Nicotinic acid adenine dinucleotide phosphate activates two-pore channel TPC1 to mediate lysosomal Ca2+ release in endothelial colony-forming cells
Abstract
Nicotinic acid adenine dinucleotide phosphate (NAADP) is the most recently discovered Ca2+-releasing messenger that increases the intracellular Ca2+ concentration by mobilizing the lysosomal Ca2+ store through two-pore channels 1 (TPC1) and 2 (TPC2). NAADP-induced lysosomal Ca2+ release regulates multiple endothelial functions, including nitric oxide release and proliferation. A sizeable acidic Ca2+ pool endowed with TPC1 is also present in human endothelial colony-forming cells (ECFCs), which represent the only known truly endothelial precursors. Herein, we sought to explore the role of the lysosomal Ca2+ store and TPC1 in circulating ECFCs by harnessing Ca2+ imaging and molecular biology techniques. The lysosomotropic agent, Gly–Phe β-naphthylamide, and nigericin, which dissipates the proton gradient which drives Ca2+ sequestration by acidic organelles, caused endogenous Ca2+ release in the presence of a replete inositol-1,4,5-trisphosphate (InsP3)-sensitive endoplasmic reticulum (ER) Ca2+ pool. Likewise, the amount of ER releasable Ca2+ was reduced by disrupting lysosomal Ca2+ content. Liposomal delivery of NAADP induced a transient Ca2+ signal that was abolished by disrupting the lysosomal Ca2+ store and by pharmacological and genetic blockade of TPC1. Pharmacological manipulation revealed that NAADP-induced Ca2+ release also required ER-embedded InsP3 receptors. Finally, NAADP-induced lysosomal Ca2+ release was found to trigger vascular endothelial growth factor-induced intracellular Ca2+ oscillations and proliferation, while it did not contribute to adenosine-5′-trisphosphate-induced Ca2+ signaling. These findings demonstrated that NAADP-induced TPC1-mediated Ca2+ release can selectively be recruited to induce the Ca2+ response to specific cues in circulating ECFCs.
1 INTRODUCTION
Endothelial colony-forming cells (ECFCs) are released in circulation from vascular stem cell niches to replace senescent/necrotic/apoptotic endothelial cells (Yoder, 2010), to regenerate blood vessels upon an ischemic insult (Tasev, Koolwijk, & van Hinsbergh, 2016), and to promote the angiogenic switch in growing tumors (Poletto et al., 2018) according to a process termed vasculogenesis (Yoder, 2010). A recent consensus paper proposed that ECFCs represent the only true endothelial progenitors, as they belong to the endothelial lineage, display robust clonal potential, and are able to self-assemble into patent vessels in vivo (Medina et al., 2017). ECFCs, isolated from human peripheral or cord blood, have been successfully employed in several models of myocardial infarction (Lee et al., 2014), hindlimb ischemia (Schwarz et al., 2012), stroke (Ding et al., 2016), and ischemic retinopathy (Sakimoto et al., 2017). However, the rather low frequency of circulating ECFCs in peripheral blood (<1 ECFC every 106 mononuclear cells [MNCs]) hampers their use for autologous stem cell transplantation (Moccia, Ruffinatti, & Zuccolo, 2015; Tasev et al., 2016). Understanding the signaling pathways that drive ECFC proliferation, homing, and retention into ischemic tissues is, therefore, mandatory to significantly enhance the clinical outcome of ECFCs-based therapy (Moccia, Berra-Romani, & Rosti, 2018; Moccia, Lucariello, & Guerra, 2018; Moccia et al., 2015; Tasev et al., 2016).
Recent studies unveiled some of the molecular mechanisms that finely regulate ECFC fate and behavior, including aldehyde dehydrogenase (Putman, Liu, Broughton, Bell, & Hess, 2012), Notch signaling (Patel et al., 2016), CD44 expression (Sakimoto et al., 2017), NOX4 (O'Neill et al., 2019), and CD133 (Rossi et al., 2019). Furthermore, we have shown that an increase in intracellular Ca2+ concentration ([Ca2+]i), a ubiquitous signaling mode across the phylogenetic tree (Berridge, Bootman, & Roderick, 2003), plays a key role in controlling ECFC proliferation, migration, tubulogenesis, and neovessel formation (Balbi et al., 2019; Guerra, Perrotta, & Testa, 2018; Lodola et al., 2017; Moccia & Guerra, 2016; Zuccolo et al., 2018). ECFCs may impinge on two main sources to shape intracellular Ca2+ signals: the virtually infinite extracellular Ca2+ pool and an intracellular Ca2+ reservoir of limited capacity, which is mainly located within the endoplasmic reticulum (ER; Moccia & Guerra, 2016; Sanchez-Hernandez et al., 2010). Extracellular growth factors and autacoids, such as vascular endothelial growth factor (VEGF) and adenosine-5′-trisphosphate (ATP), stimulate several isoforms of phospholipase C to cleave inositol-1,4,5-trisphosphate (InsP3) from phosphatidylinositol 4,5-bisphosphate, a minor phospholipid component of the plasma membrane (PM; Dragoni et al., 2013, 2015; Moccia & Guerra, 2016; Sanchez-Hernandez et al., 2010). InsP3 elicits either transient or repetitive cycles of Ca2+ release from the ER by activating three distinct isoforms of InsP3 receptors (InsP3R1–InsP3R3; Dragoni et al., 2011; Sanchez-Hernandez et al., 2010), while ryanodine receptors (RyRs) are absent in ECFCs (Zuccolo, Dragoni et al., 2016). The subsequent drop in ER Ca2+ levels activates the ER Ca2+ sensor, STIM1, to oligomerize and translocate towards ER/PM junctional domains, where it interacts with the Ca2+-permeable channels Orai1 and transient receptor potential canonical 1 (TRPC1) to mediate store-operated Ca2+ entry (SOCE; Li et al., 2011; Moccia et al., 2012). The ER, however, is not the sole endogenous Ca2+ store in mammalian cells. A recent series of studies have shown that the acidic stores of the endolysosomal (EL) system may serve as an additional intracellular Ca2+ reservoir that can be recruited by extracellular ligands through the generation of the Ca2+-releasing second messenger, nicotinic acid adenine dinucleotide phosphate (NAADP; Morgan, Platt, Lloyd-Evans, & Galione, 2011). The acidic stores contain a relatively high concentration of Ca2+, that is, ≈500 µM, which is mainly maintained by the H+ gradient established by a Ca2+/H+ exchanger that is yet to be identified in placental mammalian animals (Faris, Shekha, Montagna, Guerra, & Moccia, 2018; Melchionda, Pittman, Mayor, & Patel, 2016; Morgan et al., 2011). Other studies, however, suggested that the EL Ca2+ store is refilled by InsP3-dependent ER Ca2+ mobilization in a pH-independent manner (Atakpa, Thillaiappan, Mataragka, Prole, & Taylor, 2018; Garrity et al., 2016). NAADP evokes Ca2+ release from acidic stores by activating a novel superfamily of voltage-gated ion channels, that is, two-pore channels 1-3 (TPC1-3) (Patel, 2015). Nevertheless, only TPC1 and TPC2 are expressed in humans, mice, and rats (Morgan et al., 2011). NAADP activates TPC1 and TPC2 through the interposition of an NAADP-binding protein that could also mediate NAADP-induced RyR activation (Guse, 2012; Wolf et al., 2015). NAADP-induced mobilization of the acidic Ca2+ pool may be then amplified by InsP3Rs and RyRs through the mechanism of Ca2+-induced Ca2+ release (CICR) at the very narrow (<30 nm) membrane contact sites between EL and ER vesicles (Kilpatrick, Eden, Schapira, Futter, & Patel, 2013; Penny, Kilpatrick, Han, Sneyd, & Patel, 2014).
