Histamine induces intracellular Ca2+ oscillations and nitric oxide release in endothelial cells from brain microvascular circulation
Abstract
The neuromodulator histamine is able to vasorelax in human cerebral, meningeal and temporal arteries via endothelial histamine 1 receptors (H1Rs) which result in the downstream production of nitric oxide (NO), the most powerful vasodilator transmitter in the brain. Although endothelial Ca 2+ signals drive histamine-induced NO release throughout the peripheral circulation, the mechanism by which histamine evokes NO production in human cerebrovascular endothelial cells is still unknown. Herein, we exploited the human cerebral microvascular endothelial cell line, hCMEC/D3, to assess the role of intracellular Ca 2+ signaling in histamine-induced NO release. To achieve this goal, hCMEC/D3 cells were loaded with the Ca 2+- and NO-sensitive dyes, Fura-2/AM and DAF-FM/AM, respectively. Histamine elicited repetitive oscillations in intracellular Ca 2+ concentration in hCMEC/D3 cells throughout a concentration range spanning from 1 pM up to 300 μM. The oscillatory Ca 2+ response was suppressed by the inhibition of H 1Rs with pyrilamine, whereas H 1R was abundantly expressed at the protein level. We further found that histamine-induced intracellular Ca 2+ oscillations were initiated by endogenous Ca 2+ mobilization through inositol-1,4,5-trisphosphate- and nicotinic acid dinucleotide phosphate-sensitive channels and maintained over time by store-operated Ca 2+ entry. In addition, histamine evoked robust NO release that was prevented by interfering with the accompanying intracellular Ca 2+ oscillations, thereby confirming that the endothelial NO synthase is recruited by Ca 2+ spikes also in hCMEC/D3 cells. These data provide the first evidence that histamine evokes NO production from human cerebrovascular endothelial cells through intracellular Ca 2+ oscillations, thereby shedding novel light on the mechanisms by which this neuromodulator controls cerebral blood flow.
1 INTRODUCTION
Histamine is now recognized as a major neuromodulator which finely tunes the action of excitatory (i.e., glutamate) and inhibitory (i.e., GABA) neurotransmitters on postsynaptic targets in the brain (H. Haas & Panula, 2003; Panula & Nuutinen, 2013). Histamine-releasing neurons are located within the tuberomammillary nucleus (TMN) of the posterior hypothalamus, and extend long fibers to nearly all major brain areas, including cerebral cortex, amygdala, hippocampus, substantia nigra, striatum, and cerebellum (H. Haas & Panula, 2003; H. L. Haas, Sergeeva, & Selbach, 2008). The histaminergic TMN system regulates multiple functions such as the correct blastocyst implantation, the invasion, and proliferation of villi, and the development of an adhesive molecule leading to placenta formation (Lucariello et al., 2013; Lucariello et al., 2014; Lucariello et al., 2015; Matsuno et al., 2018), whereas within the central nervous system it regulates neuronal excitability and synaptic plasticity, sleep and wakefulness, cognition, feeding, and energy balance. Accordingly, histamine dysfunction has been reported in sleep and motor disorders, Alzheimer's disease, schizophrenia, multiple sclerosis, and addictive behaviors (H. Haas & Panula, 2003; H. L. Haas et al., 2008; Panula & Nuutinen, 2013). A common neurodegenerative process is also observed in HIV-positive patients taking antiretroviral therapy, even if the causes are still unknown. Certainly, there is an HIV–Alzheimer association and the increased longevity of patients due to current therapies increases their incidence (Coppola et al., 2018; Esposito et al., 2012aa, 2012bb, 2015; Zhou et al., 2010).
Histamine is produced in the brain by neurons and mast cells, although the contribution of the nonneuronal pool to total brain histamine is significant only during early postnatal development (Panula & Nuutinen, 2013). Histamine is synthesized from the l-amino-acid histidine through oxidative decarboxylation by the specific enzyme histidine-decarboxylase and is metabolized through ring methylation and oxidative deamination by diamine oxidase (Haas et al., 2008; Panula & Nuutinen, 2013). Histamine released by TMN neurons exerts its action through at least three types of G-protein coupled histamine receptors (HRs), namely H1Rs, H2Rs, and H3Rs, which are widely expressed in neuronal and glial cells. Conversely, H4Rs has been mainly described in peripheral tissues, such as leukocytes and bone marrow (H. Haas & Panula, 2003; Panula & Nuutinen, 2013). Notably, H1Rs and H2Rs were also found in the vascular wall of human cerebral circulation (Jansen-Olesen et al., 1997; Stanimirovic, Bertrand, Merkel, Bembry, & Spatz, 1994), and histaminergic fibers are closely apposed to cerebral blood vessels (W. W. Hu & Chen, 2012). In addition, H1R-H4R4 are all expressed and modulate the blood–brain barrier in rodent brain endothelial cells (Karlstedt, Jin, & Panula, 2013; Zhang et al., 2012). Early investigations revealed that histamine induces vasodilation in human cerebral, meningeal and temporal arteries via endothelial H1Rs which result in the downstream synthesis of nitric oxide (NO; Jansen-Olesen et al., 1997; Lassen, Christiansen, Iversen, Jansen-Olesen, & Olesen, 2003), the most powerful vasorelaxing agent in cerebral circulation (Guerra et al., 2018). However, the intracellular signaling cascade by which H1Rs stimulation leads to NO release is still unknown and histamine-induced NO production from human brain microvascular endothelial cells has never been measured.
An elevation in endothelial intracellular Ca2+ concentration ([Ca2+]i) is key to endothelial NO synthase (eNOS) activation (Blatter, 2017; Guerra et al., 2018; Khaddaj Mallat, Mathew John, Kendrick, & Braun, 2017; Zuccolo et al., 2016). Likewise, histamine activates H1Rs to elicit Ca2+-dependent NO release in endothelial cells from multiple vascular districts, including monkey cerebral and temporal arteries (Toda, Kawakami, Yamazaki, & Okamura, 1991), human umbilical vein (Sheng & Braun, 2007), bovine pulmonary artery (Lin et al., 2000), and pig thoracic aorta (Kishi et al., 1996). The intracellular signaling pathway coupling H1Rs activation to eNOS recruitment has been widely examined throughout the peripheral circulation. H1Rs are coupled to Gq/11 protein and phospholipase Cβ (PLCβ; H. Haas & Panula, 2003). The endothelial Ca2+ response to histamine is, therefore, triggered by Ca2+ mobilization from the endoplasmic reticulum (ER), the main endogenous Ca2+ reservoir (Moccia & Guerra, 2016), through inositol-1,4,5-trisphosphate (InsP3) receptors (InsP3Rs; Mikelis et al., 2015; Morgan & Jacob, 1996; Stolwijk et al., 2016), which can in turn activate adjacent ryanodine receptors (RyRs) through Ca2+-induced Ca2+ release (Paltauf-Doburzynska, Frieden, Spitaler, & Graier, 2000). The subsequent decrease in ER Ca2+ levels caused by histamine elicits extracellular Ca2+ entry through the store-operated Ca2+ entry (SOCE) pathway, which is mediated by the association between STIM1, the sensor of ER Ca2+ concentration, and the store-operated Ca2+ channel, Orai1 (Abdullaev et al., 2008; Kassan et al., 2015; Stolwijk et al., 2016). The endothelial Ca2+ response to histamine may adopt distinct intracellular Ca2+ signatures, including intracellular Ca2+ oscillations (Jacob, Merritt, Hallam, & Rink, 1988; Morgan & Jacob, 1996; Zhu et al., 2008) and biphasic Ca2+ signals (Crawford, MacCallum, & Ernst, 1992; Kassan et al., 2015; Stolwijk et al., 2016). In addition, histamine-induced Ca2+ signals in vascular endothelial cells could be also contributed by nicotinic acid adenine dinucleotide phosphate (NAADP), which gates two-pore channels 1–2 (TPC1–2) to mobilize Ca2+ from the lysosomal Ca2+ pool (Faris, Shekha, Montagna, Guerra, & Moccia, 2018; Moccia, Nusco, Lim, Kyozuka, & Santella, 2006). Recent work demonstrated that extracellular autacoids, such as acetylcholine, evoke Ca2+-dependent NO release from the human cerebral microvascular endothelial cell line hCMEC/D3 (Zuccolo et al., 2019). Acetylcholine-induced Ca2+ signals in hCMEC/D3 cells were initiated by muscarinic M5 receptors and shaped by the concerted interplay between endogenous Ca2+ release through type 3 InsP3Rs (InsP3R3) and TPC1–2 and SOCE (Zuccolo, Laforenza, et al., 2019). Similarly, extracellular Ca2+ influx through cyclic nucleotide-gated channels mediated adenosine-induced enhanced gap junction coupling (Bader et al., 2017). Therefore, hCMEC/D3 cells represent a predictive model to explore whether and how histamine induces NO release in a Ca2+-dependent manner from human cerebrovascular endothelium.
