PRR34-AS1 overexpression promotes protection of propofol pretreatment against ischemia/reperfusion injury in a mouse model after total knee arthroplasty via blockade of the JAK1-dependent JAK-STAT signaling pathway
Abstract
Long noncoding RNAs have been documented to be protective against ischemia/reperfusion (I/R) injury. However, few research works have focused on the protective effects of PRR34-AS1 on I/R injury after total knee arthroplasty (TKA). The objective of the present study was to investigate the possible effect of PRR34-AS1 on I/R injury after TKA. A mouse model with I/R injury after TKA was established. The interaction between PRR34-AS1 and Janus kinase 1 (JAK1) was examined and thoroughly investigated. Next, the effects of PRR34-AS1 on the expression of apoptosis-related proteins, JAS-signal transducer and activator of transcription (STAT) signaling pathways, and inflammation-related genes, chondrocyte proliferation, and apoptosis were analyzed after gain- and loss-of-function experiments. Attenuated symptoms were observed in mice pretreated with propofol, which was evidenced by decreased positive expression rate of JAK1 protein and superoxide dismutase content along with increased malondialdehyde content and IL-10 levels. PRR34-AS1 was poorly expressed in mice with I/R injury after TKA. JAK1 was a target of PRR34-AS1. Upregulated PRR34-AS1 diminished expression of JAK1, STAT1, JAK2, and STAT3 as well as cell apoptosis, while enhancing cell proliferation in vitro. Furthermore, JAK1 silencing could reverse the suppressed cell proliferation and enhanced cell apoptosis of chondrocytes imposed by silencing PRR34-AS1. Upregulation of PRR34-AS1 can potentially relieve I/R injury after TKA in mice pretreated with propofol through inhibition of the JAS-STAT signaling pathway by targeting JAK1.
1 INTRODUCTION
Several disorders, such as stroke, peripheral vascular disease, and myocardial infarction, which are characterized by ischemia/reperfusion (I/R), still prevail as the most prevalent causes responsible for debilitating diseases and mortality (Kalogeris, Baines, Krenz, & Korthuis, 2012). Total knee arthroplasty (TKA) is the most common remediation for knee pain from osteoarthritis, but the tourniquet, typically applied during a TKA, causes ischemia or reperfusion to the lower limbs (Hocker et al., 2013; Muyskens et al., 2016). However, to prevent I/R injury, pharmacological intervention and ischemic preconditioning have been established, with the mechanisms of cardioprotection being investigated (Lien et al., 2018). It has been revealed in previous research that several drugs can protect against I/R injury. For example, propofol, an intravenous anesthetic agent, induces cardioprotective effects in isolated hearts which are subject to I/R injury; this highlights propofol as a potential option in I/R injury treatment (Shravah et al., 2014). Subsequently, the incidence of reperfusion and postischemic damage remains universal (Wang, Wu, & Zhu, 2018).
However, recent evidence suggests that long noncoding RNAs (lncRNAs) are involved in I/R injury (Tao et al., 2019). For example, MALAT1 plays a vital role in the cardioprotective effects of fentanyl in myocardial I/R injury (Zhao et al., 2017). Moreover, lncRNA AK139328 silencing has the potential to attenuate I/R injury in mouse livers, indicating its potential as a primary target in diagnosis or treatment of a liver surgery or transplantation (Chen et al., 2013). PRR34-AS1 is closely associated to the early tumor recurrence in bile duct cancer, as Janus kinase-signal transducer and activator of the transcription (JAK-STAT) signaling pathway is the primary pathway (Becker et al., 2016; Kang et al., 2014). Therefore, the JAK-STAT signaling pathway is suggested during the period of myocardial I/R (Bolli, 2003). Additionally, the JAK-STAT signaling pathway has been demonstrated to modulate I/R injury-induced apoptosis in a mouse model (Oh et al., 2013). JAK1 has been illustrated to be activated following cerebral I/R (Huang et al., 2015). Fractional inhibition of JAK1 and JAK2 is sufficient for significant activity in autoimmune diseases (Fridman et al., 2010). JAK1 is responsible for STAT3 activation in lung cancer cells (Song, Rawal, Nemeth, & Haura, 2011). Propofol has been associated with the activation of the cytoprotective phosphoinositide 3-kinase/protein kinase B and JAK2/STAT3 signaling pathways to induce an adaptive response during cardiac surgery as a preemptive cardio-protectant (Shravah et al., 2014). It has also been reported in previous studies that propofol postcondition has the ability to regulate the JAK2/STAT3 signaling pathway to ameliorate hippocampus I/R injury in rats (Jia et al., 2017). Based on the aforementioned literature, we proposed a hypothesis that PRR34-AS1 may affect I/R injury after TKA involving the regulation of the JAK1 and JAK2/STAT3 signaling pathway. Thus, the current study aims to elucidate the effect of PRR34-AS1 on propofol-pretreated mice with I/R injury after TKA through the JAK-STAT signaling pathway by binding to JAK1.
2 MATERIALS AND METHODS
2.1 Ethics statement
This study was conducted with the approval of the Institutional Animal Care and Use Committee of Guizhou Provincial People's Hospital and in strict accordance to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The mice included in the study were treated kindly and precautions were taken for minimal animal suffering.
