Specific ablation of Hippo signalling component Yap1 in retinal progenitors and Müller cells results in late onset retinal degeneration
Abstract
Yes-associated protein (YAP) is a major component of the Hippo pathway involved in development, growth, repair and homeostasis. Nonsense YAP1 mutations in humans result in autosomal dominant coloboma. Here, we generated a conditional knockout mouse model in which Yap1 was specifically deleted in embryonic retinal progenitor cells (RPCs) and in mature Müller cells using a Chx10-Cre driver. Our data demonstrated that the conditional ablation of Yap1 in embryonic RPCs does not prevent normal retinal development and caused no gross changes in retinal structure during embryonic and early postnatal life. Nevertheless, Yap1 deficient in retinal Müller cells in adult mice leads to impaired visual responses and extensive late-onset retinal degeneration, characterized by reduced cell number in all retinal layers. Immunofluorescence data further revealed the degeneration and death of rod and cone photoreceptors, bipolar cells, horizontal cells, amacrine cells and ganglion cells to varying degrees in aged knockout mice. Moreover, alteration of glial homeostasis and reactive gliosis were also observed. Finally, cell proliferation and TUNEL assay revealed that the broad retinal degeneration is mainly caused by enhanced apoptosis in late period. Together, this work uncovers that YAP is essential for the normal vision and retinal maintenance, highlighting the crucial role of YAP in retinal function and homeostasis.
1 INTRODUCTION
The vertebrate eye contains a precise retinal circuitry for vison formation by converting light stimulus into neural impulse. The mature retina is mainly composed of seven cell types: rod and cone photoreceptors in outer nuclear layer (ONL); horizontal cells (HCs), bipolar cells (BCs) and amacrine cells (ACs) in inner nuclear layer (INL); output neurons—retinal ganglion cells (RGCs) in ganglion cell layer (GCL); and a unique glial cell type—the Müller glia. All these retinal cell types and subtypes are derived from a common pool of multipotent retinal progenitor cells (RPCs) in a precise order. RGCs are generated first, followed by HCs, cones, ACs, rods, BCs and finally Müller glia (Raeisossadati et al., 2021). In mice, retinal neurogenesis begins at the center of the optic cup at embryonic day 11 (E11) and expands peripherally, reaching the distal-most region by E16 (Hinds, 1968; Sidman, 1960). During early development period, the retina is formed from the invagination of the dorsal aspect of the optic vesicle (OV). The OV invaginates to form a two-layered tissue of neuroepithelial precursor cells referred to as the optic cup (OC). The outer layer of the OC develops into a nonneural, pigmented sheet called retinal pigment epithelium (RPE), which surrounds the entire inner layer of the neural retina (NR). NR development involves proliferation of self-renewing and multipotential retinal progenitors that eventually differentiate to give rise to all seven retinal cell types organized into a tri-laminated structure (Cepko et al., 1996).
Among the seven RPC-derived retinal cell types, Müller cells are a type of radial glia only found in the retina, which represents approximately 4%–5% of retinal cells (Jeon et al., 1998). They span the entire thickness of the retina, displaying intimate contact with adjacent neurons and retinal blood vessels. Indeed, Müller cells play a wide variety of roles that contribute to the maintenance of retinal homeostasis and visual function, including a trophic support and clearing metabolic waste for retinal neurons in the healthy retina (Bringmann et al., 2006; Reichenbach & Bringmann, 2013). When the retina is injured, they respond locally and rapidly to injury and undergo reactive gliosis, which includes changes in morphology, upregulation of various markers, dedifferentiation, nuclear migration to the apical surface (Bringmann et al., 2009; Dyer & Cepko, 2000), thus eventually safeguard the retinal structure and repair the damaged retina. If they cease to support retinal neurons, they convert to facilitating neurodegeneration (Bringmann & Wiedemann, 2012).
The Hippo signalling pathway controls development, organ growth, homeostasis, and cancer by regulating diverse biological processes including cell proliferation, differentiation, and apoptosis (Dong et al., 2007; Meng et al., 2016; Yu et al., 2015). As a transcription coactivator, Yes-associated protein (YAP), encoded by the gene Yap1, is a major component of the Hippo pathway. The Hippo pathway exerts regulation effect in response to diverse stimuli such as mechanical stress, DNA damage, or oxidative stress (Di Cara et al., 2015). When the pathway is suppressed, YAP and its homolog TAZ migrate from the cytoplasm to the nucleus and interacts with several DNA-binding partners including TEAD1-4, thereby driving transcription of target genes involved in cell proliferation and differentiation (Zhao et al., 2010). Conversely, activation of the Hippo pathway induces phosphorylation of YAP1/TAZ, leading to their return to the cytoplasm, thus inhibiting their effect on transcription (Hauri et al., 2013).
It is well documented that the Hippo signalling could regulate eye development, which dysfunction in animal models results in severe ocular defects (Lee et al., 2018; Moon & Kim, 2018). Heterozygous loss-of-function mutations in YAP1 in humans leads to autosomal dominant coloboma and the missense mutation of its cofactor TEAD1 causes Sveinsson's chorioretinal atrophy, an autosomal dominant loss of RPE, choroid, and photoreceptors radiating from the optic nerve (ON) head (Fossdal et al., 2004; Williamson et al., 2014). Of note, YAP has been reported that strongly expressed in multiple ocular tissues at different period. During retinal development, YAP is expressed in all OV compartments, becoming more prominently expressed in the RPE and the ciliary margin of the optic cup (Kim et al., 2016; Williamson et al., 2014). In adult mouse eye, YAP expression was evident within an array of ocular tissues, including the corneal epithelial and endothelial cells, lens epithelial cells, ciliary body (CB), iris, INL of the retina, and the RPE (Hamon et al., 2017). Several studies in mice and zebrafish have suggested YAP could participate in the early ocular development and corneal wound healing, and loss of Yap1 leads to complex ocular abnormalities, including microphthalmia, corneal fibrosis, anterior segment dysgenesis, and cataract (Q. He et al., 2019; Holt et al., 2017; Miesfeld et al., 2015; Raghunathan et al., 2014; Williamson et al., 2014). Recent work indicated that retinas in Yap1 heterozygous mice exhibited altered Müller cell homeostatic function, and causing late-onset cone degeneration (Masson et al., 2020). Although these data suggest YAP function in Müller cells for the preservation of cone integrity, the specific requirement for YAP in in other components of retina has not been fully explored.