Herein, we provide the first evidence that histamine elicits a dose-dependent oscillatory increase in [Ca2+]i via H1Rs activation in hCMEC/D3 cells. The repetitive Ca2+ spikes evoked by histamine are initiated by InsP3- and NAADP-dependent intracellular Ca2+ mobilization and maintained over time by SOCE. Notably, histamine-induced intracellular Ca2+ release results in robust NO release, which requires both intra- and extracellular Ca2+ sources. These results gain novel insights on the molecular mechanisms by which histamine may regulate human cerebral blood flow (CBF).
2 MATERIALS AND METHODS
2.1 Cell culture
Human cerebral microvascular endothelial cells (hCMEC/D3) were obtained from Institut National de la Santé et de la Recherche Médicale (INSERM, Paris, France) and cultured as illustrated elsewhere (Zuccolo, Kheder, et al., 2019). Only hCMEC/D3 cells between passage 25 and 35 were employed in the present investigation. As described in, the cells were seeded at a concentration of 27.000 cells/cm2 and grown in tissue culture flasks coated with 0.1 mg/ml rat tail collagen type 1, in the following medium: EBM-2 medium (Lonza, Basel, Switzerland) supplemented with 5% fetal bovine serum, 1% penicillin–streptomycin, 1.4 μM hydrocortisone, 5 μg/ml ascorbic acid, 1/100 chemically defined lipid concentrate (Life Technologies, Milan, Italy), 10 mM HEPES and 1 ng/ml basic fibroblast growth factor. The cells were cultured at 37°C, 5% CO2 saturated humidity.
2.2 Solutions
Physiological salt solution (PSS) had the following composition (in mM): 150 NaCl, 6 KCl, 1.5 CaCl2, 1 MgCl2, 10 Glucose, 10 Hepes. In Ca2+-free solution (0Ca2+), Ca2+ was replaced with 2 mM NaCl, and 0.5 mM EGTA was added. Solutions were titrated to pH 7.4 with NaOH. The osmolality of PSS as measured with an osmometer (Wescor 5500, Logan, UT) was 339.5 mOsm/l.
2.3 [Ca2+]i and NO measurements
Ca2+ imaging was carried out as described elsewhere (Zuccolo, Laforenza, et al., 2019). Briefly, hCMEC/D3 cells were loaded with 4 µM Fura-2 acetoxymethyl ester (Fura-2/AM; 1 mM stock in dimethyl sulfoxide) in PSS. The cells were maintained in the presence of Fura-2 for 30 min at 37°C, 5% CO2 saturated humidity. After de-esterification in PSS for 15 min, the coverslip (8 mm) was mounted on the bottom of a Petri dish and the cells observed by an upright epifluorescence Axiolab microscope (Carl Zeiss, Oberkochen, Germany) equipped with a Zeiss × 40 Achroplan objective (water-immersion, 2.0 mm working distance, 0.9 numerical aperture). The cells were alternately excited at 340 and 380 nM by using a filter wheel (Lambda 10, Sutter Instrument, Novato, CA). The emitted fluorescence was detected at 510 nm by using an Extended-ISIS CCD camera (Photonic Science, Millham, UK). Custom-made software, working in the LINUX environment, was used to drive the CCD camera and the filter wheel, and to measure and plot on-line the fluorescence from 15 to 25 rectangular “regions of interest” (ROI), each corresponding to a well defined single cell. (Ca2+)i was monitored by measuring, for each ROI, the ratio of the mean fluorescence emitted at 510 nm when alternately exciting at 340 and 380 nm (Ratio [F340/F380]). An elevation in (Ca2+)i induces an increase in the ratio (Zuccolo, Laforenza, et al., 2019). Ratio measurements were performed and plotted on-line every 3 s. The experiments were performed at room temperature (22°C).
NO was measured as also illustrated in (Zuccolo, Laforenza, et al., 2019). Briefly, hCMEC/D3 cells were loaded with the NO-sensitive fluorophore, 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate (10 µM) in PSS. The cells were maintained in DAF-FM for 1 hr, 37°C and 5% CO2 saturated humidity. Subsequently, the dye was de-esterified in PSS for 1 hr. DAF-FM fluorescence was measured by using the same equipment described for Ca2+ recordings but with a different filter set, that is excitation at 480 nm and emission (termed “NOi”) at 535 nm. Histamine-induced variations in DAF-FM fluorescence were recorded at room temperature (22°C) and plotted on-line every 5 s.
2.4 Immunoblotting
hCMEC/D3 cells were homogenized with a Teflon glass Potter-Elvehjem homogenizer in a solution containing: 250 mM Sucrose, 1 mM EDTA, 10 mM Tris-HCl, pH 7.6, 0.1 mg/ml phenylmethylsulfonyl fluoride, 100 mM β-mercaptoethanol, protease and phosphatase inhibitor cocktails (P8340 and P5726, P0044, Sigma-Aldrich Inc., Milan, Italy). The homogenate was heated at 80°C in Laemmli buffer (Laemmli, 1970), 30 µg proteins subjected to 12.5% SDS-polyacrylamide gel electrophoresis and transferred to the Hybond-P polyvinylidene fluoride membrane (GE Healthcare, Milan, Italy). After 1 hr blocking with Tris buffered saline containing 5% non fat dry milk (blocking solution) and 0.1% Tween (blocking solution), the membranes were incubated overnight with affinity purified anti-H1R (sc-20633; Santa Cruz Biotechnology, Dallas, TX, Inc.) diluted 1:200 in the blocking solution. The membranes were washed and incubated for 1 hr with peroxidase-conjugated goat anti-rabbit immunoglobulin (AP132P; Merck Millipore, Milan, Italy) diluted 1:100.000. The bands were detected with ECL™ Select western blot analysis detection system (GE Healthcare, Milan, Italy). Blots were stripped with the method of Yeung and Stanley (2009) and reprobed with anti β-2-microglobulin (B2M) antibody (ab75853; Abcam, Cambridge, UK) as housekeeping. The antibody was diluted 1:10000 in blocking solution.