The NAADP/TPC axis has been unequivocally linked to the control of a growing number of cellular functions in health and disease (Faris et al., 2018; Morgan et al., 2011; Patel & Kilpatrick, 2018). For instance, in vascular endothelial cells, NAADP was reported to mediate histamine-induced von Willebrand factor (vWF) release (Esposito et al., 2011), arachidonic acid-, acetylcholine-, histamine-, and glutamate-induced nitric oxide (NO) release (Berra-Romani, Faris, Negri et al., 2019; Berra-Romani, Faris, Pellavio et al., 2019; Negri et al., 2019; Zuccolo, Kheder et al., 2019; Zuccolo, Laforenza et al., 2019). In addition, NAADP gates TPC2 to sustain VEGF-dependent angiogenesis both in vitro and in vivo (Favia et al., 2014, 2016). For instance, VEGF-induced intracellular Ca2+ signals were triggered by NAADP in human umbilical vein endothelial cells (HUVECs; Favia et al., 2014), which represent a widespread model to investigate endothelial signaling (Moccia, Berra-Romani, & Tanzi, 2012). We have recently demonstrated the existence of functional EL Ca2+ stores endowed with TPC1 in circulating ECFCs (Zuccolo, Dragoni et al., 2016). Subsequently, we showed that NAADP may evoke an increase in [Ca2+]i in ECFCs that is antagonized by NED-19, an established TPC blocker (Biwer et al., 2016; Di Nezza et al., 2017). However, it remained unclear whether in circulating ECFCs: (a) the EL Ca2+ store is functionally coupled to ER-embedded InsP3Rs; (b) NAADP actually targets TPC1 and mediates Ca2+ release from the EL Ca2+ stores; and (c) VEGF also requires NAADP to stimulate NAADP proliferation. Likewise, it is still unclear whether the EL and ER Ca2+ reservoirs are functionally coupled in any endothelial cell type (Filippini, D'Amore, & D'Alessio, 2019). Understanding how endothelial Ca2+ signals are shaped is mandatory to pursue the ambitious goal to rescue endothelial dysfunction, which is a crucial hallmark of multiple cardiovascular risk factors (Filippini et al., 2019; Moccia et al., 2012; Moccia, Negri, Faris, & Berra-Romani, 2019) and is often associated to derangement of the Ca2+ machinery (Filippini et al., 2019; Khaddaj Mallat, Mathew John, Kendrick, & Braun, 2017; Moccia, Negri, Shekha, Faris, & Guerra, 2019).
The present investigation was, therefore, undertaken to uncover the aforementioned issues by adopting a multidisciplinary approach, which included: Ca2+ imaging, gene targeting through small interfering RNA (siRNA), and proliferation assays. We revealed for the first time that the EL Ca2+ mobilization requires the ER to be fully charged with Ca2+ in circulating ECFCs. As expected, NAADP-induced Ca2+ release was abolished upon genetic deletion of TPC1 and pharmacological impairment of InsP3-dependent ER Ca2+ release. Finally, while ATP-induced Ca2+ signals were independent on this signaling pathway, VEGF-induced proangiogenic Ca2+ oscillations in circulating ECFCs were triggered by NAADP. These data shed further light on the molecular mechanisms by which intracellular Ca2+ dynamics control ECFC behavior and expand the list of NAADP-regulated functions.
2 MATERIALS AND METHODS
2.1 Isolation and cultivation of ECFCs
Blood samples (40 ml) were obtained from patients who were out of ongoing cytoreductive therapy at the moment of blood sampling for ECFC isolation and from healthy donors. The ECFC samples used in the present investigation belong to the stocks recently described and characterized by Moccia et al. (2017). To isolate ECFCs, MNCs were separated from peripheral blood by density gradient centrifugation on lymphocyte separation medium for 30 min at 400g and washed twice in endothelial basal medium-2 (EBM-2) with 2% fetal bovine serum (FBS). A median of 36 × 106 MNCs (range, 18–66) were plated on collagen-coated culture dishes (BD Biosciences) in the presence of the endothelial cell growth medium EGM-2MV BulletKit (Lonza) containing EBM-2, 5% FBS, recombinant human (rh)-epidermal growth factor, rhVEGF, recombinant human fibroblast growth factor-basic, recombinant human insulin-like growth factor-1, ascorbic acid, and heparin, and maintained at 37°C in 5% CO2 and humidified atmosphere. Discard of nonadherent cells was performed after 2 days; thereafter, the medium was changed three times a week. The outgrowth of endothelial colonies from adherent MNCs was characterized by the formation of a cluster of cobblestone-appearing cells, resembling endothelial cells. That ECFCs-derived colonies belonged to endothelial lineage was confirmed as described by Moccia et al. (2017) and Sanchez-Hernandez et al. (2010). In more detail, ECFCs-derived colonies were stained with anti-CD31, anti-CD105, anti-CD144, anti-CD146, anti-vWF, anti-CD45, and anti-CD14 monoclonal antibodies and by assessment of capillary-like network formation in an in vitro Matrigel assay. For our experiments, we have mainly used endothelial cells obtained from early passage ECFCs (P1–P3, which roughly encompasses a 15–18-day period) with the purpose to avoid (or maximally reduce) any potential bias due to possible cell differentiation. As already shown by Lodola et al. (2012), the immunophenotype of both normal and tumor ECFCs does not change at different passages in culture. We also tested whether functional differences occurred when early (P2) and late (P6) passage ECFCs were used by testing the in vitro capacity of capillary network formation in a Matrigel assay and found no differences between early and late passage ECFC-derived cells (data not shown).
2.2 Solutions
Physiological salt solution (PSS) had the following composition (in millimolar): 150 NaCl, 6 KCl, 1.5 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES. In Ca2+-free solution (0Ca2+), Ca2+ was substituted with 2 mM NaCl, and 0.5 mM egtazic acid was added. Solutions were titrated to pH 7.4 with NaOH. The osmolality of PSS as measured with an osmometer (Wescor 5500; Logan, UT) was 338 mmol/kg.