Given the distinct expression pattern of YAP in embryonic RPCs and in Müller cells during adult stage, we hereby generated mice with conditional disruption of Yap1 in these retinal cells at different stage using Chx10-Cre, and assessed effects on retinal development and homeostasis in the retina. Our data demonstrated that the conditional knockout of Yap1 in embryonic RPCs caused no visible gross changes in the retinal structure during embryonic and early postnatal life. Nevertheless, Yap1 depletion in retinal Müller cells at adulthood leads to impaired visual responses and extensive late-onset retinal degeneration, characterized by reduced cell number in all retinal layers. Immunofluorescence data further manifested the degeneration and death of rod and cone photoreceptors, rod bipolar cells (RBCs), HCs, ACs and RGCs to varying degrees. Moreover, alteration of glial homeostasis and reactive gliosis were also observed. Cell proliferation and TUNEL assay revealed that the broadly retinal degeneration is mainly caused by enhanced apoptosis in late period. Overall, this work provides a novel late onset retinal degeneration model and highlights the essential roles of YAP in retinal function and homeostasis.
2 MATERIALS AND METHODS
2.1 Mouse models
All animal experiment protocols were approved by the Institutional Animal Care and Use Committee of Sichuan Provincial People's Hospital (Chengdu, Sichuan, China) and conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All animals were housed in a temperature-controlled room (24°C) with a 12-h light/dark cycle. Mice were maintained on a C57BL/6J background.
Mice with Yap1 deletion specifically in retinal in embryonic RPCs and in mature Müller glial cells were generated using the Cre-loxP system. Briefly, Yap1flox/flox mice (Yap1tm1.1Dupa/J, Jackson Laboratory, stock number: 027929) (N. Zhang et al., 2010) were crossed with the Chx10-Cre transgene (Jackson Laboratory, stock number: 005105) (Rowan & Cepko, 2004) transgenic mice to yield progeny with the genotype Yap1loxP/+; Chx10-Cre. Then, the Yap1loxP/+; Chx10-Cre mice were crossed with Yap1flox/flox animals to generate Yap1flox/flox; Chx10-Cre (Yap1ChxKO) mice. Yap1flox/flox littermates were used as controls. In addition, to monitor the expression pattern of the Cre enzyme, a tdTomato reporter gene was used (strain name: Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J; Jackson Laboratory, stock number: 007914). In the presence of the Cre enzyme, the stop codon before the tdTomato expression cassette was removed, which permits the expression of tdTomato (red fluorescence) in Cre-positive cells.
2.2 Genotyping
Mouse genomic DNA samples were extracted from mouse tails and genotyped using PCR to screen for the floxed Yap1 alleles using the primers Yap1-flox-F (5′-AGGACAGCCAGGACTACACAG-3′) and Yap1-flox-R (5′-CACCAGCCTTTAAATTGAGAAC-3′). Chx10-Cre was genotyped using the generic Cre primers Cre-F (5′-TGCCACGACCAAGTGACAGCAATG-3′) and Cre-R (5′-ACCAGAGACGCAAATCCATCGCTC-3′). The tdTomato mice were genotyped using the following primers provided by the JAX mouse service: oIMR9020, 5′-AAGGGAGCTGCAGTGGAGTA-3′; oIMR9021, 5’-CCGAAAATCTGTGGGAAGTC-3′; oIMR9103, 5′-GGCATTAAAGCAGCGTATCC-3′; and oIMR9105, 5′-CTGTTCCTGTACGGCATGG-3′. All amplification reactions were performed using a master mix (Invitrogen) according to the manufacturer's instructions. The first cycle of PCR consisted of holding at 95°C for 5 min, followed by 32 cycles of 95°C for 15 s, 60°C for 30 s and 72°C for 30 s. The PCR products were separated by DNA electrophoresis on a 3% agarose gel.
2.3 Electroretinography (ERG)
For electroretinographic evaluation, following overnight dark adaptation, mice were anesthetized with an intraperitoneal injection of xylazine (80 mg/kg) and ketamine (16 mg/kg) in normal saline. Additional anesthetic was given if akinesia was inadequate. The equipment and protocol used here have been described previously (Yang et al., 2019). Briefly, the responses of dark-adapted, rod-mediated ERGs to short-wavelength flashes generated by a photopic stimulator over 4.0-log units to the maximum intensity were recorded. Cone-mediated ERGs were recorded in response to white flashes after 10 min of complete light adaptation. The signals were sampled at 0.8 ms intervals and averaged.
2.4 Histological analysis
For hematoxylin and eosin staining (H&E), enucleated eyes from control and Yap1ChxKO mice were fixed overnight in 1.22% glutaraldehyde and 0.8% paraformaldehyde in 0.08 M phosphate buffer, embedded in paraffin and then cut into 5 μm sections. To ensure that sections used for quantification came from the same eccentricity, the globe was embedded in the same orientation. Sections that encompassed the ON were selected for staining with H&E according to a standard protocol.
2.5 Semithin-section processing of the ON
ONs were dissected from eyes and treated with a solution containing 4% paraformaldehyde and 0.2% glutaraldehyde for 24 h. After rinsing three times in 0.1 M phosphate buffer (pH 7.4) for 15 min each, tissues were fix with 1% osmic acid and 0.1 M phosphate buffer (pH 7.4) at room temperature (20°C) for 2 h. After rinsing three times in 0.1 M phosphate buffer (pH 7.4) for 15 min each. Stained tissues were dehydrated in graded concentrations of ethanol, embedded in acetone: 812 embedding medium = 1:1 for 2–4 h, and in acetone: 812 embedding medium = 1:2 infiltration, overnight, and finally in 812 embedding medium for 5–8 h. Samples were inserted into an embedding plate containing 812 embedding medium, and placed overnight in an incubator at 37°C, followed by polymerization at 60°C for 72 h. Semithin Section (1 μm) were cut transversely from segments of ONs caudal to the eyeball and stained with 1% toluidine blue.