2.5 Statistical analysis
The amplitude of histamine-induced NO production was evaluated as the difference between the maximal increase in DAF fluorescence and an average of 1 min baseline before the peak. Pooled data are given as average ± standard error (SE) and statistical significance (p < .05) was evaluated by two-tailed Student's t test for unpaired observations or one-way analysis of variance analysis followed by the post-hoc Dunnett's test as appropriate. Data relative to both Ca2+ and NO signals are presented as mean ± SE, whereas the number of cells analyzed is indicated in the corresponding bar histograms.
2.6 Chemicals
Fura-2/AM and DAF-FM were purchased from Molecular Probes (Molecular Probes Europe BV, Leiden, The Netherlands). All the chemicals were of analytical grade and obtained from Sigma Chemical Co. (St. Louis, MO).
3 RESULTS
3.1 Histamine induces intracellular Ca2+ oscillations in hCMEC/D3 cells
To assess whether histamine increases the [Ca2+]i, hCMEC/D3 cells were loaded with the Ca2+-sensitive fluorescent indicator, Fura-2/AM, as described in Materials and methods. Unlike the mouse brain endothelial cell line bEND5 (Zuccolo et al., 2017; Zuccolo, Kheder, et al., 2019), hCMEC/D3 did not undergo any unsolicited elevation in [Ca2+]i when no agonist was present in the perfusate (data not presented). Early work demonstrated that histamine could trigger repetitive Ca2+ spiking in human vascular endothelial cells at concentrations spanning from the low nanomolar up to the middle micromolar range (Q. Hu, Deshpande, Irani, & Ziegelstein, 1999; Jacob et al., 1988; Morgan & Jacob, 1996; Oike, Droogmans, & Nilius, 1994). Likewise, histamine evoked intracellular Ca2+ oscillations in hCMEC/D3 cells in a dose-dependent manner. Histamine did not elicit any discernible increase in [Ca2+]i at very low concentrations, such as 3fM (not shown). The Ca2+ response to histamine appeared at 1 pM (Figure 1 and Figure 2a), when the majority of hCMEC/D3 cells produced a single Ca2+ transient in response to agonist stimulation (Figure 1 and Figure 2a). However, a low (≈10%) percentage of hCMEC/D3 cells challenged with 1 pM histamine displayed a short train of consecutive Ca2+ transients (1–4 transients, Ca2+ traces not shown). The dose-response relationship showed that the fraction of oscillating cells augmented with a minimum effective dose of 1 pM histamine, a maximal response at 1 nM, and an EC50 of 4.26 pM (Figure 1 and Figure 2a). The dose-response relationship of the percentage of oscillating cells was fitted by a sigmoidal curve, showing that the percentage of oscillating cells did not further increase at histamine concentrations higher than 1 μM (Figure 2a). Nearly all (>92%) the cells displayed intracellular Ca2+ oscillations at histamine concentrations ranging from 1 nM to 300 μM (Figure 1 and Figure 2a). The dose-response relationship of the amplitude of the 1st Ca2+ spike and of the number of Ca2+ oscillations over 45 min also followed a sigmoidal curve. The magnitude of the 1st Ca2+ transient reached a maximal response at 1 μM and showed an EC50 of 54.07 nM (Figure 2b), whereas the number of Ca2+ oscillations over 45 min also attained a maximal response at 1 μM and displayed an EC50 of 91.83 nM (Figure 2c). The amplitudes of consecutive Ca2+ spikes and the interspike interval (ISI) of the oscillatory responses to 1 nM, 1 and 30 μM histamine were further evaluated. As shown in Figure 2d, the amplitude of the Ca2+ spikes remarkably decreased after the initial Ca2+ response (spike number 1), but it remained relatively constant during the Ca2+ train induced by each dose of histamine. Likewise, the ISI did not undergo significant variations during 45 min recording at 1 and 30 μM, while the interspike period at 1 nM was significantly larger as compared to higher doses, although the frequency of Ca2+ spikes accelerated after the eight spikes (Figure 2e). This statistical analysis, therefore, suggests that 1 μM histamine represents the most suitable dose to explore the mechanisms that underlie the Ca2+-dependent production of NO by histamine in hCMEC/D3 cells. As expected, the oscillatory Ca2+ response to 1 μM histamine ceased upon agonist removal from the bath, but rapidly resumed on its reapplication (Figure 2f), thereby confirming that histamine was necessary for the onset of the repetitive spikes.
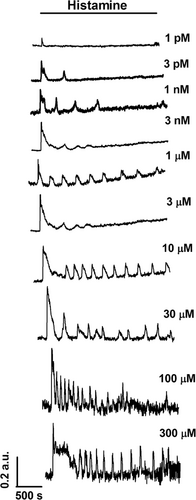
Histamine-induced intracellular Ca2+ oscillations in hCMEC/D3 cells. Dose-dependent relationship of the oscillatory Ca2+ response to histamine in hCMEC/D3 cells. In this and the following figures, histamine was added at the time indicated by the horizontal bar drawn above the Ca2+ tracings. The baseline of Ca2+ tracings has been shifted to avoid their overlapping for representation purposes. hCMEC/D3, human cerebral microvascular endothelial cells
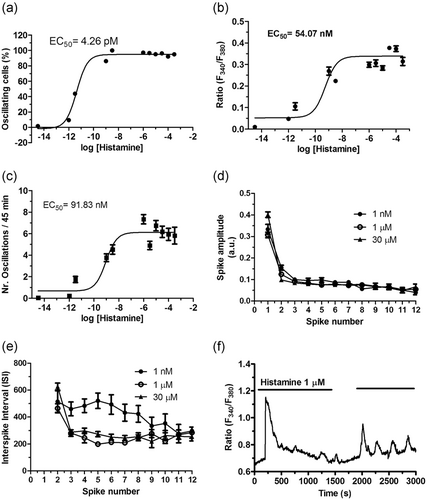
Statistical analysis of histamine-induced intracellular Ca2+ oscillations in hCMEC/D3 cells. Dose-response relationship of the percentage of responding cells (a), amplitude of the 1st spike (b), number of oscillations/45 min (c), amplitude of the 2–12th Ca2+ spikes (d) and average ISI (e). The continuous lines for the data a, b and c were obtained from a fit to a sigmoidal concentration-response curve by using Equation 1. The ISI was evaluated by measuring the peak-to-peak interval between two successive Ca2+ transients. The number of cells analyzed for each concentration ranged from 39 to 285 from no less than three independent experiments. (f) 1 µM histamine-induced intracellular Ca2+ oscillations reversely ceased upon agonist removal from the perfusate. hCMEC/D3, human cerebral microvascular endothelial cells
3.2 Histamine-induced intracellular Ca2+ oscillations are triggered by H1Rs
H1Rs have long been known to trigger the endothelial Ca2+ response to histamine due to their tight coupling to PLCβ and InsP3-dependent ER Ca2+ mobilization (Q. Hu et al., 1999; Kishi et al., 1996; Lin et al., 2000; Oike et al., 1994; Sheng & Braun, 2007; Toda et al., 1991). Likewise, histamine-induced intracellular Ca2+ oscillations in hCMEC/D3 cells were blocked by pyrilamine (10 μM, 10 min; Figure 3a,c), a selective H1R antagonist (Adderley, Zhang, & Breslin, 2015). This treatment abrogated the oscillatory Ca2+ response to histamine (1 μM) in all the cells (Figure 3b). Likewise, immunoblots displayed a major band of about 56 kDa for H1Rs (Figure 3d), as previously shown by Adderley et al. (2015). Therefore, these findings confirm that histamine-induced repetitive Ca2+ spikes are initiated by H1Rs also in hCMEC/D3 cells.