2.3 [Ca2+]i measurements
ECFCs were loaded with 4 µM Fura-2 acetoxymethyl ester (Fura-2/AM; 1 mM stock in dimethyl sulfoxide) in PSS for 1 hr at room temperature. After washing in PSS, the coverslip was fixed to the bottom of a Petri dish and the cells observed by an upright epifluorescence Axiolab microscope (Carl Zeiss, Oberkochen, Germany), usually equipped with a Zeiss ×40 Achroplan objective (water immersion, 2.0 mm working distance, and 0.9 numerical aperture). ECFCs were excited alternately at 340 and 380 nm, and the emitted light was detected at 510 nm. A first neutral density filter (1 or 0.3 optical density) reduced the overall intensity of the excitation light and a second neutral density filter (optical density = 0.3) was coupled to the 380-nm filter to approach the intensity of the 340 nm light. A round diaphragm was used to increase the contrast. The excitation filters were mounted on a filter wheel (Lambda 10; Sutter Instrument, Novato, CA). Custom software, working in the Linux environment, was used to drive the camera (Extended-ISIS Camera; Photonic Science, Millham, UK) and the filter wheel, and to measure and plot online the fluorescence from 10 to 100 rectangular “regions of interest” (ROI). Each ROI was identified by a number. Since cell borders were not clearly identifiable, an ROI may not include the whole cell or may include part of an adjacent cell. Adjacent ROIs never superimposed. [Ca2+]i was monitored by measuring, for each ROI, the ratio of the mean fluorescence emitted at 510 nm when exciting alternatively at 340 and 380 nm (shortly termed "ratio"). An increase in [Ca2+]i causes an increase in the ratio (Sanchez-Hernandez et al., 2010). Ratio measurements were performed and plotted online every 3 s. The experiments were performed at room temperature (22°C). In the figures, each Ca2+ tracing refers to an individual cell and is representative of at least three independent experiments.
2.4 Preparation of NAADP-containing liposomes
NAADP-containing liposomes were prepared from lecithin by a thin film hydration method, as recently shown by Di Nezza et al. (2017) and Faris et al. (2019). A thin film was formed by dissolving the lecithin in chloroform/methanol solution (2:1 vol/vol) in a round bottom flask and following removal of the solvent under vacuum condition at room temperature, which ensured complete removal of the solvents. The film was then hydrated with phosphate-buffered saline (PBS) buffer (10 mM, pH 7.4) to make 20 ml of lipid coarse dispersion. Liposomes were prepared by adding cholesterol in an 89:20 lecithin:cholesterol molar ratio, codissolved in chloroform, and then dried. The dried film from a flask was suspended in 4 ml of rehydration solution. The resulting liposomal dispersion was sonicated 66 for 3 min (ultrasound homogenizer; BioLogics) and extruded 21 times with 100-nm filter. Finally, the mixture was dialyzed in PBS bulk for 24 hr with three bulk changes. Properties of liposomes were modulated by varying the rehydration solution composition. Liposomes were prepared from PBS solution containing only 8.70 µg of NAADP. NAADP was diluted at 1:20, as shown by Di Nezza et al. (2017).
2.5 Membrane preparation and immunoblotting
Cells were diluted in PBS and centrifuged 1,000g for 10 min. The cell pellets were resuspended with radioimmunoprecipitation assay buffer (150 mM NaCl, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecylsulphate, 50 mM Tris-HCl, and pH 8.0) containing 0.1 mg/ml phenylmethylsulfonyl fluoride, protease inhibitor cocktails (P8340; Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitor (1 mM sodium orthovanadate). The homogenates were solubilized in Laemmli buffer (Laemmli, 1970) and 30 µg proteins were separated on precast gel electrophoresis (4–20% Mini-PROTEAN TGX Stain-Free Gels; Bio-Rad) and transferred to the Hybond-P polyvinylidene difluoride membrane (GE Healthcare, Italy) by electroelution. After 1 hr blocking with Tris-buffered saline (TBS) containing 3% bovine serum albumin (BSA) and 0.1% Tween (blocking solution), the membranes were incubated overnight at 4°C with the following antibody: affinity-purified rabbit anti-TPC1 (SAB2104213; Sigma-Aldrich), diluted 1:500 in 3% BSA in Tween-TBS. The membranes were washed and incubated for 1 hr with peroxidase-conjugated goat anti-rabbit IgG (AP132P; Chemicon), diluted 1:100,000 in blocking solution. The bands were detected with ECL™ Select western blot analysis detection system (GE Healthcare Europe GmbH, Italy). Prestained molecular weight markers (ab116028; Abcam) were used to estimate the molecular weight of the bands. Blots were stripped as shown in and reprobed with RabMAb anti-β-actin antibody (AB-81599; Immunological Sciences) as housekeeping. The antibody was diluted 1:2,000 in blocking solution.
2.6 Protein content
Protein contents of all the samples were determined by the Bradford's (1976) method using BSA as standard.
2.7 Gene silencing
siRNA targeting TPC1 (siTPC1) was purchased by Sigma-Aldrich MISSION esiRNA (human TPCA1, EHU016301). Scrambled siRNA was used as a negative control. Briefly, once the monolayer cells had reached 50% confluency, the medium was removed and the cells were added with Opti-MEM I reduced serum medium without antibiotics (Life Technologies). siRNAs (100 nM final concentration) were diluted in Opti-MEM I reduced serum medium and mixed with Lipofectamine™ RNAiMAX Transfection Reagent (Invitrogen) prediluted in Opti-MEM, according to the manufacturer's instructions. After 20 min incubation at room temperature, the mixes were added to the cells and incubated at 37°C for 5 hr. Transfection mixes were then completely removed and the fresh culture media was added. The effectiveness of silencing was determined by immunoblotting (see Figure S1) and silenced cells were used 48 hr after transfection. Before and after the transfection procedure, ECFCs were always maintained in the presence of the EGM-2MV BulletKit endothelial cell medium. The Trypan blue exclusion assay confirmed that the genetic silencing of TPC1 did not affect ECFC viability (Figure S2).
2.8 Proliferation assay
ECFCs were detached by trypsinization at 48 hr after transfection with scrambled siRNA and siTPC1 and then resuspended in the EBM-2 medium containing 2% FCS and 10 ng/ml VEGF, as shown by Dragoni et al. (2011, 2015). Cultures were incubated at 37°C, 5% CO2, and cell growth assessed every day until confluence was reached in the control dishes. Cells were then recovered by trypsinization and their number assessed by counting in a hemocytometer. Three different sets of experiments were performed.
2.9 Statistics
All the data have been collected from ECFCs deriving from at least three coverslips from three distinct donors. The amplitude of intracellular Ca2+ release in response to each agonist (NAADP or ATP) or drug (glycyl-l-phenylalanine 2-naphthylamide [GPN], nigericin, and cyclopiazonic acid [CPA]) was measured as the difference between the ratio at the peak of intracellular Ca2+ mobilization and the mean ratio of 1 min baseline before the peak. For VEGF stimulation, the percentage of oscillating the cells was evaluated. Pooled data are given as mean ± standard error (SE) and statistical significance (p < .05) was evaluated by Student's t test for unpaired observations or one-way analysis of variance followed by the post hoc Dunnett's test as appropriate. Data relative to Ca2+ signals are presented as mean ± SE, while the number of cells analyzed is indicated in the corresponding bar histograms.