2.2 Establishment of I/R injury mouse model after TKA
A total of 42 clean grade Kunming mice (weight: 18–22 g Experimental Animal Center of Guizhou University, Guiyang, Guizhou, China) were anesthetized via intraperitoneal injection of 1% sodium pentobarbital (50 mg/kg; P3761; Sigma-Aldrich, St. Louis, MO). The mice were then fixated on a surgical board; the skin on the middle of the knee to the joint capsules was incised. Next, the right knee joints were exposed and placed into steel-pin prostheses (diameter: 0.2 mm). The incisions were washed, sutured layer by layer, and covered using alcohol-soaked gauzes. Then, 36 mice were selected and evenly classified into three groups randomly: The pretreatment group (mice injected with propofol on femoral sides of the knee joint at 2 mg/kg, 30 min before I/R surgery), I/R group (mice injected with normal saline on femoral sides of the knee joint), and normal group (mice injected with normal saline on femoral sides of the knee joint).
Mice in the pretreatment and I/R groups were anesthetized and fixed using 3 ml 0.5% lidocaine as a local anesthetic. Gelatin sponges were stuffed in the medullary cavities of mice to restrict the blood supply in the marrow cavity. Femoral arteries and veins were clipped. The location of clips was replaced from proximal veins to distal veins every 2 hr for protection of intima as a precaution for thrombus formation. After the incisions were sterilized, sterile towels and gelatin sponges were discarded. Blood supply was restored and incisions were sutured. Penicillin (400,000 units, 130437; Beijing Biobw Biotechnology Co., Ltd., Beijing, China) was injected into the mice 30 min after the surgery was finished. The mice were injected with penicillin (400,000 units/d) for 1 consecutive week. Moreover, ischemia lasted for 8 hr, while reperfusion lasted for 1 week. The mice in the normal group were not touched.
Next, the mice were euthanized 1 week after surgery. Knee joint capsule of the mice was dissected to extract synovial tissues and cartilages in the weight-bearing area on the lateral femoral condyle. Synovial tissues and cartilages were refrigerated at −20°C for further experimentations. Synovial tissues (weighing approximately 0.2 g) were rinsed using normal saline, with the impurities being removed. The tissues were dried with filter paper, added with homogenate medium (weight: Volume, 1:9), incised into sections in a water bath, and reacted in a homogenizer 3–5 times until cells were disrupted. Then, the tissues were used to prepare 10% tissue homogenate, which was then centrifuged for 15 min at 1,610g. Next, the supernatant was stored in a −20°C freezer for follow-up measurement and observation. Hematoxylin-eosin (HE) staining and immunohistochemistry (IHC) were carried out as previously stated (Shi et al., 2011). The staining results were observed using an optical microscope (DSX100; Olympus, Tokyo, Japan) and photographed. At last, the mean optical density (OD) of positive staining was determined in five randomly selected fields using Image-Pro Plus 7.0 software.
2.3 Malondialdehyde and superoxide dismutase determination
Malondialdehyde (MDA) and superoxide dismutase (SOD) concentrations in the tissue homogenate were examined after kit 1 (ml531021) and kit 2 (ml001998) (all from Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China), respectively.
2.4 Enzyme-linked immunosorbent assay
The obtained tissue homogenate was used to prepare standard materials by following the strict instructions provided by the interleukin-1β (IL-1β) enzyme-linked immunosorbent assay (ELISA) kit (ml023125), IL-6 ELISA kit (ml023130), IL-10 ELISA kit (ml037873), and the tumor necrosis factor-α (TNF-α) ELISA kit (ml002098) (all from Shanghai Enzyme-linked Biotechnology Co., Ltd.). OD values were measured at 450 nm wavelength. The concentrations of IL-1β, IL-6, IL-10, and TNF-α in knee joint tissues were calculated according to the regression equation of the standard curve.
2.5 Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using an ultrapure RNA extraction kit (D203-01; GenStar Biosolutions Co., Ltd., Beijing, China). Primers were then designed and synthesized using Takara (Tokyo, Japan; Table 1). Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was constructed as per the instructions of TaqMan microRNA assays reverse transcription primer (4366596; Thermo Fisher Scientific, Waltham, MA). The reaction solution was used for fluorescent quantitation PCR in strict accordance to the instructions provided by the SYBR® Premix Ex TaqTM II kit (RR820A; Action-award Biotech Co., Ltd., Guangzhou, Guangdong, China). Fluorescent quantitation PCR was performed in the ABI PRISM® 7300 system (Shanghai Kunke Equipment Cp., Ltd.). Small nuclear ribonucleic acid 6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as internal controls.
| Gene | Sequence |
|---|---|
| PRR34-AS1 | F: 5′-AGAACCGAGGCCATCTTTGG-3′ |
| R: 5′-TAACGGAGGGGGACCAATCT-3′ | |
| JAK1 | F: 5′-AATGTTCTCTATGAGGTCATGGTGACT-3′ |
| R: 5′-CAGTTTTTTCCGCTTCAGTTTATTT-3′ | |
| STAT1 | F: 5′-GCTGGGCGTCTATCCTGTGGT-3′ |
| R: 5′-GCTCAGCTGGTCTGCGTTCA-3′ | |
| Caspase-3 | F: 5′-CTAAGCCATGGTGATGAAGGG-3′ |
| R: 5′-CTGCAAAGGGACTGGATGAAC-3′ | |
| FasL | F: 5′-AGCCCCTAAACCACAAGGTC-3′ |
| R: 5′-TGAATACTGCCCCCAGGTAG-3′ | |
| Bax | F: 5′-CTGAGCTGACCTTGGAGC-3′ |
| R: 5′-GACTCCAGCCACAAAGATG-3′ | |
| Bcl-2 | F: 5′-TGCACCTGAGCGCCTTCAC-3′ |
| R: 5′-TAGCTGATTCGACCATTTGCCTGA-3′ | |
| GAPDH | F: 5′-GAACGGGAAGCTTGTCATCAA-3′ |
| R: 5′-CTAAGCAGTTGGTGGTGCAG-3′ |
- Abbreviations: Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated protein X; FasL, CD95 ligand; F, forward; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; JAK1, Janus kinase 1; R, reverse; STAT1, signal transducer and activator of transcription 1; U6, small nuclear ribonucleic acid 6.