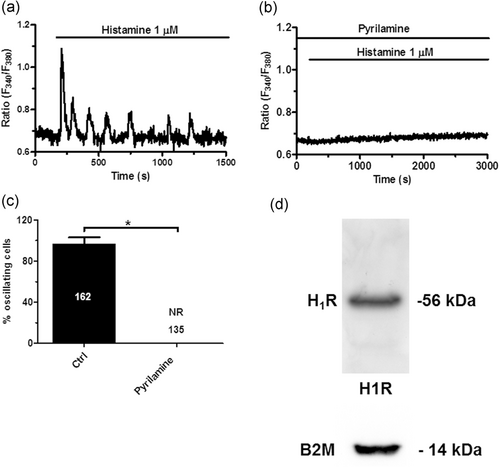
Histamine-induced intracellular Ca2+ oscillations are initiated by H1Rs. (a) Histamine-induced intracellular Ca2+ oscillations under control conditions. (b) Pyrilamine (10 μM, 10 min), a selective H1R antagonist, suppressed the oscillatory Ca2+ response to histamine. (c) Mean±SE of the percentage of oscillating cells in the presence and absence of pyrilamine. The asterisk indicates p < .05. No Ca2+ signal was detected upon pharmacological blockade of H1Rs (no response, NR). (d) H1R protein was detected by immunoblotting, as described in Materials and Methods. Lanes were loaded with 35 μg of proteins and probed with specific rabbit polyclonal antibodies. A band of about 56 kDa was found. Blots representative of three were shown. H1RS, histamine 1 receptors; SE, standard error
3.3 Histamine-induced intracellular Ca2+ oscillations require extracellular Ca2+ entry through the SOCE pathway
Extracellular stimulation with physiological autacoids, such as acetylcholine, triggers endogenous Ca2+ release followed by extracellular Ca2+ entry in hCMEC/D3 cells (Zuccolo, Laforenza et al. 2019). Therefore, to disentangle the contribution of intra- and extracellular Ca2+ sources to histamine-induced intracellular Ca2+ oscillations (Figure 4a), we stimulated the cells upon removal of extracellular Ca2+ (0Ca2+). The Ca2+ response to histamine (1 μM) always arose under 0Ca2+ conditions, but rapidly run down despite the continuous presence of agonist (Figure 4b). Intracellular Ca2+ oscillations, however, could be reinitiated by exposure to 1.5 mM Ca2+ in the presence of histamine in 142 out of 144 cells (Figure 4b). The amplitude of the first histamine-induced Ca2+ transient was not reduced by removal of extracellular Ca2+ (Figure 4c). This behavior is different as compared to acetylcholine, which did not evoke sizeable Ca2+ signals in the majority of hCMEC/D3 cells bathed under 0Ca2+ conditions (Zuccolo, Laforenza, et al., 2019). Likewise, removal of extracellular Ca2+ during ongoing oscillations reversibly suppressed histamine-induced intracellular Ca2+ spikes in 65 of 84 cells (Figure 4d). SOCE represents the major Ca2+ entry pathway underlying agonizts-evoked extracellular Ca2+ entry in cerebrovascular endothelial cells (G. C. Brailoiu et al., 2016; E. Brailoiu, Shipsky, Yan, Abood, & Brailoiu, 2017; Kito et al., 2015; Moccia et al., 2015; Zuccolo et al., 2017; Zuccolo, Kheder, et al., 2019; Zuccolo, Laforenza, et al., 2019) and is mediated by the interaction between STIM2, the ER Ca2+ sensor, and Orai1, the pore-forming subunit on the plasma membrane, in hCMEC/D3 cells (Zuccolo, Laforenza, et al., 2019). Accordingly, when extracellular Ca2+ was restituted to the bath in the absence of the agonist, the subsequent increase in [Ca2+]i did not adopt an oscillatory waveform but appeared as the Ca2+ bump typical of SOCE activation (Lodola et al., 2017; Sanchez-Hernandez et al., 2010). Therefore, we first assessed whether intracellular Ca2+ oscillations restart upon restoration of extracellular Ca2+ levels in the presence of Pyr6 (10 μM, 10 min), a selective Orai1 inhibitor (Moccia et al., 2016). This treatment, however, prevented the restart of Ca2+ oscillations following return to the Ca2+-containing perfusate in 80 of 80 cells (Figure 4e). Then, we found that the addition of Pyr6 (10 μM) during the ongoing spiking response irreversibly blocked histamine-induced intracellular Ca2+ oscillations (Figure 4f). These findings, therefore, demonstrate that histamine-induced repetitive Ca2+ transients are initiated by endogenous Ca2+ mobilization, whereas they are sustained by extracellular Ca2+ entry through the SOCE pathway.
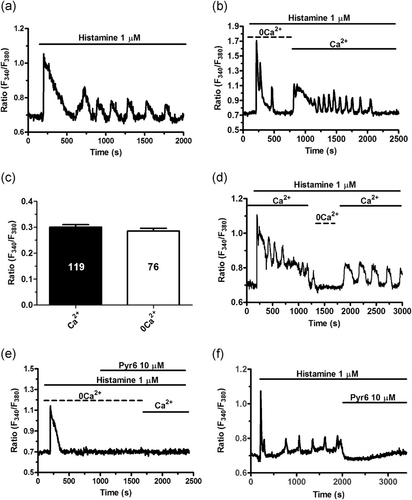
Contribution to intra- and extracellular Ca2+ sources to histamine-induced intracellular Ca2+ oscillations. (a) Intracellular Ca2+ oscillations induced by histamine in hCMEC/D3 cells bathed in the presence of extracellular Ca2+. (b) Histamine evoked only 1–2 Ca2+ spikes under 0Ca2+ conditions, but intracellular Ca2+ transients rapidly resumed upon Ca2+ restitution to the bath. (c) Mean±average of the amplitude of the 1st Ca2+ spike induced by histamine in the presence (Ca2+) and absence (0Ca2+) of extracellular Ca2+. (d) Removal of extracellular Ca2+ (0Ca2+) during ongoing oscillations caused the quick and reversible interruption of histamine-induced repetitive Ca2+ transients. (e) Pharmacological blockade of SOCE with Pyr6 (10 μM, 10 min), a selective Orai1 inhibitor, prevented histamine-induced intracellular Ca2+ oscillations upon Ca2+ readdition to the perfusate. (f) The acute addition of Pyr6 (10 μM) during an ongoing signal abrogated histamine-induced intracellular Ca2+ oscillations. hCMEC/D3, human cerebral microvascular endothelial cells
3.4 InsP3 and NAADP trigger histamine-induced endogenous Ca2+ release
We next assessed whether histamine-induced endogenous Ca2+ release was supported by InsP3Rs following PLCβ recruitment by H1Rs (Figure 5a), as demonstrated in endothelial cells from a variety of vascular beds (Q. Hu et al., 1999; Kishi et al., 1996; Lin et al., 2000; Oike et al., 1994; Sheng & Braun, 2007; Toda et al., 1991). As illustrated in Figure 5b, histamine-evoked intracellular Ca2+ mobilization was prevented by U73122 (10 µM; 30 min), a widespread employed PLC inhibitor (Dragoni et al., 2013; Zuccolo, Laforenza, et al., 2019), and 2-aminoethoxydiphenyl borate (2-APB; 50 µM, 30 min), which only blocks InsP3Rs in the absence of extracellular Ca2+ (0Ca2+) (Zuccolo, Laforenza, et al., 2019). In addition, the acute addition of 2-APB (50 μM) during an established response to histamine (1 μM) caused the immediate interruption of the spiking activity in 68 out of 70 cells (Figure 5c). It is, however, worth mentioning that under such conditions (i.e., Ca2+ present in the bath), 2-APB could also target SOCE (Moccia et al., 2014; Moccia et al., 2016). Histamine-induced intracellular Ca2+ release was also suppressed by emptying the ER Ca2+ reservoir with cyclopiazonic acid (CPA; 10 µM), which specifically interferes with sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) activity (Berra-Romani et al., 2008). As reported elsewhere (Zuccolo,Laforenza, et al., 2019), CPA induced a transient elevation in [Ca2+]i due to passive ER Ca2+ efflux followed by Ca2+ extrusion across the plasma membrane through the Na+/Ca2+ exchanger and the Plasma Membrane Ca2+-ATPase. The subsequent addition of histamine (10 µM) failed to increase the [Ca2+]i in hCMEC/D3 cells (Figure 5d). Moreover, SERCA blockade during ongoing Ca2+ oscillations caused a large elevation in [Ca2+]i followed by abrogation of histamine-evoked intracellular Ca2+ transients in 78 out of 79 cells (Figure 5e). Notably, after the Ca2+ peak caused by CPA-evoked ER Ca2+ release, the [Ca2+]i declined to a sustained plateau level which was sustained by SOCE (Zuccolo et al., 2017; Zuccolo, Kheder, et al., 2019). Figure 5f reports the statistical analysis of these experiments. Collectively, these findings indicate that histamine-induced intracellular Ca2+ oscillations require repetitive cycles of InsP3-dependent Ca2+ mobilization from the ER followed by Ca2+ reuptake and that SOCE is required to ensure proper refilling of endogenous stores to sustain the ongoing Ca2+ transients.