2.10 Chemicals
Fura-2/AM was obtained from Molecular Probes (Molecular Probes Europe BV, Leiden, The Netherlands). All the chemicals were of analytical grade and obtained from Sigma-Aldrich.
3 RESULTS
3.1 A functional EL Ca2+ pool is present in circulating ECFCs
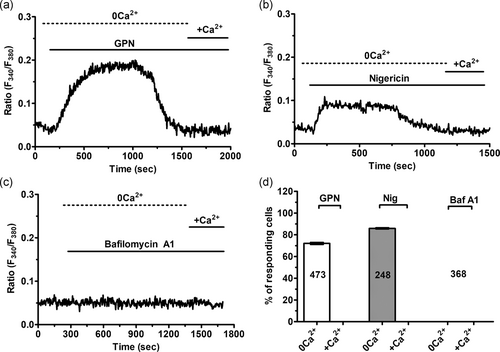
To assess whether a function EL Ca2+ store is present and able to increase the [Ca2+]i, ECFCs were loaded with the Ca2+-sensitive fluorochrome, Fura-2/AM, as shown by Di Nezza et al. (2017). Thereafter, ECFCs were challenged with the dipeptide GPN, which is cleaved by the lysosomal enzyme cathepsin C, thereby rupturing lysosomal membrane and liberating lysosomal Ca2+ into the cytosol (Faris et al., 2018). GPN has been widely exploited to analyze EL Ca2+ signaling in multiple cell types (Faris et al., 2019; Kilpatrick et al., 2013; McGuinness, Bardo, & Emptage, 2007; Moccia, Billington, & Santella, 2006; Pandey et al., 2009; Ronco et al., 2015), including vascular endothelial cells (Srinivas, Ong, Goon, Goon, & Bonanno, 2002; Zuccolo, Laforenza et al., 2019) and circulating ECFCs (Zuccolo, Dragoni et al., 2016). As expected, GPN (200 μM) caused a rapid and transient elevation in [Ca2+]i in the absence of extracellular Ca2+ (0Ca2+; Figure 1a), which reflects Ca2+ mobilization from the endogenous lysosomal pool (Kilpatrick et al., 2013; Pandey et al., 2009). The subsequent addition of Ca2+ did not cause any detectable increase in [Ca2+]i (Figure 1a), which suggests that acidic Ca2+ stores are not coupled to plasmalemmal Ca2+ entry pathways. Likewise, the H+/K+ antiporter, nigericin (50 μM), which dissipates the lysosomal proton gradient responsible for Ca2+ refilling (Faris et al., 2019, 2018), caused a transient elevation in [Ca2+]i under 0Ca2+ conditions that was not coupled to extracellular Ca2+ entry upon restoration of external Ca2+ concentration (Figure 1b). Finally, we probed the effect of bafilomycin A1 (100 nM), a selective inhibitor of the V-type H+ ATPase that drives EL acidification (Faris et al., 2018; Morgan et al., 2011). Bafilomycin A1 did not cause any detectable change in [Ca2+]i in circulating ECFCs (Figure 1c). As discussed by Faris et al. (2018) and Morgan et al. (2011), bafilomycin A1 depletes lysosomal Ca2+ only in the presence of adequate Ca2+ leakage. It is, therefore, conceivable that resting lysosomal Ca2+ permeability is low in ECFCs. The statistical analysis of these recordings is presented in Figure 1d. Overall, these observations confirm that a functional lysosomal Ca2+ store is present and able to increase the [Ca2+]i in ECFCs.
3.2 Lysosomal Ca2+ release requires an intact InsP3-sensitive ER Ca2+ pool
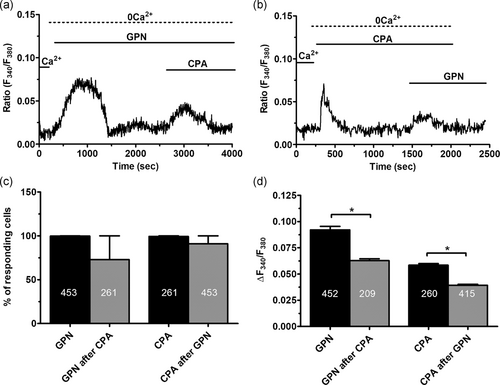
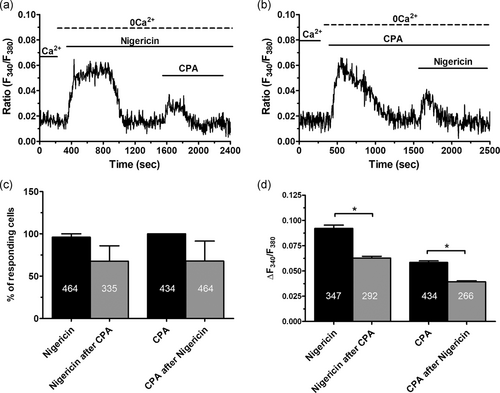
As mentioned above, lysosomal Ca2+ release may require a bidirectional coupling with the ER in which InsP3Rs play a crucial role (Atakpa et al., 2018; Kilpatrick et al., 2013; Morgan, 2016; Ronco et al., 2015). To explore the role of InsP3-dependent ER Ca2+ release in acidic Ca2+ signaling in ECFCs, we took advantage of CPA, an established inhibitor of sarco–ER Ca2+-ATPase activity, which impairs Ca2+ sequestration into the ER lumen and causes the depletion of ER Ca2+ pool. Pretreating ECFCs with CPA (10 μM) under 0Ca2+ conditions are sufficient to deplete the InsP3-sensitive ER Ca2+ pool (Sanchez-Hernandez et al., 2010). This protocol significantly (p < .05) reduced the amplitude of GPN-induced endogenous Ca2+ release (Figures 2a,b and 2d), while it did not affect the percentage of responding cells (Figure 2c). Likewise, pretreating ECFCs with GPN (200 μM) under 0Ca2+ conditions significantly (p < .05) reduced the amplitude of the endogenous Ca2+ response to CPA (Figures 2a,b and 2d), although it did not affect the percentage of responding cells (Figure 2d). GPN was maintained throughout the recording as lysosomes readily reform upon GPN washout from the bath (Pandey et al., 2009). The same results were obtained when we compared the Ca2+ signals induced under 0Ca2+ conditions by nigericin (50 μM) and CPA (10 μM; Figure 3a) and then reversing the temporal order of agonist application (Figure 3b). The previous depletion of the ER Ca2+ pool with CPA did not affect the percentage of ECFCs responding to nigericin (Figure 3c) but caused a significant (p < .05) reduction in the amplitude of the endogenous Ca2+ mobilization (Figure 3d). Vice versa, previous depletion of the lysosomal Ca2+ store with nigericin did not affect the percentage of ECFCs responding to CPA (Figure 3c), but significantly (p < .05) reduced the amplitude of endogenous Ca2+ release (Figure 3d). Overall, these findings clearly indicate that a replete ER Ca2+ pool is necessary for the lysosomal Ca2+ store to generate a full increase in [Ca2+]i in ECFCs.