2.6 Western blot analysis
The protein concentration of the supernatant in individual samples was determined following the instructions of a bicinchoninic acid protein assay kit (20201ES76, Shanghai Yeasen Biotechnology Co., Ltd., Shanghai, China). Moreover, the protein was separated using 10% separation gel and spacer gel, electroblotted onto nitrocellulose membranes and blocked with 5% skimmed milk. The overnight incubation of membrane was conducted with diluted primary rabbit polyclonal antibodies against JAK1 (1:2,000; ab47435), STAT1 (1:500; ab31369), cleaved caspase-3 (1:500; ab49822), FasL (1:1,000; ab15285), B-cell lymphoma 2 (Bcl-2; 1:1,000; ab32124), Bcl-2 associated protein (Bax; 1:2,000; ab32503), JAK2 (1:5,000; ab108596), STAT3 (1:2,000; ab109085), GAPDH (1:10,000; ab181602) (all from Abcam Inc., Cambridge, MA), phosphorylation of JAK1 (p-JAK1; 1:500, ab138005; Santa Cruz, CA), and phosphorylation of STAT1 (p-STAT1; 1:500, ab30645; Santa Cruz). Furthermore, the membrane was incubated by mixing the horseradish peroxidase-labeled secondary goat antibody against the rabbit immunoglobulin G (1:1,000; Wuhan Boster Biological Technology Co., Ltd., Wuhan, Hubei, China) at 37°C for 1 consecutive hour. The results were visualized using enhanced chemiluminescence reagent (ECL; Pierce-Thermo Fisher Scientific). The ratio of the gray value of the target band to GAPDH was representative of the relative protein expression.
2.7 Vector construction
In accordance to the known transcript sequence of PRR34-AS1 and JAK1 in GenBank, the full-length sequences of PRR34-AS1 and JAK1 were designed online (Ambion, Austin, TX) and synthesized by Shanghai GeneChem Co., Ltd., (Shanghai, China). The correctly sequenced vectors were cloned into pcDNA3.1 plasmid vector (VPI0001; Invitrogen, Carlsbad, CA), which contained restricted enzyme sites Hind III and Xho I. BLOCK-iTTM RNAi Designer (Invitrogen) was used to synthesize small interfering RNA (siRNA) and negative control (NC) sequences. Double-stranded DNA was cloned into the lentivirus vector pRNA-Lenti-GFP. All ligation products were added into Escherichia coli DH5α (D9052; Takara) for transformation.
2.8 Cell transfection and grouping
The cartilage tissue at the knee joints of the mice was isolated, cultured, and identified (Chan et al., 2018; Wang et al., 2018). Cells which were pretreated with propofol were transfected with NC plasmids, overexpressed PRR34-AS1 plasmids, overexpressed JAK1 plasmids, PRR34-AS1 siRNA plasmids, or JAK1 siRNA plasmids. After 0.25% trypsin was used for detachment, the cells were resuspended to a density of 1 × 105 cells/ml with the use of M199 culture medium containing 10% fetal bovine serum, and further seeded into a six-well plate. The cells were then cultured in serum-free M199 culture medium for 24 hr, followed by transfection according to the instructions of lipofectamine 2000 (11668-019; Invitrogen) once cell confluence reached approximately 70%. Next, and the cells underwent additional culture in the complete culture medium. The cells were harvested after 48 hr of transfection.
2.9 Dual-luciferase reporter gene assay
Target genes of PRR34-AS1 were predicted by analyzing the lncRNA Targets available at http://www.herbbol.org:8001/lrt/. HEK-293T cells (AT-1592; ATCC, Manassas, VA). They were then seeded into a 24-well plate and underwent 24 hr of culture. JAK1 vector pmiRRB-NFKBIA-3′-untranslated region (3′-UTR) was artificially constructed. PmiRRB-NFKBIA-3′-UTR was cotransfected with PRR34-AS1 vector, siRNA-PRR34-AS1, or NC, respectively into HEK-293T cells. Dual-luciferase® Reporter Assay System (E1910; Promega Corporation, Madison, WI) was utilized to measure the luciferase activity; the relative luciferase activity was expressed as the ratio of the activity of firefly luciferase to renilla luciferase.
2.10 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
Cells under the logarithmic growth phase were seeded into a 96-well plate at a density of 1 × 104 cells/well. A total of 10 μl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml; ST316; Beyotime Institute of Biotechnology, Shanghai, China) was added into each well and further incubated at 37°C respectively for 24, 48, and 72 hr once cells confluence reached approximately 70%. Next, 100 μl dimethyl sulfoxide (DMSO; D5879; Sigma-Aldrich) was then added in individual wells. OD value at a wavelength of 490 nm was measured using a microplate reader (MK3; Thermo Fischer Scientific, Pittsburgh, PA).