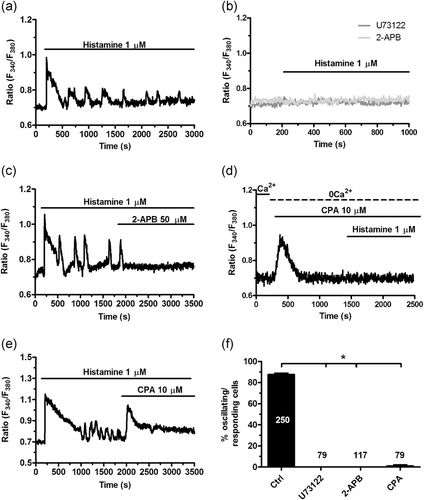
InsP3Rs support histamine-induced intracellular Ca2+ oscillations. (a) Intracellular Ca2+ oscillations induced by histamine in hCMEC/D3 cells under control conditions. (b) Histamine-induced intracellular Ca2+ oscillations were abolished by pretreating the cells with U73122 (10 μM, 30 min), a selective PLC inhibitor, or 2-APB (50 μM, 30 min), which blocks InsP3Rs under 0Ca2+ conditions. For this reason, the effect of 2-APB was monitored by removing extracellular Ca2+ 100 s before stimulation with histamine. (c) The acute addition of 2-APB (50 μM) during an ongoing signal abrogated histamine-induced intracellular Ca2+ oscillations. (d) Depleting the ER Ca2+ pool with CPA (10 μM), which selectively impairs SERCA activity, under 0Ca2+ conditions prevented the onset of the Ca2+ response to histamine. (e) The acute addition of CPA (10 μM) during histamine-induced repetitive Ca2+ spikes caused an immediate elevation in [Ca2+]i due to passive depletion of the ER Ca2+ store followed by interruption of ongoing oscillations. (f) Mean±average of the percentage of oscillating (in the presence of extracellular Ca2+) or responding (under 0Ca2+ conditions) upon preincubation with, respectively, U73122 (10 μM, 30 min), 2-APB (50 μM, 30 min), and CPA (10 μM, 20 min). The asterisk indicates p < .05. 2-APB, 2-aminoethoxydiphenyl borate; CPA, cyclopiazonic acid; hCMEC/D3, human cerebral microvascular endothelial cells; InsP3Rs, inositol-1,4,5-trisphosphate (InsP3) receptors; SERCA, sarco-endoplasmic reticulum Ca2+-ATPase
Recent studies revealed that NAADP-induced lysosomal Ca2+ release through TPC1–2 supports agonist-induced intracellular Ca2+ signals in multiple types of endothelial cells (Brailoiu et al., 2010; Gambara et al., 2008; Zuccolo, Kheder, et al., 2019), including the hCMEC/D3 cell line (Zuccolo, Laforenza, et al., 2019). In agreement with these observations, histamine-evoked intracellular Ca2+ transients were suppressed by NED-19 (100 µM, 30 min), a selective TPC1–2 blocker (Di Nezza et al., 2017; Pitt, Reilly-O'Donnell, & Sitsapesan, 2016) (Figure 6a, b and f). Moreover, the acute addition of NED-19 (100 μM) abrogated the established spiking response to histamine (1 μM) in 60 of 71 cells (Figure 6c). Subsequently, we depleted the lysosomal Ca2+ store with glycyl-l-phenylalanine 2-naphthylamide (GPN), which is selectively hydrolyzed by cathepsin C to induce the osmotic lysis of acidic vesicles and liberate their Ca2+ content (Moccia, Billington, & Santella, 2006; Ronco et al., 2015). As also shown in (Zuccolo, Laforenza et al., 2019), GPN (200 μM) elicited a transient elevation in [Ca2+]i due to lysosomal Ca2+ disruption (Figure 6d). The following addition of histamine (1 μM) failed to induce intracellular Ca2+ oscillations (Figure 6d,f) thereby confirming that NAADP sustains the spiking response by inducing EL Ca2+ release through TPC1–2. Consistently, the acute addition of GPN (200 μM) during an established oscillatory Ca2+ signal caused a transient elevation in [Ca2+]i followed by the interruption of Ca2+ activity (Figure 6e).