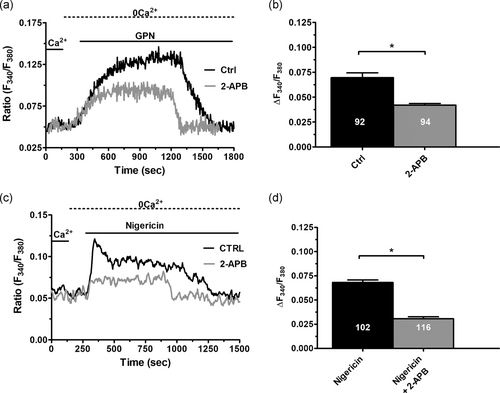
InsP3Rs may play an intermediary role in the Ca2+-dependent cross-talk between the lysosomal and ER Ca2+ stores (Atakpa et al., 2018; Churchill & Galione, 2001; Garrity et al., 2016; Kilpatrick et al., 2013). Therefore, we then measured the Ca2+ response to either GPN (200 μM) or nigericin (50 μM) in the presence of 2-aminoethoxydiphenyl borate (2-APB; 50 μM, 30 min), which selectively blocks InsP3Rs in the absence of extracellular Ca2+ (Dragoni et al., 2011). This treatment significantly (p < .05) reduced both GPN- and nigericin-evoked increase in [Ca2+]i under 0Ca2+ conditions (Figure 4), thereby confirming that functional InsP3Rs are required to sustain intracellular Ca2+ signaling upon the liberation of the lysosomal Ca2+ store also in ECFCs.
3.3 TPC1 mediates NAADP-induced lysosomal Ca2+ release
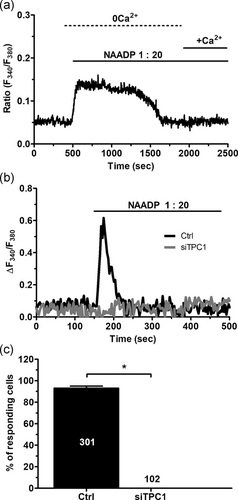
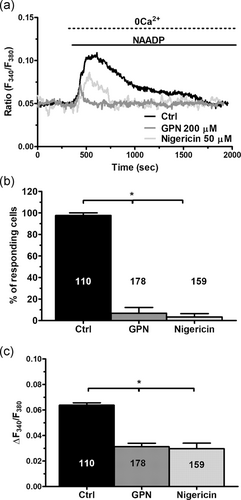
A recent investigation revealed that liposomal delivery of NAADP (at 1:20 dilution) caused an increase in [Ca2+]i that was prevented by NED-19 (Di Nezza et al., 2017), a selective TPC antagonist (Patel, 2015). In agreement with these findings, this NAADP-containing liposomal preparation induced a Ca2+ signal which decayed to the baseline under 0Ca2+ conditions, while restitution of extracellular Ca2+ did not evoke any detectable change in intracellular Ca2+ levels (Figure 5a). Therefore, NAADP does not elicit Ca2+ entry in ECFCs. The Ca2+ response to NAADP-containing liposomes displayed different kinetics, ranging from long lasting (≈1,500 s; Figure 5a) to transient (≈200 s; Figure 5b), which are likely to reflect different rates of NAADP release from the liposomes. To confirm that TPC1 mediates the intracellular Ca2+ response to NAADP, TPC1 was knocked down by using a specific siRNA (siTPC1), as recently shown in metastatic colorectal cancer cells (Faris et al., 2019). The efficacy of gene silencing on the expression of TPC1 protein has been displayed in Figure S1. The genetic suppression of TPC1 completely suppressed the Ca2+ response to NAADP, while ECFCs transfected with a scrambled construct were still responsive to this second messenger (Figure 5b,c). Resting Fura-2 fluorescence was not affected by genetic silencing of TPC1 (control: 0.120 ± 0.007 arbitrary units [a.u.], n = 301 vs. siTPC1: 0.109 ± 0.026 a.u., n = 102) Subsequently, we found that NAADP-evoked endogenous Ca2+ mobilization was impaired by preincubating the cells with either GPN (200 μM, 30 min) or nigericin (50 μM, 30 min; Figure 6a). Disruption of acidic Ca2+ stores significantly (p < .05) reduced the percentage of responding cells (Figure 6b) and the amplitude of the Ca2+ response to NAADP (Figure 6c). Taken together, these findings convincingly demonstrated that TPC1 mediates NAADP-induced lysosomal Ca2+ release in ECFCs.
3.4 InsP3-dependent ER Ca2+ release is necessary for NAADP-induced lysosomal Ca2+ mobilization in ECFCs
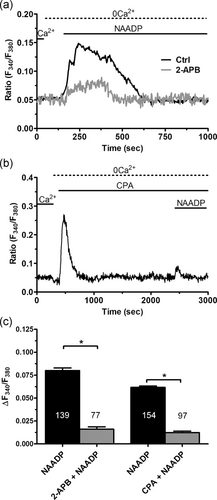
In the next set of experiments, the role of InsP3-dependent ER Ca2+ release in NAADP signaling was explored by exploiting the strategy introduced by Kilpatrick et al. (2013); Kilpatrick, Yates, Grimm, Schapira, and Patel, (2016). We found that pharmacological blockade of InsP3Rs with 2-APB (50 μM, 30 min) significantly (p < .05) reduced the Ca2+ response to NAADP-containing liposomes (1:20), as illustrated in Figure 7a and summarized in Figure 3c. Likewise, depletion of the ER Ca2+ pool with CPA (10 μM, 30 min) in the absence of extracellular Ca2+ (0Ca2+) significantly (p < .05) decreased NAADP-induced intracellular Ca2+ release by diminishing the percentage of responding cells (Figure 7b,c). Overall, these observations endorse the notion that functional InsP3Rs are required for the lysosomal Ca2+ store to generate a full increase in [Ca2+]i in response to NAADP.