2.11 Flow cytometry
The cells were detached using 0.25% trypsin, and further centrifuged for 5 min (178g) at 4°C, after which the supernatant was discarded. This procedure was repeated several times, followed by 10 μl RNase enzyme being added in the cells. Next, the cells were incubated for 5 min at 37°C, and 1% propidium iodide (PI; 40710ES03; Qcbio Science & Technologies Co., Ltd., Shanghai, China) was added to the cells for 30-min staining in avoidance to light. Furthermore, cell apoptosis was detected using the Annexin V-fluorescein isothiocyanate/PI apoptosis detection kit (CA1020; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). Finally, cell cycle distribution along with cell apoptosis was measured using a flow cytometer.
2.12 Statistical analysis
SPSS 21.0 statistical software (International Business Machines Corporation, Armonk, NY) was employed to conduct data statistical analyses. The measurement data were expressed as mean ± standard deviation. Data between two groups were compared using t test. Data among multiple groups were analyzed using one-way analysis of variance. The enumeration data were expressed as rate or percentage and analyzed by applying the χ2 test. A value of p < .05 was considered to be indicative of a significant difference.
3 RESULTS
3.1 Pretreatment of propofol ameliorates I/R injury after TKA
Histopathological differences of knee joint tissues were observed using HE staining. As depicted in Figure 1, I/R mice pretreated with propofol exhibited mild edema in the synovial mesenchyme with no capillary congestion or endothelial cell injury. No necrosis or exfoliation of the superficial synovial cells was shown besides minor inflammatory cell infiltration. Therefore, in I/R mice, hemangiectasis and capillary congestion were displayed in the synovial joint with visible edema in interstitial substance. In addition, the synovial cells were necrotic and detached from the surface, and uneven distribution of inflammatory cells in the mesenchyme was observed. Clear chondrocytes in knee joint tissues of mice were observed in normal mice, therefore indicating that no significant changes were present.
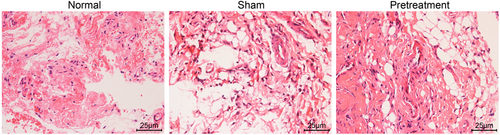
HE staining analysis of the knee joint tissues in I/R mice with propofol pretreatment. HE, hematoxylin-eosin; I/R, ischemia/reperfusion [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Pretreatment of propofol decreases the positive expression rate of JAK1 protein in mice with I/R injury after TKA
Subsequent IHC results displayed the lowest positive JAK1 rate in normal mice. The cytoplasm of the nerve cells in I/R mice was brown-stained, which indicated a highly positive rate. The positive expression rate of JAK1 was relatively lower in propofol-pretreated I/R mice in comparison to I/R mice (p < .05; Figure 2a,b). Therefore, pretreatment of propofol helps reduce the positive expression rate of JAK1 in mice suffering I/R injury after TKA.
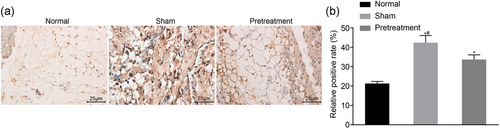
Propofol pretreatment slightly reduces the positive expression rate of JAK1 protein in I/R mice after TKA. (a) IHC analysis of JAK1-positive cells in the normal, I/R and pretreatment groups. (b) positive expression of rate of JAK1 protein in the normal, I/R, and pretreatment groups. IHC, immunohistochemistry; I/R, ischemia/reperfusion; JAK, Janus kinase; TKA, total knee arthroplasty. *p < .05 versus the normal group; #p < .05 versus the pretreatment group [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Pretreatment of propofol lessens oxidative stress in mice with I/R injury after TKA
The results collected from the measurement of both MDA and SOD contents indicated that in comparison to normal mice, MDA content was upregulated while SOD content was downregulated in all I/R mice (all p < .05). However, in comparison with propofol-pretreated I/R mice, increased MDA content and decreased SOD content were distinguished in I/R mice (p < .05; Table 2). It can be concluded that pretreatment of propofol could mitigate oxidative stress in mice with I/R injury after TKA.
| Group | Case | SOD (U/mg) | MDA (nmol/mg) |
|---|---|---|---|
| Normal | 12 | 243.67 ± 21.32 | 8.45 ± 1.03 |
| I/R | 12 | 189.49 ± 16.37*, ** | 21.87 ± 1.93*, ** |
| Pretreatment | 12 | 217.26 ± 19.58* | 15.72 ± 1.46* |
- Abbreviations: I/R, ischemia/reperfusion; MDA, malondialdehyde; SOD, superoxide dismutase.
- * p < .05 versus the normal group.
- ** p < .05 versus the pretreatment group.
