prnp gene (ENSRNOG00000021259) was performed. Two putative binding sites for Odd-skipped related-1 transcription factor (GCT/CNCTG; O'Brien et al., 2018) were found at 732 bp and 3.2 kb upstream of the transcription start site (Figure 4a) of Prnp. Full-length Osr1 cDNA (NM_001106716.3) was cloned and expressed in A7r5 cells (Supporting Information: Figure S2A). Elevated levels of Osr1 mRNA and proteins were confirmed by real-time and western blot analysis, respectively (Figure 4b,c). Ectopic overexpression of OSR1 led to elevated levels of Prnp transcripts and protein in A7r5 cells (Figures 4b,c). Figure 4d depicts quantification of western blot analysis shown in Figure 4c. To further authenticate the overexpression data, Osr1 knocked down in A7r5 cells using short-hairpin RNA containing lentiviral particles (Figure 4e and Supporting Information: Figure S2b). As expected, downregulation of Osr1 (Figure 4f,g) resulted in abrogation of Prnp transcripts and protein expression (Figure 4f,g). Figure 4h depicts quantification of western blot analysis shown in Figure 4g.
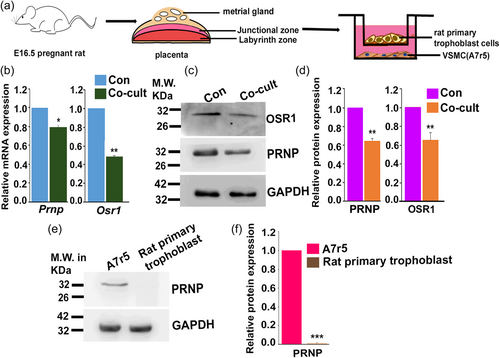
3.5 Trophoblast cells influence Osr1 and PRNP expression in VSMCs
Invasive trophoblast cells are known to influence the phenotypic changes in metrial gland VSMCs located surrounding the uterine spiral arteries (Nandy et al., 2020). We, therefore, assessed the influence of trophoblast cells on OSR1 and PRNP expression by VSMCs by co-culturing VSMCs with trophoblast cells (Figure 5a). Co-culture of VSMCs with rat primary trophoblast cells for 48 h led to downregulating Osr1 and Prnp transcripts (Figure 5b) and protein (Figure 5c,d). As it is evident from the immunoblot assay (Figure 5e,f) that rat primary trophoblast cells do not express PRNP protein, therefore, the observed alteration of Prnp transcript and protein levels during co-culture can be attributed to the VSMC pool only. These data indicate that trophoblast cells might inhibit VSMC migration through OSR1–PRNP axis.
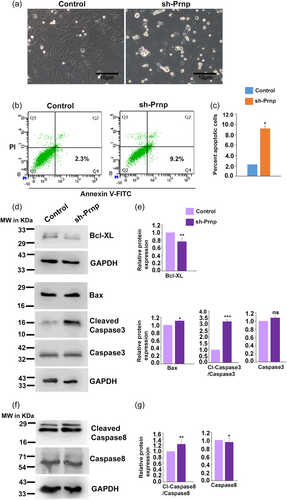
3.6 RNA interference of Prnp promotes apoptosis in VSMC
Apoptosis of VSMCs during pregnancy-related vascular reorganization is a well-characterized phenomenon (Bulmer et al., 2012; Harris et al., 2006; Helwig & Le Bouteiller, 2007; Liu et al., 2022; Whitley & Cartwright, 2009). Leading on from PRNP's ability to promote migratory and invasive properties of A7r5 cells, we postulated that PRNP facilitates the VSMCs to migrate away from the arterial wall, thus averting them from undergoing apoptosis. To test this hypothesis, Prnp was downregulated in A7r5 cells using RNA interference, and morphologically many dead cells were observed in the culture plate as compared to control (Figure 6a). Furthermore, early signs of apoptosis by Annexin-V-PI staining were tested in PRNP-knocked down A7r5 cells using flow cytometric analysis. Knockdown of PRNP substantially increased the percentage of Annexin-V positive and PI negative cells (9.2%) as compared to control (2.3%) cells (Figure 6b,c). Analysis of apoptotic markers was done to further validate this observation. Substantial elevation of the cleaved form of caspase-3 and caspase-8 was observed in PRNP-downregulated cells (Figure 6d–g). Even though total caspase3 was observed to increase in PRNP-downregulated cells, the change is nonsignificant and does not affect the overall rise of its cleaved isoform. However, this phenomenon also aptly points out that overall apoptotic signals are activated in these cells when PRNP is depleted. In line with this, proapoptotic marker Bax increased, although in less amount, and antiapoptotic marker Bcl-XL decreased in PRNP-knocked down VSMCs (Figure 6d–g).