2.6 Immunohistochemistry
For immunohistochemistry, eyes were removed from euthanized mice by intraperitoneal injection of pentobarbital (75 mg/kg) and by cervical dislocation and fixed in 4% paraformaldehyde in 100 mM phosphate buffer (pH 7.4) for 1 h at 4°C, followed by cryoprotection in 30% sucrose for 2 h. The lenses were removed, and the eyes were embedded in optimal cutting temperature (OCT) solution and sectioned at a 10 μm thickness. After blocking and permeabilization with 10% normal donkey serum and 0.2% Triton X-100 in phosphate buffer for 1 h, the sections were labeled with the primary antibody overnight at 4°C. The primary antibodies used are shown in Table S1. The sections were rinsed in phosphate-buffered saline (PBS) three times, Alexa Fluor 594/488-conjugated goat anti-mouse/rabbit secondary antibody (Cat #A11005 and A11008, 1:500 dilution; Invitrogen) was applied, and nuclei were counterstained with DAPI (Cat #D8417; Sigma) for 1 h at room temperature. Images were captured on a Zeiss LSM 800 confocal scanning microscope. In addition, the fluorescence intensity was quantified using ZEN 2.3 imaging software.
2.7 Retinal flat mounts and cell count
Retinas were dissected from enucleated eyes and flattened. Retinas were immersed in 4% paraformaldehyde for 24 h at 4°C, then blocked in PBS containing 1% bovine serum albumin and 0.5% Triton X-100 and incubated with polyclonal rabbit anti-NeuN antibody (Cat #24307, 1:200 dilution; CST) for 12 h at 4°C. After several washes, the retinas were incubated with the secondary antibody, Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (Cat #A11008, 1:250 dilution; Invitrogen) for 4 h at room temperature, and subsequently washed with PBS. To precisely evaluate the loss of RGCs, each retinal quadrant was divided into four zones—nasal, temporal, dorsal, and ventral—each located 1 mm from the ON head. Cells in GCL were counted in each unit area by two investigators in a blinded fashion, and the scores were averaged.
2.8 5-Ethynyl-2′-deoxyuridine (EdU) labeling assay
To detect cell proliferation in retinas, mice at P3, P30, 12-month old and pregnant females were first weighed and injected intraperitoneally with EdU (Cat #E10187; Thermo) (100 mg/gm of body weight) in PBS. The treated mice were killed after 3 h. EdU-positive cells were subsequently stained with the Click-iT EdU Alexa Fluor-488 Imaging Kit (Cat #C10337; Thermo). For cell cycle exit analysis, sectionswere double labeled with EdU (S-phase marker) and cell proliferation marker (Ki67).
2.9 Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End labeling (TUNEL) assay
Apoptotic cell death was detected by the TUNEL assay according to the manufacturer's protocol (Cat #11684795910; Roche Diagnostics) using prepared frozen sections. Images were captured on a Zeiss LSM 800 confocal scanning microscope.
2.10 Western blot
Tissues were lysed in radioimmunoprecipitation assay buffer containing a protease inhibitor cocktail (Cat #11697498001; Roche) and a phosphatase inhibitor (Cat #4906845001; Roche). The protein concentration of the lysates was determined using a DC Protein Assay according to the manufacturer's instructions (Cat #500-0122; Bio-Rad). Equal amounts of protein were separated on SDS polyacrylamide gels and transferred onto NC membranes (Cat #HATF00010; Millipore). The blots were blocked with 8% nonfat dry milk in Tris-buffered saline solution with Tween® 20 detergent for 2 h at room temperature. Then, the membranes were incubated with the primary antibodies in blocking solution overnight at 4°C. The primary antibodies used for western blotting are shown in Table S1. The primary antibodies were detected with anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:5000; Bio-Rad), and the signal was developed using SuperSignal™ West Pico PLUS Chemiluminescent Substrate (Cat #34577; Thermo). The relative intensity of the immunoreactive bands was quantified using the gel analysis tool provided in ImageJ software. The intensity of the proteins of interest was normalized to that of glyceraldehyde 3-phosphate dehydrogenase. At least three independent western blots were conducted, and one typical blot is presented.
2.11 Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software. The datasets were tested for normal distribution using Shapiro–Wilk test. For normally distributed data, statistical significance was determined by Student's t test or analysis of variance (ANOVA). If the data was not normally distributed, nonparametric statistic was used. All p values were calculated by Student's t test or ANOVA followed by a, Tukey, Dunnett or Sidak's multiple comparisons test as appropriate. A p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Generation of Yap1 conditional knockout mice using Chx10-Cre lines
Previous studies have demonstrated that Yap1 is widely expressed in developing eyes as well as in RPE, CB, cornea and retinal Müller cells in adult mouse (Hamon et al., 2017; Williamson et al., 2014). To confirm the expression pattern of Yap1 in both embryonic and adult retinas, cryosections of E14.5 mouse embryos and retinas from postnatal 30 (P30) mouse were prepared and immunostained with the primary antibodies anti-YAP. In wildtype embryonic E14.5 retinae, YAP exhibit ubiquitous expression in the NR and in particular were enriched in neuroblastic layers (NBL) (Figure 1a), where mainly retinal progenitors reside. In contrast, post-mitotic GCL cells presented little YAP expression (Figure 1a), suggesting specific localization of YAP in retinal progenitors. In adult mouse retina, the signal intensity of YAP was restricted in the ON, CB, as well as INL (Figure 1b, upper panels), and their position and morphology in INL strongly suggests that YAP could be specifically expressed in Müller glial cells (Figure 1b, lower panels). Additionally, we analysed the protein expression profiles of YAP in mouse retina at different ages. As shown in Figure 1c, yap was steady expressed during embryonic (E14.5) and early postnatal life (P3), while it showed approximately 1.67-fold increase in maturated retinal neurons (2-month old) (Figure 1c,d). Interestingly, YAP expression level was decreased in aged retinas (12-month old) compared to that in 2-month-old mice, while it's still slightly higher than that at embryonic stage (Figure 1c,d).