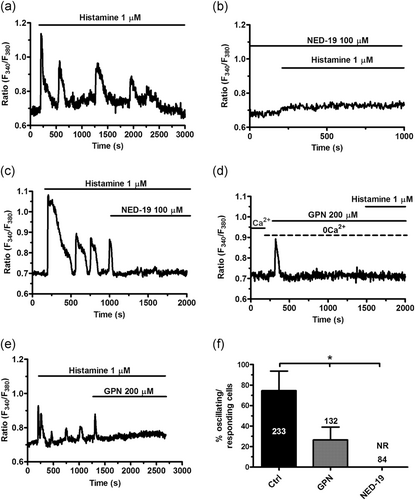
NAADP supports histamine-induced intracellular Ca2+ oscillations. (a) Intracellular Ca2+ oscillations induced by histamine in hCMEC/D3 cells under control conditions. (b) Histamine-induced intracellular Ca2+ oscillations were abolished by pretreating the cells with NED-19 (100 μM, 30 min), which selectively blocks TPC1–2. (c) The acute addition of NED-19 (10 μM) during an ongoing signal abrogated histamine-induced intracellular Ca2+ oscillations. (d) Depleting the EL Ca2+ pool with GPN (200 μM), a selective lysosomotropic agent, under 0Ca2+ conditions prevented the onset of the following Ca2+ response to histamine. As described elsewhere (Kilpatrick, Eden, Schapira, Futter, & Patel, 2013), GPN induced a transient elevation in [Ca2+]i because of liberation of the lysosomal Ca2+ content. (e) The acute addition of GPN (200 μM) during histamine-induced repetitive Ca2+ spikes caused the expected elevation in [Ca2+]i followed by blockade of ongoing oscillations. (f) Mean±average of the percentage of oscillating (in the presence of extracellular Ca2+) or responding (under 0Ca2+ conditions) upon preincubation with, respectively, NED-19 (100 μM, 30 min) and GPN (200 μM, 20 min). The asterisk indicates p < .05. GPN, glycyl-l-phenylalanine 2-naphthylamide; hCMEC/D3, human cerebral microvascular endothelial cells; NAADP, nicotinic acid adenine dinucleotide phosphate, NR, no response
3.5 Histamine-evoked intracellular Ca2+ oscillations drive NO production in hCMEC/D3 cells
With the aim to evaluate whether histamine-induced intracellular Ca2+ oscillations stimulate NO production, hCMEC/D3 cells were led with the NO-sensitive fluorescent indicator, DAF-FM, as illustrated in Materials and Methods. Histamine (1 µM) evoked a rapid elevation in DAF-FM fluorescence that was prevented by N(ω)-nitro-l-arginine methyl ester (L-NAME) (100 µM, 1 hr; Figure 7a), an established NOS inhibitor, or BAPTA (30 µM, 2 hr; Figure 7a), an intracellular Ca2+ buffer (Zuccolo, Laforenza, et al., 2019). In addition, histamine-induced NO release was abrogated by pyrilamine (10 μM, 10 min), which inhibits H1Rs (Figure 7b). Moreover, histamine-induced NO release occurred also under 0Ca2+ conditions, although DAF-FM fluorescence underwent a significantly (p < .05) lower increase compared to that recorded when Ca2+ was present in the perfusate (Figure 7c,e). Accordingly, the subsequent addition of Ca2+ to the recording chamber caused a further NO signal (Figure 7c), which confirms that both intra- and extracellular Ca2+ sources support histamine-evoked NO production in hCMEC/D3 cells. In agreement with this finding, blockage of SOCE with Pyr6 (10 μM; 10 min) attenuated, but not did fully suppress, histamine-induced NO production (Figure 7d,e). Figure 7e reports the statistical analysis of these experiments. These data, therefore, demonstrate that histamine impinges on intracellular Ca2+ signals to stimulate NO release via H1Rs also in endothelial cells from human brain microcirculation.
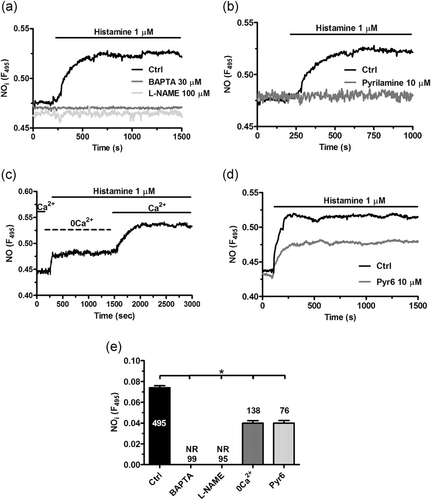
Histamine induces Ca2+-dependent NO release via H1Rs in hCMEC/D3 cells. (a) Histamine-induced NO release in hCMEC/D3 cells loaded with the NO-sensitive fluorophore, DAF-FM, which was suppressed by buffering intracellular Ca2+ levels with BAPTA (30 μM, 2 hr) and by blocking eNOS with L-NAME (100 μM, 1 hr). (b) Histamine-induced NO release was eliminated by inhibiting H1Rs with pyrilamine (10 μM, 10 min). (c) Histamine evoked a sizeable, although reduced, elevation in DAF-FM fluorescence in the absence of extracellular Ca2+ (0Ca2+), whereas NO release quickly resumed upon Ca2+ restitution to the perfusate. (d) pharmacological blockade of Orai1 with Pyr6 (10 μM, 10 min) attenuated but did prevent histamine-induced NO release. (e), mean ± SE of histamine-induced NO production under the designated treatments. The asterisk indicates p < .05. DAF-FM, 4-amino-5-methylamino-2',7'-difluorofluorescein; hCMEC/D3, human cerebral microvascular endothelial cells; NAADP, nicotinic acid adenine dinucleotide phosphate; SE, standard error
As expected based upon the Fura-2/AM recordings in 0Ca2+, histamine (1 μM) failed to induce NO synthesis in the presence of U73122 (10 µM, 30 min), 2-APB (50 µM, 30 min), and CPA (10 μM) (Figure 8a,b). Moreover, histamine-evoked NO release was abrogated by emptying the lysosomal Ca2+ store with GPN (200 µM, 30 min) and by blocking TPC1–2 with NED-19 (100 µM, 30 min; Figure 8c). Figure 8d reports the statistical analysis of these experiments. It is, therefore, possible to conclude that histamine-induced NO release in endothelial cells from human brain microcirculation is supported by intracellular Ca2+ oscillations supported by NAADP, InsP3, and SOCE.
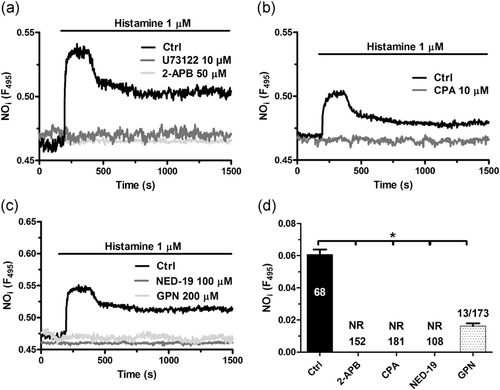
InsP3 and NAADP support histamine-induced NO release in hCMEC/D3 cells. (a) Histamine-induced NO release was suppressed by pretreating the cells with U73122 (10 μM, 30 min) or 2-APB (50 μM, 30 min). The effect of 2-APB was monitored by removing extracellular Ca2+ 100 s before stimulation with histamine. (b) Histamine-induced NO release was abolished by depleting the ER Ca2+ store with CPA (10 μM, 20 min). (c) Histamine-induced NO release was abrogated by blocking TPC1–2 with NED-19 (100 μM, 30 min) or by depleting the EL Ca2+ pool with GPN (200 μM, 20 min). (d) Mean ± SE of histamine-induced NO release under the designated treatments. The asterisk indicates p < .05. 2-APB, 2-aminoethoxydiphenyl borate; hCMEC/D3, human cerebral microvascular endothelial cells; NAADP, nicotinic acid adenine dinucleotide phosphate; SE, standard error
4 DISCUSSION
The neuromodulator histamine is known to increase CBF in human brain circulation by stimulating endothelium-dependent NO release via H1Rs (Jansen-Olesen et al., 1997; Lassen et al., 2003). Nevertheless, the signaling pathway by which H1Rs activation leads to endothelial NO production in the brain is unclear. Herein, we found that histamine evokes repetitive oscillations in [Ca2+]i in a human cerebrovascular cell line that are abrogated by pharmacological blockade of H1Rs. Histamine-evoked intracellular Ca2+ oscillations require periodical cycles of endogenous Ca2+ release which are supported by InsP3 and NAADP and sustained by SOCE. Histamine-evoked repetitive Ca2+ spikes drive robust NO release and represent, therefore, the Ca2+ waveform by which histamine induces relaxation and increases CBF through human cranial arteries (Jansen-Olesen et al., 1997; Lassen et al., 2003).