3.5 TPC1 triggers VEGF-induced proangiogenic Ca2+ oscillations, but not ATP-induced Ca2+ release
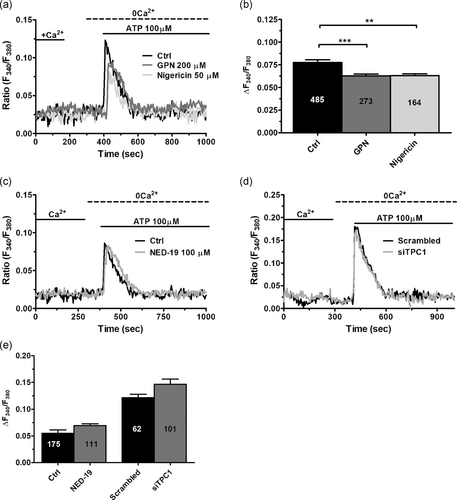
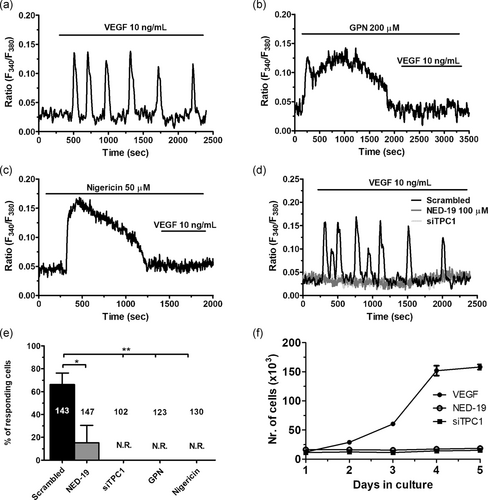
To assess the physiological role of NAADP-induced Ca2+ release in circulating ECFCs, we focused on the Ca2+ response to two distinct physiological autacoids, that is, ATP (100 μM) and VEGF (10 ng/ml), which trigger intracellular Ca2+ signals by, respectively, activating G-protein-coupled receptors (GPCRs) and tyrosine kinase receptors (Dragoni et al., 2011; Sanchez-Hernandez et al., 2010). ATP has long been used to assess the activation of GPCRs in circulating ECFCs in healthy and pathological ECFCs (Lodola et al., 2012; Sanchez-Hernandez et al., 2010; Zuccolo, Bottino et al., 2016). Depletion of the lysosomal Ca2+ pool with either GPN (200 μM, 30 min) or nigericin (50 μM, 30 min) induced a modest, but statistically significant, reduction in ATP-induced intracellular Ca2+ release (Figure 8a,b). However, the Ca2+ response to ATP was not affected by the pharmacological blockade of TPC1 with NED-19 (100 μM, 30 min; Figure 8c,d). Likewise, genetic silencing of NAADP-evoked lysosomal Ca2+ mobilization with the siTPC1 did not affect ATP-induced endogenous Ca2+ release (Figure 8d,e). VEGF, in turn, elicits long-lasting oscillations in [Ca2+]i that stimulate ECFCs to undergo proliferation and tube formation (Dragoni et al., 2011, 2015; Lodola et al., 2017). Unlike ATP, pretreatment with either GPN (200 μM, 30 min) or nigericin (50 μM, 30 min) abolished the oscillatory Ca2+ response to VEGF in circulating ECFCs (Figures 9a–c and 9e). Moreover, VEGF-induced intracellular Ca2+ oscillations were fully prevented by pharmacological and genetic blockade of TPC1 with, respectively, NED-19 (100 μM, 30 min) and the siTPC1 (Figure 9d,e). Therefore, we examined the effect of TPC1 inhibition on VEGF-induced ECFC proliferation in the presence of EBM-2 and 5% FBS, as described by Dragoni et al. (2011, 2015) and Lodola et al. (2017). As described in Figure 9f, both NED-19 (100 μM, 30 min) and the siTPC1 prevented ECFCs from reaching the confluence after 5 days in culture in the presence of 10 ng/ml VEGF. Overall, these experiments demonstrate that NAADP-induced lysosomal Ca2+ release is selectively recruited by VEGF to trigger proangiogenic Ca2+ oscillations in ECFCs, whereas it does not support the Ca2+ response to ATP.
4 DISCUSSION
Growing evidence reported the crucial role played by acidic Ca2+ stores and their Ca2+-releasing second messenger, NAADP, in all the main cellular constituents of the cardiovascular system, including cardiac myocytes (Collins, Bayliss, Churchill, Galione, & Terrar, 2011), vascular smooth muscle cells (Fameli, Ogunbayo, van Breemen, & Evans, 2014), and vascular endothelial cells (Favia et al., 2014). Herein, we showed for the first time that the lysosomal Ca2+ pool is functionally coupled to the largest ER Ca2+ store via InsP3Rs in ECFCs. This observation also represents the first proof in favor of a Ca2+-mediated cross-talk between EL vesicles and ER cisternae in any endothelial cell type. We further showed that NAADP-induced Ca2+ release from the acidic Ca2+ organelles is mediated by TPC1 and requires a replete InsP3-releasable ER Ca2+ pool. Finally, we provided the first evidence that NAADP-induced lysosomal Ca2+ mobilization selectively triggers VEGF-induced proangiogenic Ca2+ oscillations in circulating ECFCs. These findings extend to vasculogenesis the range of physiological functions regulated by NAADP and hint at TPC1 as a suitable candidate to target to improve the efficiency of autologous cell based therapy (Moccia, Berra-Romani et al., 2018; Moccia, Lucariello et al., 2018; Moccia et al., 2015).
4.1 The acidic Ca2+ store is functionally coupled to the ER via InsP3Rs in ECFCs
The acidic Ca2+ stores, including acidocalcisomes, lysosomes, lysosome-related organelles, secretory vesicles, and vacuoles (Morgan et al., 2011; Patel & Docampo, 2010), are emerging as an additional endogenous Ca2+ reservoir that contributes to Ca2+ signals in response to extracellular stimulation by establishing a bidirectional Ca2+-dependent cross-talk with the ER (Faris et al., 2018; Morgan, 2016; Morgan et al., 2011; Penny, Kilpatrick, Eden, & Patel, 2015). For instance, pharmacological disruption of the lysosomal Ca2+ store with GPN evokes long-lasting intracellular Ca2+ oscillations in human fibroblasts that are curtailed by emptying the ER Ca2+ store or inhibiting InsP3Rs (Kilpatrick et al., 2013). Likewise, GPN- and nigericin-induced intracellular Ca2+ release in metastatic colorectal carcinoma cells were attenuated by preventing InsP3-dependent ER Ca2+ release (Faris et al., 2019). Herein, we demonstrated for the first time in an endothelial cell type that the endogenous Ca2+ response to either GPN or nigericin was curbed by depleting the ER Ca2+ pool with CPA and inhibiting InsP3Rs with 2-APB. This finding endorses the emerging view that acidic and neutral Ca2+ stores are intimately connected at the MCSs between lysosomal vesicles and ER cisternae that have been described in multiple cell types (Penny et al., 2015). This issue remains to be explored in endothelial cells, although mitochondria-associated ER membranes were recently reported in HUVECs (Yu et al., 2019). The Ca2+-dependent cross-talk between lysosomes and ER is largely interpreted within the framework of the “trigger hypothesis” originally put forward by Churchill and Galione (2001). These authors proposed that the Ca2+ response to multiple extracellular stimuli is triggered by local Ca2+ release from the acidic stores and then propagated into a regenerative Ca2+ wave by recruiting ER-embedded InsP3Rs and RyRs through the CICR process (Churchill & Galione, 2001; Galione, 2015). Subsequently, it was found that lysosomal Ca2+ refilling was impaired by preventing ER-dependent Ca2+ release through InsP3Rs (Garrity et al., 2016; Ronco et al., 2015) and that InsP3Rs may concentrate at ER-lysosomes contact sites, thereby delivering Ca2+ directly into the lysosomal lumen (Atakpa et al., 2018). It turns out that when lysosomal Ca2+ uptake is prevented, the increase in [Ca2+]i observed in response to ER store depletion is larger as compared to control cells (Lopez-Sanjurjo, Tovey, Prole, & Taylor, 2013). The finding that GPN and nigericin remarkably reduced ER Ca2+ release in response to CPA, as well to VEGF (see below), strongly suggests that acidic Ca2+ stores are more prone to trigger, rather than tempering, ER-derived Ca2+ signals in circulating ECFCs (Patel, 2019). Conversely, depletion of lysosomal Ca2+ did not result in extracellular Ca2+ entry, as also reported in human fibroblasts (Kilpatrick et al., 2013), mouse pancreatic acinar cells (Menteyne, Burdakov, Charpentier, Petersen, & Cancela, 2006), and metastatic colorectal cancer cells (Faris et al., 2019), while GPN-evoked Ca2+ entry has been reported in bovine corneal endothelial cells (Srinivas et al., 2002). The ER, which is seemingly recruited by lysosomal-derived Ca2+ in ECFCs, is a heterogeneous organelle that can be subdivided into distinct functional compartments (Berridge, 2002). For instance, a recent investigation revealed that InsP3Rs are closely coupled to SOCE only when they are located in close proximity of the PM (Thillaiappan, Chavda, Tovey, Prole, & Taylor, 2017). It is, therefore, conceivable that in ECFCs, lysosomal Ca2+ release recruits a pool of InsP3Rs which is located deeper within the cell and is, therefore, not coupled to SOCE (Taylor & Machaca, 2018).