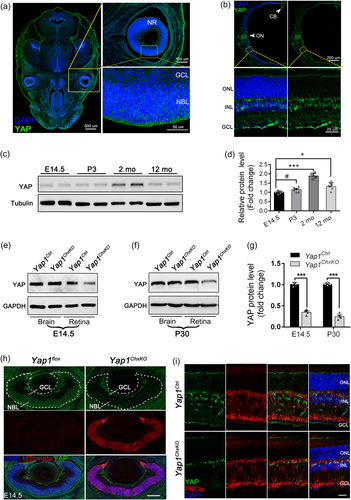
To investigate the underlying role of YAP specifically in embryonic and adult mouse retina, we developed Yap1 conditional knockout mice by crossing Yap1 exon 1–2 floxed mice (Yap1flox/flox) with BAC-Tg Chx10-Cre mice (Figure S1a), which droves Cre expression in the majority of E9-P2 RPCs, as well as in a subset of retinal Müller cells at adult stage (Kowalchuk et al., 2018; Lu et al., 2013; Ueki et al., 2009). Their progenies were intercrossed to yield littermate Yap1flox/flox; Chx10-Cre (hereafter Yap1ChxKO), Yap1flox/+; Chx10-Cre (hereafter Yap1Het) and Yap1flox/flox (hereafter Yap1Ctrl, served as control) mice (Figure S1b). Moreover, a strong Cre reporter line, ROSA26-tdTomato, was introduced to monitor the specific expression of Chx10-Cre. We crossed ROSA26-tdTomato reporter mice with Yap1flox/flox; Chx10-Cre mice to generate Yap1flox/flox; Chx10-Cre; Rosa mice (Figure S2a). A loxP-flanked STOP sequence before the tdTomato expression cassette is deleted in Cre-expressing cells, and subsequently, tdTomato is expressed.
Immunostaing data of tdTomato-expressing retinas from E14.5 and P30 mice suggested that the tdTomato was expressed distinctly in the NR as well as Müller glial cells in E14.5 and P30 mice retina respectively (Figure S2b,c), verifying the specific expression of this Cre line. Besides, we found that YAP distinctly expressed in the ROSA+ Müller glial cells in adult Chx10-Cre; ROSA mice (Figure S2d), confirming the specific YAP expression in mature retinas. To further evaluate the knockout efficiency of YAP in these cells, we prepared cerebral and ocular/retinal lysates from E14.5/P30 Yap1ChxKO mice and subjected to immunoblotting analysis. The expression level of YAP in E14.5 Yap1ChxKO eyes was reduced to approximately 36% of that in controls (Figure 1e and 1g), and reduced to approximately 27% in P30 Yap1ChxKO retina (Figure 1f,g), while the YAP expression in both Yap1ChxKO cerebral cortex did not change (Figure 1e,f). Considering that YAP is also expressed in other retinal cells, the excision efficiency was fairly good. The immunostaining results further verified this. The YAP signals were robust in the tdTomato expressed NR in control retinas but were almost absent in the Yap1ChxKO NR (Figure 1h). Besides, the sections of P30 mice retinas were co-immunostained with YAP and PKCα, a marker for RBCs. Although still expressed in PKCα marked by RBCs, YAP staining disappeared in areas where Müller cells are mainly occupied (Figure 1i). Together, these results suggested the precise excision of YAP in embryonic RPCs and retinal Müller cells.
3.2 Yap1 depletion in RPCs has no detectable effect on retinal structure in embryonic and neonatal mice
Previous studies have implicated YAP in retinal development by modulate retinal cell proliferation (Kim et al., 2016; H. Zhang et al., 2012). In present study, Cre-mediated recombination in Yap1ChxKO mice led to YAP ablation in the majority of retinal progenitors in E9-P2 mice, therefore we set out to assess the consequences of the specific excision of YAP in developing retinas. We first compared histologic sections from E14.5 control and Yap1ChxKO retinas at the early stages of retinal development (Figure S3a). In control retinas, the NBL zone was occupied by proliferating progenitor cells and the inner retina distributed postmitotic neurons. Regrettably, the Yap1ChxKO retina presented no visible defects on the gross retinal mmorphology compared with controls (Figure S3a, upper panels). To examine the differentiation of retinal progenitors, position-matched sections of the H&E staining were immunostained with Brn3a, which marks the postmitotic neurons in embryonic retina (Wang et al., 2002). However, in line with histologic result, E14.5 Yap1 deficient and control retinas contained comparable proportions of Brn3a positive postmitotic neurons (Figure S3a, lower panels), indicated that there was no detectable abnormality in retinal development of Yap1 mouse in this stage.
We next examine the P9 retina to evaluate the role of Yap1 at the late periods of retinal development after birth. Likewise, the Yap1ChxKO retina appeared to resemble the control retinas in morphology, exhibiting comparable thicknesses of outer and INLs (Figure S3b). Surprisingly, no obvious alterations was observed neither in the thickness of ONL/INL nor in cell number of GCL in Yap1ChxKO mouse retina at 4-mouths age, compared to those in control littermates (Figure S4). Overall, these observations suggested that Yap1 depletion in RPCs has no detectable effect on retinal structure in embryonic and neonatal mice.
3.3 YAP is essential for the normal visual function and retinal maintenance in adult retinas
Since YAP is also expressed in Müller cells which maintain retinal function and homeostasis at the adult period of mice, we next evaluate the physiological status of the retina at the adult period using photopic and scotopic electroretinograms (ERGs) of control and Yap1ChxKO mice at 10 months of age. Scotopic ERG recordings, which detect the responses of rod-driven circuits, revealed reduced amplitudes of both a-waves and b-waves compared with those of the controls at each flash intensity (Figure 2a). The mean amplitudes of the a- and b-waves of Yap1ChxKO mice were decreased by approximately 56.7% and 52.5%, respectively (Figure 2c,d). The dark-adapted amplitude reductions for both a- and b-waves suggested that Yap1ChxKO retinas not only have photoreceptor dysfunction, but also deficits in visual signal transmission from photoreceptors to inner neurons. Likewise, the photopic b-wave and flicker amplitudes, which mainly reflect the status of cone cells, were also decreased in Yap1ChxKO mice (Figure 2b and 2e).