4.1 Histamine-induced intracellular Ca2+ oscillations via H1Rs in hCMEC/D3 cells
Intracellular Ca2+ oscillations control a plethora of endothelial functions (McCarron, Lee, & Wilson, 2017), including angiogenesis (Noren et al., 2016), vasculogenesis (Dragoni et al., 2013), gene expression (Zhu et al., 2011), vascular tone (Francis et al., 2016), and NO release (Zuccolo et al., 2017; Zuccolo, Kheder, et al., 2019). Likewise, histamine-induced repetitive Ca2+ spikes were reported in multiple types of endothelial cells, such as human umbilical vein endothelial cells (HUVEC; Jacob et al., 1988; Morgan & Jacob, 1996; Oike et al., 1994), HUVEC-derived EA.hy926 cells (Hadri, Pavoine, Lipskaia, Yacoubi, & Lompre, 2006) and human aortic endothelial cells (Zhu et al., 2008). The present investigation demonstrated that histamine evoked intracellular Ca2+ oscillations also in the human cerebrovascular endothelial cell line hCMEC/D3. Previous observations on human cranial arteries (Jansen-Olesen et al., 1997) demonstrated H1R expression at the protein level, a finding that was confirmed in the present investigation. Furthermore, pyrilamine, a selective H1R antagonist, prevented histamine-induced intracellular Ca2+ oscillations, which is fully consistent with the tight coupling between this receptor and PLCβ (H. Haas & Panula, 2003). Likewise, pharmacological blockade of H1Rs inhibited histamine-induced oscillatory and biphasic Ca2+ signals in, respectively, HUVEC (Ikeda et al., 1999), EA.hy926 cells (Hadri et al., 2006) and bovine corneal endothelial cells (Crawford et al., 1992). The dose-response relationship disclosed that the oscillatory Ca2+ signal arose at 1 pM histamine, whereas the percentage of oscillating cells attained a peak at 1 nM. However, the amplitude of the 1st spike and the number of Ca2+ oscillations over 45 min achieved their peak at 1 µM. The percentage of oscillating cells, the magnitude of the 1st Ca2+ spike and the frequency of Ca2+ transients did not significantly increase by raising histamine concentration above 1 µM. These observations concur with existing data showing that low micromolar doses of histamine are already able to induce repetitive Ca2+ spikes in vascular endothelial cells (Q. Hu et al., 1999; Jacob et al., 1988; Morgan & Jacob, 1996; Oike et al., 1994). A recent investigation measured histamine levels in the cerebrospinal fluid and in the parenchyma of human brain reporting a nanomolar concentration which is well above the threshold required to induce the spiking Ca2+ response documented here (Croyal et al., 2011). Nevertheless, histamine levels may vary depending on the brain area, reaching a concentration as high as ≈1.4 μg/g in the hypothalamus (Lipinski, Schaumberg, & Baldessarini, 1973). Furthermore, histamine levels in the brain dramatically fluctuate according to the circadian rhythm as the firing of TMS neurons significantly increases during waking (John, Wu, Boehmer, & Siegel, 2004). Finally, varicosities of histaminergic fibers are closely apposed to cerebral blood vessels, including arteries, arterioles, and capillaries (W. W. Hu & Chen, 2012; Wada, Inagaki, Yamatodani, & Watanabe, 1991). It is, therefore, likely that local histamine concentration undergoes a significant increase following the activation of TMN neurons, as demonstrated for glutamatergic synapses (Lee et al., 2007). These observations prompted us to use 1 μM histamine to stimulate hCMEC/D3 cells, as reported in several types of vascular endothelial cells (Q. Hu et al., 1999; Jacob et al., 1988; Oike et al., 1994).
4.2 InsP3, NAADP, and SOCE mediate histamine-evoked intracellular Ca2+ oscillations
A number of observations support the view that InsP3- and NAADP-driven periodical Ca2+ mobilization initiates histamine-induced repetitive Ca2+ spikes, whereas SOCE maintains the oscillatory signal over time. First, histamine triggered 1–2 Ca2+ transients in a Ca2+-free solution, whereas Ca2+ transients immediately resumed when extracellular Ca2+ was restituted to the bath. This observation demonstrates that endogenous Ca2+ release initiates the oscillatory Ca2+ response, whereas extracellular Ca2+ entry supports the Ca2+ transients over time, as previously demonstrated for acetylcholine-induced Ca2+ transients in the hCMEC/D3 cell line (Zuccolo, Laforenza, et al., 2019) and glutamate-evoked intracellular Ca2+ oscillations in mouse cerebrovascular endothelial cells (Zuccolo, Kheder, et al., 2019). Second, histamine-induced extracellular Ca2+ entry was inhibited upon pharmacological blockade of SOCE with Pyr6. Likewise, SOCE inhibition resulted in complete abrogation of the histamine-induced intracellular Ca2+ oscillations. This observation concurs with the notion that hCMEC/D3 cells lack transient receptor potential (TRP) canonical (TRPC) channels (Zuccolo, Laforenza, et al., 2019), which contribute to mediate Ca2+ influx following PLC recruitment in vascular endothelial cells (Moccia, Berra-Romani, & Tanzi, 2012). It should be pointed out that neither removal of extracellular Ca2+ nor acute addition of Pyr6 resulted in the immediate inhibition of ongoing oscillations, which persisted for at least one spike. As discussed in (Berra-Romani et al., 2012; Berridge, 2007), this finding indicates that, rather than triggering repetitive Ca2+ release from the oscillating stores, SOCE is required to recharge the endogenous stores during prolonged stimulation. We have previously shown that SOCE is constitutively activated in hCMEC/D3 cells and may be further recruited by physiological depletion of the ER Ca2+ pool with acetylcholine (Zuccolo, Laforenza, et al., 2019). These findings, therefore, indicate that constitutive SOCE can be also engaged by histamine. Third, the spiking Ca2+ response elicited by histamine was initiated by H1Rs, which are coupled by Gq/11 to PLCβ and InsP3-dependent ER Ca2+ mobilization (H. Haas & Panula, 2003). Fourth, histamine-induced intracellular Ca2+ oscillations were suppressed by inhibiting PLCβ with U73122, by blocking InsP3R3, the only InsP3R isoform present in the hCMEC/D3 cell line (Zuccolo, Laforenza, et al., 2019), with 2-APB and by emptying the ER Ca2+ pool with CPA. Fifth, histamine-induced intracellular Ca2+ oscillations were suppressed by blocking NAADP-gated TPC1–2 with the selective inhibitor, NED-19 (Pitt et al., 2016), and by emptying the lysosomal Ca2+ pool with GPN. NAADP has also been shown to gate type 1 RyRs (Hohenegger, Suko, Gscheidlinger, Drobny, & Zidar, 2002; Wolf et al., 2015). However, RyRs are absent and caffeine did not trigger an increase in [Ca2+]i in hCMEC/D3 cells. It is, therefore, possible to conclude that TPC1–2 are targeted by NAADP to shape histamine-induced intracellular Ca2+ oscillations. Notably, earlier work demonstrated that H1Rs are also coupled to NAADP production and lysosomal Ca2+ release in HUVEC and EA.hy926 (Esposito et al., 2011). The ongoing intracellular Ca2+ spikes were also abrogated by preventing SERCA-mediated Ca2+ sequestration into the ER with CPA and by impeding Ca2+ reuptake into acidic vesicles with GPN. As discussed (Dai et al., 2007; Dai, Kuo, Leo, van Breemen, & Lee, 2006), these findings suggest that once delivered into the cytosol, Ca2+ must be sequestered back into endogenous organelles to give rise to the next cycle of Ca2+ mobilization. These findings are in strong agreement with the pioneering proposal by Galione and coworkers according to which the finely tuned interplay between NAADP and InsP3 is the most suitable mechanism to initiate recurrent sequences of Ca2+ fluxes via the two-pool (i.e., lysosomal and ER Ca2+ stores) model (Churchill & Galione, 2001). Likewise, NAADP and InsP3 were recently found to mediate repetitive Ca2+ oscillations also in mouse cerebrovascular endothelial cells challenged with acetylcholine and glutamate (Zuccolo et al., 2017; Zuccolo, Kheder et al., 2019). The exact mechanism by which these two Ca2+-releasing second messengers interact to shape intracellular Ca2+ signals is yet to be fully elucidated. It has been originally suggested that NAADP-mediated local Ca2+ release is amplified by the recruitment of juxtaposed InsP3Rs and/or RyRs into a regenerative cytosolic Ca2+ wave (Morgan, 2016; Morgan, Platt, Lloyd-Evans, & Galione, 2011). Recent studies, however, demonstrated that InsP3-dependent ER Ca2+ release may, in turn, be necessary to refill the EL Ca2+ pool (Atakpa, Thillaiappan, Mataragka, Prole, & Taylor, 2018; Ronco et al., 2015). Therefore, one could envisage that InsP3-induced Ca2+ release serves to load the lysosomal Ca2+ store, thereby sustaining Ca2+ release through TPC1–2. Moreover, an increase in intraluminal Ca2+ could, in turn, activate TPC2 and, sometimes, also TPC1 (Pitt et al., 2016). Alternately, InsP3-induced ER Ca2+ release could induce the Ca2+-dependent production of NAADP (Morgan et al., 2013), the physiological agonist of TPC1–2.