3.4 Pretreatment of propofol alleviates inflammatory injury in I/R mice after TKA
Furthermore, the levels of TNF-α, IL-6, IFN-γ, and IL-10 were assessed in I/R mice pretreated with propofol. The results were as follows: elevated levels of TNF-α, IL-6, and IFN-γ and lower IL-10 level were observed in all I/R mice versus normal mice (all p < .05). In comparison to I/R mice pretreated with propofol, the levels of TNF-α, IL-6, and IFN-γ were elevated, while IL-10 level was reduced in I/R mice (p < .05; Table 3). Collectively, pretreatment of propofol can disrupt the inflammatory response in I/R mice following TKA.
| Group | N | TNF-α (pg/mL) | IL-6 (pg/mL) | IFN-γ (pg/mL) | IL-10 (pg/mL) |
|---|---|---|---|---|---|
| Normal | 12 | 113.46 ± 9.63 | 26.54 ± 2.42 | 66.64 ± 5.82 | 46.48 ± 4.06 |
| I/R | 12 | 137.65 ± 10.58*, ** | 52.36 ± 4.87*, ** | 107.34 ± 9.34*, ** | 21.24 ± 1.68*, ** |
| Pretreatment | 12 | 125.58 ± 10.14* | 38.39 ± 3.64* | 84.86 ± 8.12* | 34.56 ± 2.87* |
- Abbreviations: IFN-γ, interferon-γ; IL, interleukin; I/R, ischemia/reperfusion; TNF-α, tumor necrosis factor-α.
- * p < .05 versus the normal group.
- ** p < .05 versus the pretreatment group.
3.5 Pretreatment of propofol increases PRR34-AS1 expression and inactivates the JAK-STAT signaling pathway
RT-qPCR and western blot analysis were employed to detect PRR34-AS1 expression, cell apoptosis-, and JAK-STAT signaling pathway-related genes in I/R mice receiving propofol pretreatment. In comparison to normal mice, PRR34-AS1 and Bcl-2 expressions were reduced, while the expression of JAK1, STAT1, cleaved caspase-3, FasL, Bax, JAK2, and STAT3, as well as the extent of JAK1 and STAT1 phosphorylation, was increased in all I/R mice (all p < .05). Subsequently, in comparison with I/R mice, PRR34-AS1 and Bcl-2 expression was elevated, while the expression of JAK1, STAT1, cleaved caspase-3, FasL, Bax, JAK2, and STAT3, as well as the extent of JAK1 and STAT1 phosphorylation, was reduced in propofol-pretreated I/R mice (all p < .05; Figure 3a,c). Thus, pretreatment of propofol can elevate both PRR34-AS1 and Bcl-2 expression, while displaying a reduced expression of JAK1, STAT1, cleaved caspase-3, FasL, Bax, JAK2, and STAT3.
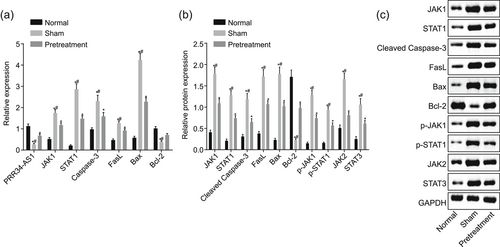
Propofol pretreatment increases expression of PRR34-AS1 and Bcl-2, while decreasing expression of JAK1, STAT1, cleaved caspase-3, FasL, Bax, JAK2, and STAT3 in I/R mice after TKA. (a) PRR34-AS1 expression and mRNA expression of Bcl-2, JAK1, STAT1, cleaved caspase-3, FasL, and Bax detected using RT-qPCR. (b and c) Western blot analysis of Bcl-2, JAK1, STAT1, cleaved caspase-3, FasL, Bax, JAK2, and STAT3 proteins. Bax, Bcl-2 associated protein X; Bcl-2, B-cell lymphoma 2; I/R, ischemia/reperfusion; JAK, Janus kinase; mRNA, messenger RNA; RT-qPCR, quantitative reverse transcription-quantitative polymerase chain reaction; STAT, signal transducer and activator of transcription. *p < .05 versus the normal group; #p < .05 versus the pretreatment group
3.6 PRR34-AS1 targets JAK1 and inhibits its activity
Online analyses predicted the binding sites between PRR34-AS1 and JAK1 3′-UTR, indicating that JAK1 was a target gene of PRR34-AS1 (Figure 4a). Dual-luciferase reporter gene assay results revealed that relative luciferase activity was inhibited in the presence of PRR34-AS1 vector, while that in the absence of PRR34-AS1 was enhanced (p < .05; Figure 4b). The aforementioned results demonstrated that JAK1 was the target of PRR34-AS1 and downregulated by PRR34-AS1.
