Proinflammation effect of Mst1 promotes BV-2 cell death via augmenting Drp1-mediated mitochondrial fragmentation and activating the JNK pathway
Abstract
Inflammation has been increasingly studied as part of the pathophysiology of neurodegenerative diseases. Mammalian Ste20-like kinase 1 (Mst1), a key factor of the Hippo pathway, is connected to cell death. Unfortunately, little study has been performed to detect the impact of Mst1 in neuroninflammation. The results indicated that Mst1 expression was upregulated because of LPS treatment. However, the loss of Mst1 sustained BV-2 cell viability and promoted cell survival in the presence of LPS treatment. Molecular investigation assay demonstrated that Mst1 deletion was followed by a drop in the levels of mitochondrial fission via repressing Drp1 expression. However, Drp1 adenovirus transfection reduced the protective impacts of Mst1 knockdown on mitochondrial stress and neuronal dysfunction. Finally, our results illuminated that Mst1 affected Drp1 content and mitochondrial fission in a JNK-dependent mechanism. Reactivation of the JNK axis inhibited Mst1 knockdown-mediated neuronal protection and mitochondrial homeostasis. Altogether, our results indicated that Mst1 upregulation and the activation of JNK-Drp1-mitochondrial fission pathway could be considered as the novel mechanism regulating the progression of neuroninflammation. This finding would pave a new road for the treatment of neurodegenerative diseases via modulating the Mst1-JNK-Drp1-mitochondrial fission axis.
1 INTRODUCTION
Parkinson's disease (PD) is an inflammation-induced central nervous system (CNS) disorder. Although several theories have been proposed to explain the pathogenesis of PD, the inflamamtion-mediated neuronal death has been reported to be the key factor involving PD development and progression. Unfortunately, the detailed molecular basis by which neuroinflammation mediates the neuronal damage has not been fully addressed (Man, Sanchez Duffhues, Ten Dijke, & Baker, 2019).
At the molecular levels, mitochondria have been found to be associated with neuronal dysfunction. Healthy mitochondria constantly produce ATP to ensure neuronal metabolism (Coverstone et al., 2018). Interestingly, under inflammation conditions, mitochondria are the potential target and undergo abnormal mitochondrial fission to transmit the inflammatory signals to cells (Erland, Yasunaga, Li, Murch, & Saxena, 2018). For example, in inflammation-treated alveolar epithelial cells, mitochondrial fission is induced in the LPS-mediated inflammation microenvironment and is responsible for the cell damage (Bittremieux et al., 2019). Besides, in LPS-treated microglial cells, Drp1-induced mitochondrial dynamics disorder modulates p62-required autophagy and this effect is connected to cell damage as well as mitochondrial dysfunction (Chandra et al., 2018). Besides, in BV2 neuronal cells, oxygen glucose deprivation-induced inflammation augments neuronal energy disorder and dysfunction via controlling TLR4/Myd88/Drp1-related mitochondrial fission (Zhou, Wang et al., 2018). The above information indicates that inflammation-mediated neuronal dysfunction seems to be regulated by mitochondrial fission, which contributes mitochondrial stress and neuronal death via multiple effects (Zhang, Jin, & Faber, 2019). However, the molecular affecting the mitochondrial fission has been not explored in inflammation-modulated neuronal damage.
Mammalian Ste20-like kinase 1 (Mst1), a key factor of the Hippo pathway, is handled by inflammation damage (Dickinson et al., 2018). For example, in LPS-induced hepatocyte damage, Mst1 is activated and augments the mitochondrial damage via reducing mitochondrial membrane potential and augmenting caspase activation (Merz et al., 2018). Besides, Mst1 also modulates post-infarction myocardial damage through controlling the JNK-Drp1 pathway. The impacts of Mst1-Hippo on mitochondrial dynamics have also been validated in liver cancer, hyperglycemia-attacked retinal pigmented epithelial cells, and renal ischemia-reperfusion injury. Herein, we conducted cellular assay to explore whether Mst1 is connected to mitochondrial fission under neuroinflammation (Wei et al., 2018).
Mechanistically, fission is post-transcriptionally handled by a key mitochondrial fission factor and that is Drp1 (Saito et al., 2019). Drp1 activation and translocation from cytoplasm onto the outer membrane of mitochondria seem to be a key upstream event. During this process, activated JNK could indirectly and/or directly elevate the transcription and/or expression of Drp1, amplifying Drp1-dependent mitochondrial fission (Zhou, Zhu et al., 2018). Accordingly, JNK protein is thought as to be the critical controlled in the processes of Drp1-mediated fission and JNK's action is validated by several studies including rectal cancer cells, cardiac reperfusion injury, and endometrial stromal cells (Zhu, Jin et al., 2018). Herein, we conducted cellular assays to understand whether inflammation-activated Drp1 fission is modulated by the JNK pathway.
2 MATERIALS AND METHODS
2.1 Cell line and inflammation model
Murine microgalia BV2 were cultured with Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2 mM glutamine, and 200 mM streptomycin/penicillin and maintained in 5% CO2 at 37°C. These cells were cultured with LPS (10 μg/ml) for 12 hr according to a recent report (Sajib, Zahra, Lionakis, German, & Mikelis, 2018).
2.2 MTT assay and TUNEL staining
Approximately, 8 × 104 cells in logarithmic growth were seeded in a 96-well culture plate. Twenty microliters of MTT solution (5 mg/ml) was added to each well, and then the cells were cultured for 4–6 hr. After removal of the supernatant, 150 μl of dimethyl sulfoxide was added. The 96-well culture plate was shaken for 10 min till crystal was completely dissolved. The optical density of dimethyl sulfoxide at 570 nm was measured on a plate reader. Cell survival rate was calculated as follows: (OD sample − OD sample blank) / (OD control − OD control blank) × 100%(Mehra et al., 2018). After treatment with LPS, the samples were stained with 50 μl of TUNEL reaction mixture. After washing with PBS, the samples were scanned by a confocal microscope (TCS SP5, Leica) at room temperature (Magni et al., 2019).