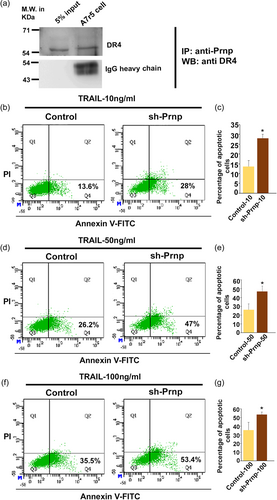
3.7 PRNP inhibits VSMC apoptosis by blocking TRAIL-DR4 ligand–receptor complex formation
Previous studies from our laboratory demonstrated that trophendothelial cells secrete TRAIL, which causes selective demise of endothelial cells but not trophoblast cells (Paul et al., 2022). We, therefore, tested whether PRNP has any role in TRAIL-induced apoptosis. PRNP, being located in the outer leaflet of the plasma membrane, we first tested whether it interacts with TRAIL receptor DR-4. Interestingly, DR4 co-immunoprecipitated with PRNP in A7r5 cells (Figure 7a). This led us hypothesize that PRNP protects VSMCs from TRAIL-mediated apoptosis. Treatment of A7r5 cells with increasing doses of TRAIL (10, 50, and 100 ng/mL) induced more apoptotic death in PRNP-downregulated cells (~15%–20% more) as compared to control (Figure 7b–g). These data indicate that PRNP might prohibit TRAIL binding to its receptor on VSMCs leading to their protection from TRAIL-induced apoptosis.
To summarize, cellular Prion protein expression in the metrial gland of rat is initiated on E11.5. It peaks on E16.5, whereby its interaction with pro-migratory proteins PDGFRβ and FAK potentiate cell migration. Its antiapoptotic property appears to be mediated via its antagonistic effect toward TRAIL-DR4 signaling. An overview of the above concepts has been illustrated in Figure 8.

4 DISCUSSION
In the present study, we have demonstrated the presence of PRNP in VSMCs located at the rat metrial gland, the entry point of uterine arteries into the maternal–fetal interface. The metrial gland is a complex modification of the myometrium of the pregnant uterus which has endocrinological and immunological functions during pregnancy (Ain & Soares, 2004) and the primary site for trophoblast invasion and SAR (Rosario et al., 2008). Spatio-temporal expression analysis of PRNP during gestation showed the highest expression on E16.5, which coincided with maximum trophoblast invasion, as demonstrated previously (Ain et al., 2003). This observation and localization of PRNP in VSMCs, which seem to migrate away from the arterial wall, led us to hypothesize the role of PRNP in uSAR. Interestingly, VSMCs, which are yet to be detached from the tunica media of the spiral arteries, only express both caldesmon and PRNP. The fact that only actively motile VSMCs express prion and not the smooth muscle gene caldesmon refers to a plausible phenomenon where VSMCs achieve migratory potential by switching on endogenous Prion expression. Thus, this finding might project PRNP as a potent marker to characterize these cells.
The mechanism of VSMC migration under normal and diseased conditions has been well studied and elegantly reviewed (Gerthoffer, 2007). Small bio-molecules belonging to diverse chemical and functional families constitute a large consortium of anti-migratory and pro-migratory signaling factors. For example, while PDGF-BB has pro-migratory effect, PDGF-AA is anti-migratory; thus, the net effect on migration depends upon the integration of both the signals (Koyama et al., 1992). Activation of PDGFRβ leads to activation of ERK signaling, which eventually culminates in extension of new actin filaments (Bornfeldt et al., 1995). Formation and degradation of focal contacts is a continuous process that propels the cell forward while still attached to the substratum. Recruitment and activation of FAK by integrin clustering enables cytoskeletal remodeling (Bornfeldt et al., 1995). Activated FAK also forms a complex with the activated PDGFRβ and positively contributes to PDGFBB-induced VSMC migration (Hauck et al., 2000). PRNP is known to promote cancer cell invasion and metastasis by regulating various pathways (Cha et al., 2021; Pan et al., 2006; Wang et al., 2012). Knockdown of PRNP in colorectal cancer cells resulted in reduced metastatic activity both in vitro and in vivo (Pan et al., 2006). In our study, the presence of PRNP in migrating VSMCs led to analysis of ability of PRNP in affecting VSMC migration and as expected, PRNP promotes VSMC migration. It was quite intriguing that PRNP formed a ter-molecular complex with PDGFRβ-FAK. These data clearly indicate that migration promoting function of PRNP is executed through the well-established PDGFRβ–FAK complex. Furthermore, given that PRNP is exclusively located in the outer leaflet of the plasma membrane, our data establish the role of PRNP in regulating “outside-in” migratory signals promoting VSMC migration at the entry site of uterine spiral arteries.
Our finding on OSR-1 transcription factor in regulating PRNP is rather novel. OSR1 was shown to be the earliest marker for intermediate mesoderm, required for patterning and differentiation of kidney precursor cells into nephrons (James et al., 2006), but its expression and role in VSMCs were not known. Our data on expression of OSR-1 in VSMCs indicate that it might have other functions in regulating VSMCs. It is evident that OSR1 indirectly regulates VSMC migration via PRNP and thus plays an important role in uSAR.