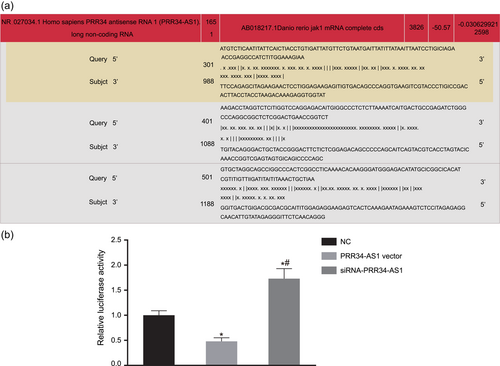
JAK1 is a target gene of PRR34-AS1. (a) The binding site between PRR34-AS1 and 3′-UTR of JAK1 predicted by the target prediction program. (b) Relative luciferase activities detected by dual-luciferase reporter gene assay. 3′-UTR, 3′-untranslated region; JAK, Janus kinase; NC, negative control. *p < .05 versus cells treated with NC; #p < .05 versus cells transfected with PRR34-AS1 vector [Color figure can be viewed at wileyonlinelibrary.com]
3.7 Upregulation of PRR34-AS1 elevates Bcl-2 expression while decreasing the expression of JAK1, STAT1, cleaved caspase-3, FasL, Bax, JAK2, and STAT3 in chondrocytes
To elucidate the effect of PRR34-AS1 on cell apoptosis and the JAK-STAT signaling pathway, the expression of related genes was evaluated in propofol-pretreated cells treated with overexpressed or silenced PRR34-AS1. As depicted in Figure 5a,c, PRR34-AS1 and Bcl-2 expressions were increased, while JAK1, STAT1, cleaved caspase-3, FasL, Bax, JAK2, and STAT3 expression, as well as the extent of JAK1 and STAT1 phosphorylation, was decreased in all chondrocytes that were pretreated with propofol. PRR34-AS1 and Bcl-2 expression was upregulated, while JAK1, STAT1, cleaved caspase-3, FasL, Bax, JAK2, and STAT3 expression, as well as the extent of JAK1 and STAT1 phosphorylation, was downregulated in response to transfection of PRR34-AS1 vector and siRNA-JAK1; transfection of siRNA-PRR34-AS1 and JAK1 vector showed a contradictory tendency. siRNA-PRR34-AS1 effects on these genes were abrogated by siRNA-JAK1. On the basis of the aforementioned evidence, overexpressed PRR34-AS1 can inhibit the expression of JAK1, STAT1, cleaved caspase-3, FasL, Bax, JAK2, and STAT3 and promote Bcl-2 expression in chondrocytes.
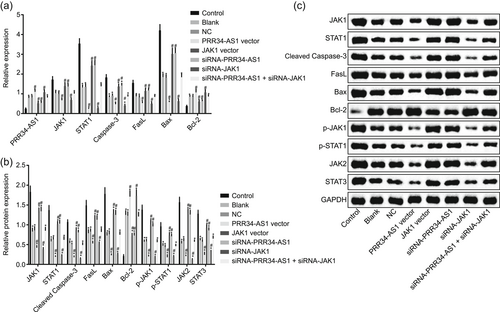
PRR34-AS1 overexpression inhibits the expression of JAK1, STAT1, cleaved caspase-3, FasL, Bax, JAK2, and STAT3 yet accelerates Bcl-2 expression in chondrocytes. (a) PRR34-AS1 expression and mRNA expression of Bcl-2, JAK1, STAT1, cleaved caspase-3, FasL, and Bax detected using RT-qPCR. (b and c) Western blot analysis of Bcl-2, JAK1, STAT1, cleaved caspase-3, FasL, Bax, JAK2, and STAT3 proteins. Bax, Bcl-2 associated protein X; Bcl-2, B-cell lymphoma 2; JAK, Janus kinase; mRNA, messenger RNA; NC, negative control; RT-qPCR, quantitative reverse transcription-quantitative polymerase chain reaction; siRNA, small interfering RNA; STAT, signal transducer and activator of transcription. *p < .05 versus control cells; #p < .05 versus cells treated with blank and NC
3.8 Upregulation of PRR34-AS1 or JAK1 silencing accelerates chondrocyte proliferation
To elucidate the effect of PRR34-AS1 on the proliferation of chondrocytes, MTT assay was employed in propofol-pretreated cells after being treated with overexpressed or silenced PRR34-AS1. As depicted in Figure 6, cell proliferation displayed no obvious differences after various treatments at 24 hr (p > .05). Moreover, cell proliferation was increased at 48 and 72 hr in comparison to that at the 24th hour (p < .05). Accelerated cell proliferation was detected in response to the pretreatment of propofol (p < .05). PRR34-AS1 vector transfection and siRNA-JAK1 exhibited increased cell proliferation, while transfection of siRNA-PRR34-AS1 and JAK1 vector displayed decreased cell proliferation in propofol-pretreated cells. The effect of siRNA-PRR34-AS1 on cell proliferation was reversed with siRNA-JAK1. In conclusion, overexpressed PRR34-AS1 and JAK1 silencing could promote chondrocyte proliferation.
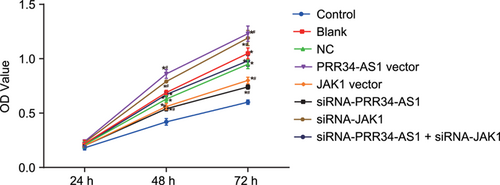
Upregulation of PRR34-AS1 or silencing JAK1 promotes chondrocyte proliferation. OD values at 24, 48 and 72 hr were measured using MTT assay. JAK, Janus kinase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NC, negative control; OD, optical density; siRNA, small interfering RNA. *p < .05 versus control cells; #p < .05 versus cells treated with blank and NC [Color figure can be viewed at wileyonlinelibrary.com]
3.9 Upregulation of PRR34-AS1 or JAK1 silencing results in less G0/G1 phase-arrested cells and more S phase-arrested cells of chondrocytes
To clarify the possible influence PRR34-AS1 has on the cell cycle, flow cytometry was employed in propofol-pretreated cells in response to transfection of overexpressed or silenced PRR34-AS1. As illustrated in Figure 7a,c, after pretreatment of propofol, less G0/G1 phase-arrested cells and more the S phase-arrested cells were exhibited. Less the G0/G1 phase-arrested cells and more the S phase-arrested cells were detected in propofol-pretreated cells upon transfection of PRR34-AS1 vector and siRNA-JAK1. Subsequently, the transfection of siRNA-PRR34-AS1 and JAK1 vector displayed contradictory results in comparison to the aforementioned factors. The effect of siRNA-PRR34-AS1 on cell cycle distribution was reversed with siRNA-JAK1. Collectively, PRR34-AS1 overexpression and JAK1 silencing can assist in the cell cycle entry of chondrocytes.