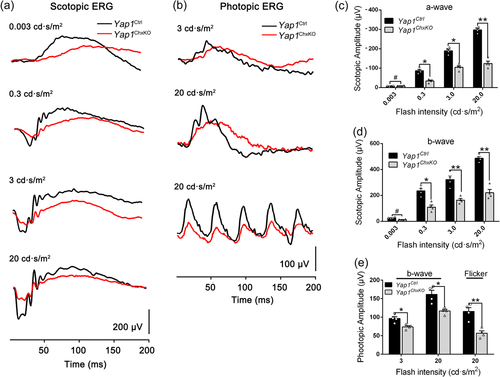
Visual functional analysis ofYap1ChxKOmice. (a) Representative electroretinogram (ERG) traces of retinas in Yap1Ctrl and Yap1ChxKO mice (10-month old) under scotopic conditions at flash intensities from 0.003 to 20 cd·s/m2. (b) Representative ERG traces corresponding to responses elicited by photopic conditions at flash intensities of 3 and 20 cd s/m2 in mice at 10 months of age. (c, d) Statistical analysis of the amplitudes of the (c) a-waves, (d) b-waves under scotopic conditions were presented (n = 4 in each group). (e) Statistical analyses were performed for the amplitudes of b-wave and flicker in photopic conditions (n = 4 in each group). *p < 0.05; **p < 0.01. #, no significant difference. The data are represented as means ± SEM.
Moreover, the total amplitude of oscillatory potentials (OPs), which serves as a sensitive functional indicator of inner retina (Heynen et al., 1985), was reduced to 43%, 49%, 57% of that of controls in 0.3, 3, and 20 cd·s/m2, respectively, in Yap1ChxKO mice (Figure S5). The reduction of OPs amplitude provided additional evidence for the impaired physiological status within the Yap1ChxKO inner retina. Together, ERG data suggested that the visual function of Yap1ChxKO mice was significantly compromised by the loss of YAP.
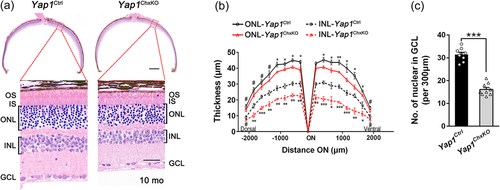
To determine the pathological changes underlying the abnormal ERG results, histologic analysis was performed to examine the retinal morphology in Yap1ChxKO retinas at 10 months of age. In accordance with the ERG results, the Yap1ChxKO mice showed a remarkable thinning of outer and INLs that worsened toward the central retina, accompanied by a shorter outer segment (OS) (Figure 3a,b). Besides, a 53.2% decrease in GCL cell number was observed compared to that in control retinas (Figure 3c), indicating significant RGC loss. Moreover, this retinal degeneration worsened in Yap1ChxKO mice at 14 months of age (Figure S6). We, however, did not detect any alterations neither in thickness of ONL/INL nor in cell number of GCL in YapHet mouse retina, compared to those in control littermates (Figure S6).
3.4 Degeneration of rod and cone photoreceptors in Yap1ChxKO retinas
Retinal ONL consists of the nuclei of photoreceptors, which are classified into two types according to differences in morphology and function: rods for dim light vision and cones for daylight and high-acuity vision. To characterize the specific cell types affected in ONL of Yap1ChxKO retina, immunostaining was performed against antibodies of Rhodopsin (rod OS marker) and NaK pump (rod IS marker) to analyze the subcellular changes in rods using retinal sections from 10-month-old mice. Though the rhodopsin marked OS was well organized and the signal was strictly restricted to the rod OS, the Yap1ChxKO mice showed a decrease in the length of the OS in the central retina, with a nearly 50% reduction (9.73 ± 0.6 vs. 17.36 ± 0.9 mm) in OS length near the ON (Figure 4a,b). This result was further confirmed by the immunoblotting: a approximately 43% reduction rhodopsin content was observed in Yap1ChxKO retinas (Figure 4c), suggesting degeneration of rod cells.
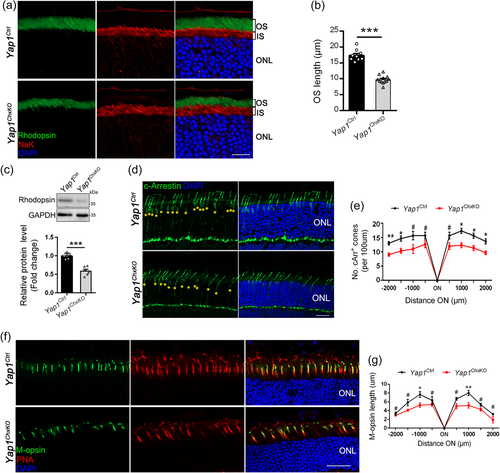
To determine the effect of YAP deficiency on cone photoreceptors, we immunolabeled retinal cryosections from 10-month-old control and Yap1ChxKO mice with cone arrestin (cArr), which revealed a reduction in cone number throughout the Yap1ChxKO retina (Figure 4d,e), compared to that of controls. Moreover, the retinal sections were coimmunostained with an L/M-opsin antibody to mark the cone OS and Alexa Fluor-594-conjugated PNA, which mainly labels the cone matrix sheaths associated with all cones. As expected, a mildly decrease in the number of M-cone cells was observed in the Yap1ChxKO retina, with a shortened, disorganized, and misshapen cone OS compared to that of controls (Figure 4f,g), suggesting that YAP depletion also results in the degeneration of cone photoreceptors.
3.5 Yap maintains the survival of multiple cell types in INL
INL, the second-order neurons layer in retinal system, plays essential roles in transmitting and integrating visual information from photoreceptors to third-order retinal neurons, such as the ganglion cells. ERG recordings revealed the reduced amplitudes of scotopic b-wave and OPs, suggesting dysfunction of inner retina. We, therefore, used cell type specific antibody markers to label and quantify RBCs, HCs, and ACs, which constitutes the major neuronal circuits in INL. Immunostaining using RBC specific marker PKCα revealed the malformation and loss of RBCs in Yap1ChxKO retina compare to that of controls (Figure 5a). The mean numbers of PKCα-positive cells in the control and Yap1ChxKO mice were 19.8 ± 0.8 and 12.9 ± 1.3 cells/200 μm of retina length, respectively (Figure 5a,b), suggesting a nearly 35% reduction in PKCα-positive RBC number.