4.2 NAADP-induced lysosomal Ca2+ release is mediated by TPC1 and requires InsP3-dependent ER Ca2+ mobilization
NAADP has recently been shown to play a crucial role in endothelial Ca2+ signaling (Moccia, Berra-Romani et al., 2018). For instance, NAADP mediates acetylcholine-induced Ca2+ signaling and NO release in human aortic endothelial cells (Brailoiu et al., 2010) and human brain microvascular endothelial cells (Zuccolo et al., 2017). In addition, NAADP triggers Ca2+-dependent NO production also in human brain microvascular endothelial cells challenged with glutamate (Negri et al., 2019), histamine (Berra-Romani, Faris, Pellavio et al., 2019), and arachidonic acid (Berra-Romani, Faris, Negri et al., 2019). Likewise, NAADP drives glutamate-induced intracellular Ca2+ oscillations and NO production in mouse brain microvascular endothelial cells (Zuccolo, Kheder et al., 2019) as well as histamine-dependent Ca2+ release and vWF liberation in EA.hy926 cells (Esposito et al., 2011). Finally, a recent series of investigations revealed that NAADP promotes VEGF-induced Ca2+ release, proliferation, and tube formation in HUVECs and promoted angiogenesis in vivo (Favia et al., 2014, 2016; Moccia, Negri, Shekha et al., 2019). Distinct TPC isoforms were identified in mature endothelial cells depending on both species and vascular district under examination. TPC1 was the main isoform detected in mouse brain microvascular endothelial cells (Zuccolo, Kheder et al., 2019), while TPC2 was the most expressed isoform in HUVECs (Favia et al., 2014). Conversely, transcripts encoding for both TPC1 and TPC2 were found in human brain microvascular endothelial cells (Zuccolo, Laforenza et al., 2019). Preliminary observations showed that TPC1 messenger RNA was the most abundant isoform present in circulating ECFCs (Zuccolo, Dragoni et al., 2016), a finding that was confirmed by immunoblotting in the present investigation. The following pieces of evidence indicate that TPC1 mediates NAADP-evoked Ca2+ release from lysosomes and is then amplified into a regenerative Ca2+ wave by InsP3Rs. First, NAADP-evoked intracellular Ca2+ mobilization was abolished by depletion of the lysosomal Ca2+ pool with either GPN or nigericin. Second, pharmacological (with NED-19) and genetic (through a specific siTPC1) blockade of TPC1 prevented the endogenous Ca2+ response to NAADP. Third, depletion of the ER Ca2+ reservoir with CPA and pharmacological inhibition of InsP3Rs with 2-APB dramatically reduced NAADP-induced intracellular Ca2+ mobilization. Fourth, RyRs are absent in ECFCs (Sanchez-Hernandez et al., 2010; Zuccolo, Dragoni et al., 2016). As discussed above, ER Ca2+ mobilization is clearly tempered by lysosomal disruption. Therefore, it is reasonable to conclude that InsP3Rs are necessary to amplify NAADP-induced local Ca2+ release in ECFCs, although we cannot rule out the possibility that a discrete pool of lysosomes is refilled by closely apposed InsP3Rs in a silent manner. Early work disclosed that NAADP gates an inwardly-rectifying Ca2+-selective current which displayed biophysical properties similar to the Ca2+ release-activated Ca2+ current in starfish oocytes (Moccia, Billington et al., 2006; Moccia, Nusco, Lim, Kyozuka, & Santella, 2006). However, NAADP did not evoke extracellular Ca2+ entry in circulating ECFCs, thereby confirming that the acidic Ca2+ store recruited by this second messenger is uncoupled from SOCE. Collectively, these data demonstrate for the first time that EL Ca2+ release is functionally coupled to ER-embedded InsP3Rs also in the endothelial lineage and suggest that the Ca2+-dependent interplay between TPC1 and InsP3Rs may shape NAADP-induced endothelial Ca2+ signals.