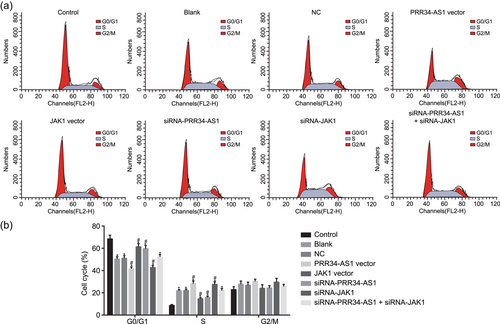
Upregulation of PRR34-AS1 or silencing JAK1 produces less G0/G1 phase-arrested cells and more the S phase-arrested cells in chondrocytes. (a) Flow cytometry analysis of PI-stained cells at G0/G1, S, and G2/M phases. (b) The percentage of PI-stained cells at G0/G1, S, and G2/M phases. JAK, Janus kinase; NC, negative control; PI, propidium iodide; siRNA, small interfering RNA. *p < .05 versus control cells; #p < .05 versus cells treated with blank and NC [Color figure can be viewed at wileyonlinelibrary.com]
3.10 Upregulation of PRR34-AS1 or JAK1 silencing inhibits chondrocyte apoptosis
To demonstrate the possible effect PRR34-AS1 has on cell apoptosis, flow cytometry was employed in propofol-pretreated cells in the presence of overexpressed or silenced PRR34-AS1. The results displayed that cell apoptosis rates after pretreatment of propofol were relatively lower. In propofol-pretreated cells, cell apoptosis rates decreased upon transfection of PRR34-AS1 vector and siRNA-JAK1, while they increased in response to transfection of siRNA-PRR34-AS1 and JAK1 vector. SiRNA-JAK1 abrogated the effect of siRNA-PRR34-AS1 on cell apoptosis (Figure 8). These abovementioned findings provided evidence suggesting that PRR34-AS1 overexpression and JAK1 silencing can impede chondrocyte apoptosis.
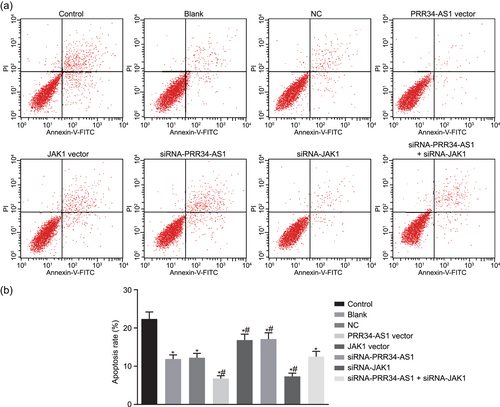
Upregulation of PRR34-AS1 or silencing JAK1 inhibits chondrocyte apoptosis. (a) Flow cytometric scatter plots of cells. (b) Cell apoptosis rates among different treatment detected by flow cytometry. JAK, Janus kinase; NC, negative control. *p < .05 versus control cells; #p < .05 versus cells treated with blank and NC [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
I/R injury primarily occurs due to the activation of leukocytes, endothelial dysfunction, and production of reactive oxygen species (Campanholle et al., 2010). Accumulating evidence has reported several lncRNAs to protect against I/R injury; for example, lncRNA AK12348 has been proven to be a potential candidate of an upcoming therapeutic target for myocardial I/R treatment (Yu, Tang, & Zhou, 2018; Zheng et al., 2018). Additionally, poorly expressed lncRNA H19 has been specified to exert cerebroprotective effects against I/R injury (Wang, Cao, Han, Sun, & Feng, 2017). Therefore, the current study aimed to explore the roles PRR34-AS1 has in I/R injury progression in mice after TKA. Altogether the findings suggested that upregulated PRR34-AS1 could potentially exercise its protective properties against I/R injury after TKA via downregulation of JAK1 and inhibition of the JAK-STAT signaling pathway.
We initially verified the high expression of JAK1 and low expression of PRR34-AS1 in mice with I/R injury after TKA. Similarly, JAK1 has been found to be activated post cerebral I/R (Huang et al., 2015). The pharmacologic inhibition of JAK1 has been highlighted to be a promising therapeutic approach to achieve the beneficial antileukemia effect and overcome human lymphocyte antigen-barriers in allogeneic hematopoietic stem cell transplantation (Choi et al., 2014). Moreover, lncRNA-N1LR has been found to be expressed at a relatively low level in a mouse model of cerebral I/R injury (Wu et al., 2017). Besides, lncRNA MEG3 was significantly downregulated in infarction lesion in comparison to hepatic I/R mice (Huang, Gao, Qin, & Lu, 2018). In the present study, online analyses confirmed binding sites between PRR34-AS1 and JAK1 3′-UTR in addition to the inhibited luciferase activity upon overexpressed PRR34-AS1 yet enhanced luciferase activity upon PRR34-AS1 silencing examined by dual-luciferase reporter gene assay results. Therefore, JAK1 was concluded to be a target gene of PRR34-AS1.