As mentioned above, histamine may induce vasorelaxation in human cerebral, meningeal and temporal arteries, thereby increasing CBF (Jansen-Olesen et al., 1997; Lassen et al., 2003). The hemodynamic response to histamine is triggered by endothelial H1Rs, which promote the release of NO, the most powerful vasorelaxing mediator in the brain (Guerra et al., 2018; Lourenco, Ledo, Barbosa, & Laranjinha, 2017; Mapelli et al., 2017). Oscillations in [Ca2+]i stimulate eNOS to synthesize NO in multiple types of vascular endothelial cells (Kasai, Yamazawa, Sakurai, Taketani, & Iino, 1997; Sandow, Senadheera, Grayson, Welsh, & Murphy, 2012), including mouse cerebrovascular endothelial cells (Zuccolo et al., 2017; Zuccolo, Kheder, et al., 2019). Histamine-evoked NO production in hCMEC/D3 cells was suppressed by inhibiting H1Rs with pyrilamine and by interfering with the activity of the main components of the endothelial Ca2+ machinery, that is InsP3R3, TPC1–2, and SOCE. Unlike acetylcholine (Zuccolo, Laforenza, et al., 2019), histamine was able to induce NO release under 0Ca2+ conditions, whereas restitution of extracellular Ca2+ evoked the second bump in intracellular NO release. Therefore, both endogenous Ca2+ mobilization and SOCE are required to activate eNOS in hCMEC/D3 cells exposed to histamine. These observations suggest that the pool of endogenous Ca2+-releasing channels, that is InsP3R3 and/or TPC1–2, engaged by histamine is also coupled to plasmalemmal eNOS and is, therefore, distinct from that recruited by acetylcholine. Consistently, emerging evidence has been provided in favor of the heterogeneity of ER microenvironment and that, for instance, InsP3Rs can be coupled to distinct effectors and/or plasmalemmal proteins (Berridge, 2002; Taylor & Machaca, 2018). Moreover, functionally distinct eNOS pools have been described in the vascular wall (Biwer et al., 2016). In addition, endothelial Ca2+ signals could engage vasorelaxing pathways other than eNOS, including prostacyclin and endothelium-dependent hyperpolarization (Behringer, 2017; Guerra et al., 2018). For instance, a recent investigation demonstrated that cultured human brain microvascular endothelial cells release considerable amounts of prostacyclin (Yoshida, Kurita, Eto, Kumagai, & Kaji, 2017), although its role in histamine-induced vasodilation in human brain circulation remains to be elucidated. Furthermore, H1Rs selectively contributed to the decrease in transendothelial resistance elicited by histamine in monolayers of human cardiac endothelial cells (Adderley et al., 2015). Conversely, H2R, H3R, and H4R contribute to regulating the endothelial permeability in HUVEC (Adderley et al., 2015) and rat brain microvascular endothelial cells (Karlstedt et al., 2013). The increase in endothelial permeability is mediated by the Ca2+-sensitive effector, myosin light chain kinase, thereby suggesting that the intracellular Ca2+ oscillations described in the present investigation also control the blood–brain barrier permeability. Future work might be devoted to manipulating the intracellular Ca2+ machinery to assess the role of each signaling pathway, that is InsP3R3, TPC1–2, and SOCE, in the regulation of endothelial permeability by histamine.
5 CONCLUSIONS
In conclusion, this report unveiled the mechanism by which histamine stimulates NO production in human cerebrovascular endothelial cells. Histamine acts by inducing intracellular Ca2+ oscillations that are triggered by H1Rs, supported by rhythmical Ca2+ release through InsP3R3 and TPC1–2 and maintained over time by SOCE. These repetitive oscillations in [Ca2+]i, in turn, recruit eNOS to induce robust NO release, thereby vasorelaxing brain blood vessels and increasing CBF. Whereas these observations reinforce the notion that endothelial Ca2+ signals may actively contribute to neurovascular coupling (Guerra et al., 2018; Hannah, Dunn, Bonev, & Nelson, 2011; Harraz, Longden, Hill-Eubanks, & Nelson, 2018), they also pave the way to deepen our understanding of the cerebrovascular dysfunction. For instance, the brain endothelial Ca2+ toolkit is severely compromised by the amyloid-beta (Aβ) peptide (Fonseca et al., 2015) and the hemodynamic response to histamine is deteriorated in Alzheimer's disease (Fernandez-Novoa, Corzo, Zas, Alvarez, & Cacabelos, 1997; Higuchi et al., 2000). Future work might investigate whether rewiring of the endothelial Ca2+ handling machinery is involved in Alzheimer's disease pathogenesis.
ACKNOWLEDGEMENTS
This research was funded by Italian Ministry of Education, University and Research (MIUR), Dipartimenti di Eccellenza Program (2018–2022)(–), Dept. of Biology and Biotechnology “L. Spallanzani”, University of Pavia (F.M. and L.B.), and by Fondo Ricerca Giovani from the University of Pavia (F.M. and L.B.). P.F. was supported by MAECI (Ministero degli Affari Esteri e della Cooperazione Internazionale).
CONFLICTS OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
R. B. R., P. F., G. P., M. O., S. N., G. F., L. B., and U. L. performed and analyzed the experiments. V. V. G. and M. G. C. analyzed Ca2+ imaging experiments. R. B. R., G. S., and U. L. provided materials and overviewed their experimental setting. F. M. supervised the work, wrote the manuscript, and finalized the submission. All the authors approved the final version of the manuscript.