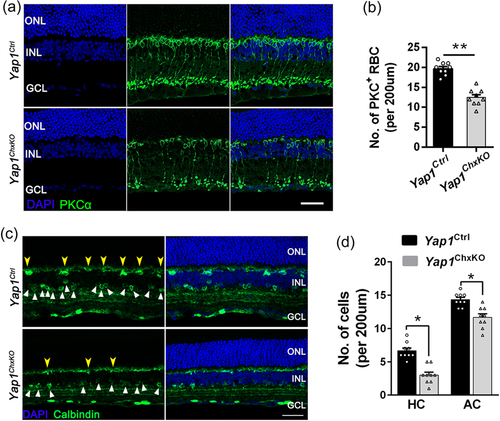
To detect the status of HCs and ACs, we performed immunostaining on retina sections from 10-month-old mice for anti-Calbindin, which marks HC and AC somata and dendrites in retina (Soto et al., 2013). In Yap1Ctrl retinas, intensely stained Calbindin-positive HC somata and dendrites are positioned at the border between INL and OPL, while lightly-stained Calbindin-positive AC somata are located in the inner INL and IPL (Figure 5c). Compared to the controls, Yap1ChxKO retinas had fewer and fainter Calbindin-positive HCs (Figure 5c,d, yellow arrowheads) and ACs (Figure 5c,d, white triangles). Together, these observations revealed the death of multiple neuronal cell types in Yap1ChxKO INL, which led to the significantly thinner INL and compromised inner circuits in aged Yap1ChxKO retinas.
3.6 RGC death and ON degeneration in Yap1ChxKO retinas
Given impaired OPs amplitude and remarkably cell loss in GCL were observed in Yap1ChxKO mice, we next set out to investigate the GCL pathologies. To directly visualize the cell content in GCL of control and Yap1ChxKO mice, wholemount retinas from 10-month-old mice were immunostained with a specific neuronal marker NeuN, which labels cells resided predominantly in the GCL (Tan et al., 2020). To this end, we imaged four equivalent prescribed areas each located 1 mm from the ON head (nasal, temporal, ventral, and dorsal) per retina and quantified the cell density of GCL by determining NeuN-positive cells per unit area. In Yap1ChxKO retinas, a significant decrease in NeuN-positive cells in all four quadrants was observed (Figure 6a). Quantitative analysis revealed a 28.6%; 26.7%; 25.4%, and 26.1% reduction of NeuN-positive cells compare to that of WT in nasal, temporal, ventral, and dorsal area, respectively (Figure 6b). To observe RGCs in the GCL, immunohistochemical analysis of Brn-3a (RGC marker) was performed in 10 months Yap1Ctrl and Yap1ChxKO retinas. The Brn3a immunofluorescence was precisely restricted within the GCL in the two groups (Figure 6c). Consistent with the results of NeuN immunofluorescence, the Brn3a staining revealed that the mean numbers of Brn-3a-positive cells were 19.7 ± 1.2 and 13.8 ± 0.8 cells/200 μm of retina length in the Yap1Ctrl and Yap1ChxKO groups, respectively, indicating a significant reduction of RGCs in Yap1ChxKO retinas (Figure 6d).
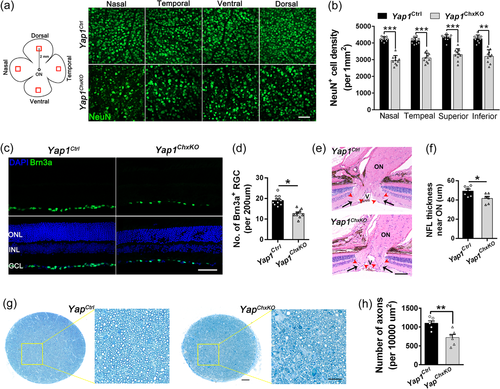
Injury to RGCs, central nervous system neurons that relay visual information to the brain, often leads to RGC axon degeneration. This prompted us to further evaluate the ON status in degraded Yap1ChxKO retinas. Histological analysis revealed the thinning of the NFL near ON in Yap1ChxKO mice at 12 months of age (Figure 6e,f). To determine details of axonopathy, toluidine blue-stained semithin sections of the ON from 12-month-old animals were prepared and the number of axons were quantified. Compared with control mice, the axons density was clearly lower in Yap1ChxKO mice and accompanied with axonal swelling and extensive myelin debris (Figure 6g,h), indicating apparent axonal damage.
3.7 Müller glial malformation and reactive gliosis in Yap1ChxKO retinas
Given the Yap1 was specifically removed in the mature retinal Müller cells, we next sought to investigate the pathologic changes of Müller glia cells in 10-months-old Yap1ChxKO retinas, through using antibodies directed against glutamine synthetase (GS) to visualize cell bodies and processes of Müller glia cells. In control retinas, Müller glia cell bodies were orderly aligned in the INL, and their cell processes span across the retina and form the outer and inner limiting membranes (Figure 7a). By contrast, uneven distribution of GS-stained cell bodies was indicated in the Yap1ChxKO retina, accompanied by the fewer and disorganized Müller glial cells (Figure 7a). The mean numbers of GS-positive Müller cells were 49.8 ± 1.1 and 40.9 ± 0.7 cells/250 μm of retina length in the Yap1Ctrl and Yap1ChxKO retinas, respectively (Figure 7b), indicated dysfunction and loss of Müller cells.
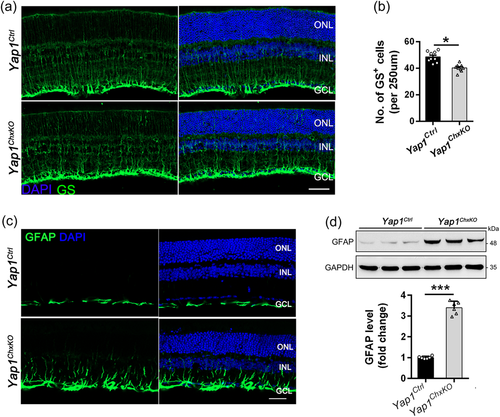
Meanwhile, immunostaining of retinal sections also revealed that the expression of glial fibrillary acidic protein (GFAP) was dramatically upregulated in degenerating Yap1ChxKO retinas compared to control retinas (Figure 7c). Western blot analysis further confirmed this result, as revealed by the increased GFAP protein level in the Yap1ChxKO retina (Figure 7d). These results suggested reactive gliosis and that Müller cells were activated as an indicator of retinal damage.