Another significant finding obtained from the study was that pretreatment of propofol exerted protective effects against I/R injury after TKA. Propofol, an intravenous anesthetic, has been demonstrated to attenuate hepatic I/R injury due to its ability in reducing oxidative stress (Ge et al., 2017). Moreover, a functional study has elucidated that cardiomyocyte apoptosis was inhibited along with a lower reactive oxygen species expression after myocardial I/R injury after propofol pretreatment (Seo et al., 2018). Another study conducted has also shown that pretreatment of propofol could promote the alleviation of myocardial I/R injury in a rat model (Sun et al., 2017). Propofol has been found to be renoprotective against I/R injury due to its ability in maintaining antioxidation ability and attenuation in the inflammatory response (Yoo et al., 2013). Propofol also exerts neuroprotective function in focal I/R injury through its inhibitory role in the inflammatory reaction by disrupting IL-1, TNF-α, and ICAM-1 serum levels (Feng, Ma, Yue, Zhang, & Qu, 2004). The aforementioned findings were consistent with the present study, which was as follows: Propofol pretreatment decreased MDA content and serum levels of TNF-α, IL-6, and IFN-γ while increasing SOD content and IL-10 level, ultimately preventing I/R injury after TKA.
Additionally, the present study demonstrated that PRR34-AS1 blocked the activation of the JAK-STAT signaling pathway, which was evidenced by decreased JAK1, STAT1, FasL, JAK2, and STAT3 expression in chondrocytes transfected with overexpressed PRR34-AS1. LncRNAs are able to work as downstream effectors of the JAK-STAT signaling pathway and/or exert effects on signaling pathway through modulating the expression level of JAK-STAT components (Witte & Muljo, 2014). The hippocampal glial cells are activated by lncRNA H19 through its negative regulation on the JAK/STAT signaling pathway, bringing new and hopeful advancements for epilepsy therapy (Han et al., 2018). LncRNA PMS2L2 exhibits a protective role in lipopolysaccharide-induced inflammatory injury in chondrocytes via blocking the activation of the JAK-STAT signaling pathway (Li et al., 2019). Besides, activating the JAK/STAT signaling pathway is paramount in I/R-elicited intestinal injury due to increased oxidative stress, inhibition of which prevents intestinal mucosal epithelial apoptosis and reduces infiltration of intestinal mucosal neutrophil (Wen et al., 2012). JAK/STAT is of vital importance in regulating stress-responsive gene expression via transducing signals and protecting against myocardial I/R injury (Smith et al., 2010).
Additionally, it was found that overexpressed PRR34-AS1 and JAK1 silencing could accelerate chondrocyte proliferation and decelerate chondrocyte apoptosis, which was indicated by inhibited cleaved caspase-3 and Bax expression, as well as promoted Bcl-2 expression. LncRNAs have the therapeutic potential to improve clinical outcomes in myocardial I/R injury patients (Ong et al., 2018). Upregulated lncRNA CasC7 is capable of reducing nerve cell apoptosis in the spinal cord of I/R rats (Liu, Pan, Jiang, & Yin, 2018). LncRNA-N1LR reduces nerve cell apoptosis and nerve cell loss in I/R-induced mouse brains while enhancing cell cycle progression and cell proliferation in vitro (Zhao et al., 2017). Downregulated JAK1 through RNA interference possesses the ability to suppress cell proliferation in ncRNA, which in turn, inhibits tumorigenicity both in vitro and in vivo (Liu et al., 2011). In addition, restrained proliferation and cardioprotective effects have been illustrated in cells transfected with tetrandrine which attenuates cardiomyocyte apoptosis in I/R injury after treatment with JAK inhibitor (Zhang, Guo, Li, Wang, & Li, 2017). Cleaved caspase-3, an activated form of caspase-3 that is an apoptosis-related gene, is an important indicator of apoptosis (Fan, Han, Cong, & Liang, 2005). Remote preconditioning has been suggested to prevent hepatic I/R injury by reducing both Bax and cleaved caspase-3 expression (Park et al., 2016). Ethyl pyruvate has the capacity of inhibiting the apoptosis of cardiomyocytes via upregulating Bcl-2, downregulating Bax, and decelerating oxidative stress (Guo, Zhang, Ji, Jiang, & Zuo, 2008).
Based on the in vitro and in vivo experiments, it can be concluded that overexpressed PRR34-AS1 can protect against I/R injury after TKA via disrupting JAK1-dependent JAK-STAT signaling pathway activation. Therefore, PRR34-AS1 holds therapeutic potential in the future as a promising target for I/R injury treatment after TK.
ACKNOWLEDGMENT
We acknowledge and appreciate our colleagues for their valuable efforts and comments on this paper.
FUNDING INFORMATION
This study was supported by Guizhou Provincial Science and Technology Foundation (GZSYQCC[2014]004) and National Key R&D Program of China (2018YFC2001800).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
H. F., F-X. Z., H-F. L., and M. Y. designed the study. H. F., F-X. Z., R. L., R-R. W., and Q-Y. W. collated the data, carried out data analyses, and produced the initial draft of the manuscript. P-C. Z. and J-P. Z. contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Open Research
DATA AVAILABILTY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.