Previous studies from our laboratory have shown that VSMCs surrounding the spiral arteries typically remain in a contractile state. VSMC de-differentiation is primarily directed by the invading interstitial trophoblast cells (Ain et al., 2003; Nandy et al., 2020) and trophendothelial cells (Paul et al., 2022). Trophoblast cell-induced de-differentiation leads to phenotypic switching in VSMCs that involve suppression of contractile markers expression and elevation of synthetic state markers (Nandy et al., 2020). During rat gestation, E16.5 is the time point wherein maximum trophoblast invasion occurs and by E19.5 it nears completion. Our data revealed that PRNP expression in the VSMCs is highest at E16.5 and declines at E19.5. This apparent association of trophoblast invasion into metrial gland and PRNP expression in the metrial gland was recapitulated in the in vitro coculture experiments. Primary rat trophoblast cells, isolated from E16.5 placentae, cultured with rat VSMC (A7r5 cells) for 48 h led to significant downregulation PRNP. These observations led to the hypothesis that trophoblast cells regulate PRNP expression and/or function. Interestingly, co-culture of trophoblast cells with VSMCs resulted in downregulation of both OSR1 and PRNP. It is therefore evident that trophoblast cells surveil PRNP expression via OSR1. Furthermore, previous studies from our laboratory demonstrated that co-culture of trophoblast cells with VSMCs resulted in activation of PDGFRβ in VSMCs. It may therefore be inferred that (a) on E16.5 heightened PRNP expression and PDGFRβ activation led to migration of VSMCs away from the uterine spiral arteries, whereas (b) trophoblast surveilled temporal dampening of PRNP expression led to retardation of VSMC movement away from the uterine spiral arteries so as to facilitate the post-partum remodeling of uterine spiral arteries (Rosario et al., 2009).
Previous studies demonstrated that trophoblast cells isolated from first trimester human placenta expressed membrane-associated TRAIL and induced apoptosis of human aortic VSMC (Keogh et al., 2007). Expression of TRAIL in trans-differentiated trophendothelial cells was demonstrated by our laboratory (Paul et al., 2022). Our observation that RNA interference of PRNP in VSMCs caused apoptotic death led to our hypothesis that PRNP protects VSMCs from apoptotic cell death. Furthermore, our data on formation of immune-complex with PRNP and TRAIL receptor, DR4, led us to test whether PRNP can protect VSMCs from TRAIL-induced apoptosis. Antiapoptotic property of prion in neurons has been previously documented (Bragason & Palsdottir, 2005; Roucou et al., 2003). In these studies, using peptide-mimics of PRNP in cytosol they demonstrated its effect on Bax, or its interaction with a proapoptotic protein NRAGE. Since PRNP is typically located in the outer leaflet of the plasma membrane with almost no cytosolic face, our observation on PRNP interaction with DR4, thereby protecting VSMCs from TRAIL-induced apoptosis, seems more realistic. Therefore, our observation certainly put forward a more preferable mechanism by which PRNP confers its antiapoptotic activity. These data suggest that, associated with the onset of immigration of the trophoblast cells to the metrial gland, PRNP levels increase in the VSMCs (E13.5), preventing their untimely death. Concurrently, PRNP potentiates de-differentiated VSMC migration through the surrounding matrix away from the arterial wall, thereby lowering the vessel resistance. VSMC exodus surrounding the spiral arteries creates more space for the trophoblast cells to maximally invade (E16.5), following which trophoblast-surveilled suppression of PRNP expression in the VSMCs, through Osr1, makes VSMCs susceptible to apoptosis.
Taken together, we unveiled hitherto-unknown cellular localization of PRNP and its regulator OSR-1. PRNP's function in various other cell types is well documented (Bendheim et al., 1992). We have demonstrated how PRNP is mechanistically involved in transforming the VSMCs from a static to a motile phase and how PRNP provides protection from TRAIL-induced apoptosis. Our data highlight a novel role of PRNP in VSMC fate that determines the dynamics of uSAR, a hallmark developmental event in placental morphogenesis, surveilled by various functions of invading interstitial trophoblast cells and trophendothelial cells (Ain & Soares, 2004).
AUTHOR CONTRIBUTIONS
Rumela Bose and Rupasri Ain designed research. Rumela Bose performed all experiments. Sarmita S. Jana cloned shRNAs and Rumela Bose cloned full-length Osr1 cDNAs used in the manuscript. Data were analyzed by Rumela Bose and Rupasri Ain. The first draft of the manuscript was written by Rumela Bose. The manuscript was edited and finalized by Rupasri Ain. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank the flow cytometry core facility of CSIR-IICB for aiding in data acquisition. Financial support from CSIR in the form of Shyama Prasad Mukherjee fellowship to Rumela Bose and Research Associateship to Dr. Sarmita S. Jana is gratefully acknowledged. This work was supported by a grant from the Indian Council of Medical Research (2019-1323 to Rupasri Ain).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data presented in the study are included in the article and in the Supplementary Material.