3.8 Yap1ChxKO retinas showed normal cell proliferation during early retinogenesis but increased apoptosis at late stage
We further assessed the proliferation potential of RPCs using an in vivo EdU labeling assay. We pulsed E14.5 pregnant females with EdU followed by embryo harvest after 3 h. Retinal sections from E14.5 embryos and P3 mice were subjected to immunostaining with Ki67, along with EdU detection (Figure 8a and 8d). The numbers of Ki67+ cells exhibited no difference between the Yap1Ctrl and Yap1ChxKO samples at both ages, indicating that RPCs maintain the normal ability to proliferate in Yap1ChxKO NBL, although with a slightly reduced rate (Figure 8a,b and 8d,e). Moreover, we quantified EdU+ (S-phase) RPCs as a percentage of total Ki67+ RPCs, which also showed no difference in S-phase RPCs in Yap1ChxKO retinas compared to Yap1Ctrl (Figure 8a–d and 8f). In addition, P30 and 8-month-old littermates were also accessed, while no proliferating cells was detected in both Yap1ChxKO and Yap1Ctrl retinas (Figure S7).
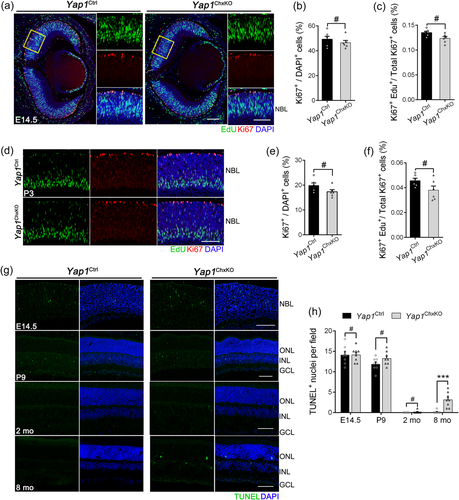
To investigate whether the YAP deficiency-induced retinal degeneration observed in aged mice could be linked to an increased cell death of the retinal cells, we investigated the extent of apoptosis in retina by TUNEL staining. Although no significant difference between Yap1ChxKO and Yap1Ctrl mice in E14.5, P9 and 2-month old, our TUNEL assay data showed that apoptotic cells were dramatically increased in 8-month-old Yap1ChxKO mice retinas, especially in the ONL and INL, compared to that of Yap1Ctrl mice (Figure 8g,h). These data indicated that the broad retina degeneration in aged Yap1ChxKO mice should be mainly caused by increased cell apoptosis at late stage, rather than abnormal retinal cell proliferation during embryonic and early postnatal life.
4 DISCUSSION
It is well documented that Yap1 is expressed in NBL of the embryonic retina where mainly retinal progenitors reside, while its expression is restricted to Müller glia in adult retina (Hamon et al., 2017; Kim et al., 2016). We show here that the specific depletion of Yap1 in retinal progenitors and Müller cells using the Chx10-Cre driver results in late onset retina degeneration, characterized by reduced cell number of multiple cell types in varying retinal layers. However, we did not observe any detectable changes in the retina structure between the control and Yap1ChxKO mice at either embryonic or postnatal time point even until 4 months of age (Figures S3 and S4), and thus we hypothesized that the late onset retinal degeneration was more likely to be caused by the later Müller dysfunction instead of early developmental disability.
Müller cells perform a diverse range of functional and supportive roles for the surrounding retinal neurons, being involved in potassium siphoning, glutamate and GABA turnover, chromophore recycling, energy metabolism and maintenance of the blood retinal barrier (Bringmann et al., 2006; Newman & Reichenbach, 1996). Müller cell loss or dysfunction in humans has been implicated in several retinal diseases including macular telangiectasia type 2 (MacTel2) and diabetic retinopathy (Mizutani et al., 1998; Powner et al., 2010, 2013), featured by vascular pathology and photoreceptor degeneration. In mice, conditional Müller cell ablation leads to structural and functional changes in the mouse retina (Bachleda et al., 2016; Byrne et al., 2013; MacDonald et al., 2015). Moreover, Müller glia offer neuroprotection to RGCs, facilitating their sprouting and neurite regeneration through the secretion of specific factors (Ruzafa et al., 2018). Additionally, Müller cell remodeling is seen in transgenic models of retinal degenerative disease, and undergo reactive gliosis in response to infection and certain inflammatory scenarios (Fernández-Sánchez et al., 2015; Jones et al., 2003). These data indicate that Müller cells are key to maintaining normal retinal morphology and function. Our results showed that YAP deletion in Müller cells in adult retina causes atrophy of Müller glia, accompanied by extensive late-onset retinal degeneration and cell death in entire retinal layer. Considering that YAP is mainly expressed in Müller cells at adulthood (Figure S1b) and Müller cells maintain retinal homeostasis, it is conceivable that YAP could regulate the proper function and survival of Müller cells and thus participates in maintaining retinal homeostasis.