4.3 NAADP mediates VEGF-induced intracellular Ca2+ oscillations and proliferation, but not ATP-induced Ca2+ release
NAADP-induced endothelial Ca2+ release may be selectively recruited by specific extracellular autacoids. For instance, while NAADP supported the Ca2+ response to acetylcholine in human aortic endothelial cells, ATP-induced Ca2+ signals were unaffected by bafilomycin A1 and NED-19 (Brailoiu et al., 2010). Likewise, pharmacological blockade of TPCs with NED-19 blocked histamine-, but not thrombin-induced Ca2+ release and vWF release in Ea.hy926 cells (Esposito et al., 2011). Therefore, we sought to assess the role of NAADP in the Ca2+ response to ATP and VEGF, which evoke intracellular Ca2+ signals in ECFCs by, respectively, activating purinergic P2Y receptors and VEGF receptor-2 (Dragoni et al., 2011; Sanchez-Hernandez et al., 2010). ATP-evoked intracellular Ca2+ mobilization was weakly affected by depleting the lysosomal Ca2+ pool with either GPN or nigericin, but it was unaffected by pharmacological (with NED-19) and genetic (with the selective siTPC1) blockade of TPC1. These findings demonstrate that TPC1 does not contribute to ATP-induced increase in [Ca2+]i in circulating ECFCs, while the modest inhibition exerted by GPN and nigericin reflects the mobilization of the ER Ca2+ pool. As already mentioned above, this finding also shows that lysosomes do not buffer Ca2+ delivered into the cytosol through InsP3Rs. We then focused on VEGF, which stimulates circulating ECFCs to proliferate and assembly into bidimensional tubular networks in Matrigel by inducing repetitive oscillations in [Ca2+]i (Dragoni et al., 2011, 2015; Lodola et al., 2017). As reported in HUVECs for TPC2 (Favia et al., 2014), the Ca2+ response to VEGF in ECFCs was fully prevented by pharmacological and genetic deletion of TPC1. In addition, VEGF failed to induce intracellular Ca2+ oscillations upon depletion of the lysosomal Ca2+ store with nigericin and GPN. These findings strongly suggest that NAADP triggers repetitive Ca2+ spikes by stimulating TPC1 to mediate lysosomal Ca2+ release. Previous work revealed that VEGF-induced intracellular Ca2+ oscillations are shaped by rhythmical Ca2+ release through InsP3Rs and are sustained over time by SOCE (Dragoni et al., 2011; Lodola et al., 2017). On the basis of evidence provided in the present investigation, the oscillatory Ca2+ activity could be initiated by NAADP via TPC1 and then transformed into global Ca2+ waves by InsP3Rs, while SOCE prevents the ER (and possibly the EL) Ca2+ pool from depleting. The functional interaction between NAADP, InsP3, and SOCE is necessary to promote in vitro angiogenesis as the pharmacological and genetic blockade of TPC1 prevents VEGF-induced ECFC proliferation. These findings extend to circulating ECFCs the requirement of NAADP signaling for VEGF to trigger a proangiogenic signal. It has recently proposed that genetic manipulation of the Ca2+ toolkit could represent an alternative strategy to boost the reparative potential of autologous ECFCs and improve the regenerative outcome of cell based therapy of ischemic diseases (Moccia, Berra-Romani et al., 2018; Moccia, Lucariello et al., 2018). Future work could challenge this hypothesis by assessing whether TPC1 overexpression in ECFCs increases their angiogenic activity in preclinical models of hindlimb ischemia or acute myocardial infarction. Interestingly, preliminary evidence demonstrated that liposomal delivery of NAADP was per se able to deliver a Ca2+-dependent mitogenic signal to circulating ECFCs (Di Nezza et al., 2017).
4.4 Limitations of the investigation
The present investigation demonstrated that NAADP activates TPC1 to induce intracellular Ca2+ release and initiated VEGF-induced proangiogenic Ca2+ oscillations in circulating ECFCs. A number of caveats, however, will certainly require future investigation.
First, single-channel analysis on lipid bilayers revealed that TPC1 displays the following permeability sequence: H+ >> K+ > Na+ ≥ Ca2+ (Pitt, Lam, Rietdorf, Galione, & Sitsapesan, 2014; Rybalchenko et al., 2012). Thus, its Ca2+ permeability is limited as compared to TPC2 (Pitt et al., 2010). Nevertheless, as recently reviewed (Galione, 2019), even a small TPC1-mediated Ca2+ flux could be sufficient to generate local Ca2+ microdomains that are then amplified by the Ca2+-dependent recruitment of adjoining InsP3Rs. Indeed, assuming that TPC1-mediated Na+ currents drive EL Ca2+ release is unlike because of the unfavorable effect of the following lysosomal membrane depolarization on Ca2+ egress into the cytoplasm (Galione, 2019; Morgan & Galione, 2014). Patch-clamping enlarged lysosomes is required to confirm that TPC1 conducts intraluminal Ca2+ also in circulating ECFCs.
Second, NAADP-induced increase in Fura-2 fluorescence was fully abolished despite the 60% reduction in TPC1 expression achieved by the specific siTPC1 employed in the present investigation. Three mechanisms could be invoked to explain this seemingly contradictory result. First, the remaining TPC1 protein (≈40%) is not expressed in EL membranes. Second, our epifluorescence imaging system does not detect subcellular increases in intracellular Ca2+ concentration. Thus, we cannot rule out that some TPC1-mediated Ca2+ release events still occurred but were missed by our charge-coupled device camera. This hypothesis would be further consistent with the notion that, although lysosomes contain ≈500 µM free Ca2+, they occupy ≈3% of the total endothelial cell volume and are sparse throughout the cytoplasm (Lloyd-Evans & Waller-Evans, 2019). Thus, reducing TPC1 expression by 60% could lead to discrete subcellular Ca2+ signals that are detectable only by confocal or 2-photon microscopy (Boittin, Galione, & Evans, 2002; Kinnear et al., 2008), especially if the remaining TPC1 are uncoupled from the ER. For instance, NAADP was found to induce discrete Ca2+ signals that did not significantly increase Fura-2 fluorescence until they let to ER Ca2+ mobilization in rat pulmonary artery vascular smooth muscle cells (Kinnear et al., 2008). Third, TPC1 is sensitive to cytosolic Ca2+ (Lagostena, Festa, Pusch, & Carpaneto, 2017; Pitt et al., 2014). It has been postulated that upon NAADP-induced EL Ca2+ release, the local elevation in cytosolic Ca2+ could prolong TPC1 activation (Pitt et al., 2014). Thus, even a 60% reduction in TPC1 expression could reduce the Ca2+ response to NAADP to such an extent to ablate the Ca2+-dependent activation of the remaining TPC1 proteins (Pitt et al., 2014), which would, therefore, become unresponsive to NAADP stimulation. This hypothesis is further supported by the earlier report that NAADP-induced EL Ca2+ release in sea urchin eggs was ablated by chelating cytosolic Ca2+ with BAPTA (Morgan et al., 2013).
Finally, it is still unclear how NAADP gates TPCs. It has been suggested that NAADP acts through the interposition of a NAADP-binding protein, which could enable this versatile second messenger to activate either TPCs or RyR1 (Guse, 2012). Photoaffinity labeling studies revealed that a doublet of 22/23 kDa accessory proteins, rather than TPCs, bind to NAADP in naive mammalian cells (Lin-Moshier et al., 2012). It is, however, still unclear whether these NAADP-binding proteins are also present in circulating ECFCs.
5 CONCLUSION
The present investigation demonstrated that the lysosomal and ER Ca2+ stores are functionally coupled by InsP3Rs in circulating ECFCs. We proposed that the main function of ER-embedded InsP3Rs is to amplify lysosomal Ca2+ mobilization into a global elevation in [Ca2+]i through the process of CICR. We further showed that NAADP-evoked Ca2+ release in circulating ECFCs is triggered by TPC1-mediated lysosomal Ca2+ release and sustained by InsP3-dependent Ca2+ mobilization. Finally, we showed that NAADP is selectively recruited to initiate VEGF-induced intracellular Ca2+ oscillations, but not ATP-induced Ca2+ release. These findings support the emerging evidence about the crucial role played by NAADP in blood vessel formation and in the cardiovascular system by demonstrating that it could also support VEGF-dependent vasculogenesis.
ACKNOWLEDGMENTS
Francesco Moccia was supported by Italian Ministry of Education, University and Research: Dipartimenti di Eccellenza Program (2018–2022)—Department of Biology and Biotechnology "L. Spallanzani," University of Pavia; by Fondo Ricerca Giovani from the University of Pavia; and by the EU Horizon 2020 FETOPEN-2018-2020 Program under Grant Agreement No. 828984 (LION-HEARTED).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