Studies in various species have implicated YAP activity in embryonic retinal development. In zebrafish, YAP regulates the balance between proliferation and differentiation of retinal progenitors (Asaoka et al., 2014; Jiang et al., 2009) and is involved in RPE genesis (Miesfeld et al., 2015). In Xenopus tadpoles, YAP is required in retinal stem cells for postembryonic retinal growth (Cabochette et al., 2015). In mouse, YAP was shown to promote the proliferation of retinal progenitors and inhibits their cell cycle exit (Kim et al., 2016; H. Zhang et al., 2012). Although these studies suggested that the developmental outcomes determined by the Hippo-YAP pathway can be modulated in different ocular cells and species, the specific requirement for YAP in each step of mammalian eye development has not been fully explored. During embryogenesis, neuroretina originates from evaginations of the anterior neural plate, which form the bilayered optic cup at E10.5 in the mouse. The inner layer of the cup comprises multipotential RPCs. All retinal cell types and subtypes are generated orderly from this same population of RPCs in a relatively short time window. The Chx10-cre mouse line used in present study directs Cre expression started from E11.5, which induced specific excision in virtually all RPC-derived cells (Rowan & Cepko, 2004). This determines that Chx10-Cre participates entirely in retinogenesis at both embryonic and early postnatal period. In fact, we detected the early Cre expression in the RPC resided NBL of E14.5 mice (Figure S2b). However, we found that the no gross abnormality showed throughout the retina from early (E14.5) to late (P9) stages of retinal development (Figure S3), accompanied with normal retinal cell proliferation in embryonic and postnatal in Yap1ChxKO mice (Figure S8a–f). Indeed, our analysis did not include several immunological markers that label different retinal neuron subtypes presented in the neonatal retina, which may show detailed defects in proliferation or differentiation. Moreover, previous study indicated that the Chx10-Cre expressed with a varying degree of mosaicism, which could complicate analyses (Rowan & Cepko, 2004). Although we did not see any ectopic expression of Chx10-Cre in optic area, the possibility that ectopic excision in other tissues contribute to the final phenotype cannot be ruled out. It is also possible that penetrance of retinal phenotypes, if any, in these Yap1ChxKO mice is not high enough to be revealed by the number of the Yap1ChxKO mice we examined. Thus, our present study does not exclude the possibility of the existence of unaware development abnormality which also contributes to the late onset degeneration phenotype in aged Yap1ChxKO retinas.
Indeed, tissue-dependent and stage-specific involvement of YAP is an important feature during mouse eye development. For example, YAP functions as an essential regulator of RPE regional specification at developing OV stages, which absent leads to prospective RPE transdifferentiates into NR, thus inducing retinal duplication (H. Zhang et al., 2012). Unlike previous study, the Chx10-Cre line used here deletes genes merely in the E9-P2 RPCs (Kowalchuk et al., 2018), our existing data suggest that YAP deficiency in RPCs at this stage is not sufficient to cause significant retinal structural damage, implying a more cryptic function of YAP at earlier stages in ocular development. The potential mechanisms of YAP in retinal development warrant further investigation.
Our results support and extend a prior study that demonstrated Yap1+/− mice showed impaired Müller cell homoeostasis and contribute to the cone dystrophy. However, no difference in the thickness of either the outer or the INLs was observed between Yap1+/− and control retinas (Masson et al., 2020). In this context, the Yap1ChxKO mice presented obvious late onset retina dystrophy characterized by not only photoreceptor degeneration, but also atrophy of many other neuronal cell types in all retinal layers to varying degrees. Since YAP is dosage sensitive and alteration in the content of functional protein (either increased or decreased) could be a disease-causative mechanism (Miesfeld et al., 2015; Sakabe et al., 2017), the phenotypic inconsistency between the two may be due to the dose effect: the Yap1ChxKO mice in present study are homozygous knockout, thus leading to a more severe phenotype in the retina.
The OS and IS of rods and cones are connected by a highly modified primary cilium, also known as the connecting cilium (CC). The CC serves as a conduit to mediate the transport of photopigment molecules, phototransduction proteins and phospholipid components of the discs from the IS to the OS, which dysfunction could lead to aberrant protein accumulation. Previous study suggested that YAP is also involved in ciliogenesis by modulating actin remodeling factors (Jun, Lee, Park, Ko, & Park, 2022; Kai et al., 2016; Kang, Beak, Kim, Herbert, & Jetten, 2009). In present study, however, we didn't see any mislocalization of rod Rhodopsin and cone opsin protein in Yap1ChxKO retinas (Figure 4a and 4f), suggesting that ciliary protein transport appears normal in Yap1ChxKO retinas.
YAP is a key regulator of tissue homeostasis and essential for organogenesis. Homozygous knockout of the mouse Yap1 results in early embryonic lethality (H. Zhang et al., 2012). Even heterozygous YAP loss-of-function mutations in humans can result in autosomal dominant coloboma (Oatts et al., 2017; Williamson et al., 2014). We, however, did not detect any defects in optic morphology of both Yap1ChxKO and Yap1Het mouse (data not shown). Given YAP immunolabeling was also detected in several nonneural ocular tissues, including the RPE, cornea, lens, CB, iris, and vascular endothelial cells (Hamon et al., 2017), and exerts vital role in this tissues or cells to regulate the overall ocular homeostasis and function. For instance, conditional knockout of YAP in lens epithelium induces cataract in mice (Q. He et al., 2019). Mice deficient in YAP are characterized by abnormal angiogenesis in the postnatal retina (J. He et al., 2018). Moreover, YAP was also been shown to play a vital role on contact guidance of corneal epithelial cells (Raghunathan et al., 2014). Therefore, heterozygous knockout of Yap1 could also lead to severe ocular phenotype. Instead, we provide a more accurate model of retinal degeneration, which is achieved by exactly knockout Yap1 in two types of retinal cells that presents abundant YAP expression at different stages of retina development, providing direct evidence of the requirement of YAP on retinal homeostasis.
In summary, our findings revealed that YAP plays an essential role in retina function and homeostasis in mouse. This work provides a novel late onset retinal degeneration model and gives insight into the development of retinal degenerative diseases as a consequence of YAP deficiency.
ACKNOWLEDGEMENTS
This study was supported by: The National Natural Science Foundation of China (82121003, 81970841 to Xianjun Zhu, 82101160 to Yeming Yang), and the Department of Science and Technology of Sichuan Province (2021YFS0386, 2021YFS0369, and 2021JDGD0036), the CAMS Innovation Fund for Medical Sciences (2019-12M-5-032), the Chinese Academy of Medical Sciences (2018PT31049, 2019PT310020), the Department of Science and Technology of Qinghai Province (2022-HZ-814), the fund for Sichuan Provincial People's Hospital (2021QN01), the Department of Chengdu Science and Technology (2021-YF05-01316-SN). The funders had no role in the study design, data collection and analysis, or preparation of the manuscript. The authors would like to thank Chengdu LiLai Biotechnology Co., Ltd., for technical assistance with histology analysis.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.