2.3 ROS flow cytometry analysis
After treatment with LPS, samples were harvested using enzyme digestion and prepared as a single-cell suspension. Subsequently, the single-cell suspension was stained with mROS probe (Molecular Probes) for 15 min at room temperature. Then, the single-cell suspension was washed with PBS, and FACSCaliber flow cytometry was applied to quantify the content of mROS. The relative content of mROS was measured by recording the percentages of positive and negative cells (Mantovani, Collavin, & Del Sal, 2019).
2.4 Enzyme-linked immunosorbent assay
The levels of cellular antioxidants, including SOD, GSH, and GPX, were analyzed through enzyme-linked immunosorbent assay (ELISA) kits. In addition, LDH content, caspase-3/9 activities were analyzed through ELISA kits (R&D Systems, Abingdon, UK) on the basis of a recent report (Shen et al., 2018).
2.5 mPTP opening detection and mitochondrial membrane potential observation
The mitochondrial potential was observed through the JC-1 probe (Beyotime, China, Cat. No:C2006). mPTP opening was evaluated according to a previous study using tetramethylrhodamine ethyl ester (Renn et al., 2018). The relative mPTP opening was measured as a ratio to that of the control group (Sinha et al., 2018).
2.6 RNA interference and transfection
BV-2 cells were transfected with Mst1-siRNA (5′-GATTAGCAGCCAGTGTCTTAG-3′) and negative control (si-RNA) (5′-TTACCGAGACCGTACGTAT-3′) synthesized by GenePharma (Shanghai, China), and were selected in DMEM containing 2 μg/ml puromycin. The pcDNA3.1-Drp1 were bought from Cyagen Company (Cyagen Biosciences Inc., Guangzhou, China; Masaldan et al., 2018), and the cells in the six-well plate were transfected 2 μg plasmid using Lipofectamine 2000 reagent, and complete medium was added after 6 hr transfection (Oh & Mouradian, 2018).
2.7 Western blot analysis
2.7.1 Western blot assay
Whole protein was extracted from whole cell lysate using RIPA buffer and the concentration was measured using a BCA assay. The protein (30 μg) was separated by 12.5% SDS-PAGE. Proteins were transferred to PVDF membranes (EMD Millipore, Billerica, MA) at 300 mA for 90 min. The membranes were then blocked at room temperature for 1 hr with TBST containing 0.1% Tween-20% and 5% dry milk and incubated overnight with primary antibodies at 4°C (Paul, Gangwar, Bhargava, & Ahmad, 2018). Membranes were washed in triplicate with TBST and incubated for 2 hr with secondary antibodies at room temperature. The western blot was performed using a Luminescent Image Analyzer (Protein Simple, San Jose, CA; Jeelani et al., 2018), and the optical densities of antibody-specific bands were analyzed using the ImageJ software (Version 1.37 for Windows, National Institutes of Health, Bethesda, MD). The optical densities of antibody-specific bands were analyzed using a Luminescent Image Analyzer (Protein Simple; Serrato, Romero-Puertas, Lazaro-Payo, & Sahrawy, 2018).
2.8 Statistical analysis
Prism 6 (version 6.01, GraphPad Software, Inc., San Diego, CA) was used for data analysis. Results in our study were shown as mean ± standard deviation (SD) of at least three independent experiments (Fan et al. (2018). Differences between multiple groups were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc. p < .05 was considered statistically significant.
3 RESULTS
3.1 Inflammation-activated Mst1 promotes BV-2 cell death
First, LPS was used to mimic the inflammation damage in BV-2 cells. Then, the protein analysis was used to detect the content of Mst1 under inflammation damage. In Figure 1a,b, LPS treatment elevated the transcription and expression of Mst1 in BV-2 cells. To figure out the action exerted by Mst1 in inflammation-mediated BV-2 cell damage (Kiel, Goodwill, Baker, Dick, & Tune, 2018), Mst1 siRNA against was used to silence inflammation-mediated Mst1 upregulation and the protein knockdown was observed through western blot analysis (Figure 1a,b). After the loss of Mst1, cell viability, as determined through the MTT assay, was reversed to near-normal levels under LPS treatment (Figure 1c). These data were further validated by analyzing the released LDH content in the medium induced by LPS. In Figure 1d, LPS treatment promoted BV-2 cells release LDH in the medium, indicative of cell membrane breakage because of LPS stress. However, Mst1 knockdown inhibited the LDH release, suggestive of the pro-survival effect exerted by Mst1 deletion in LPS-treated BV-2 cells. Moreover, the TUNEL assay was applied to further analyze the cell survival rate. In Figure 1e,f, LPS upregulated the ratio of TUNEL-positive cells whereas Mst1 knockdown reduced cell death in BV-2 cells. To observe whether death is proceeded by apoptosis, caspase-3 expression and activity were measured. In Figure 1g,h, the expression of cleaved caspase-3 was downregulated by LPS treatment (Higgs et al., 2019). In addition to capsase-3 cleavage, the content of cleaved PARP was also increased after exposure to LPS stress, reconfirming the activation of caspase-3 by LPS treatment. Interestingly, Mst1 knockdown repressed the content of cleaved caspase-3 and PARP. In addition to the protein's expression, we also found that the caspase-3 activity was augmented due to LPS stress (Figure 1i), this action could be reversed by Mst1 knockdown. Altogether, the above data indicate the critical action played by Mst1 in augmenting LPS-mediated neuronal death in BV-2 cell in vitro.
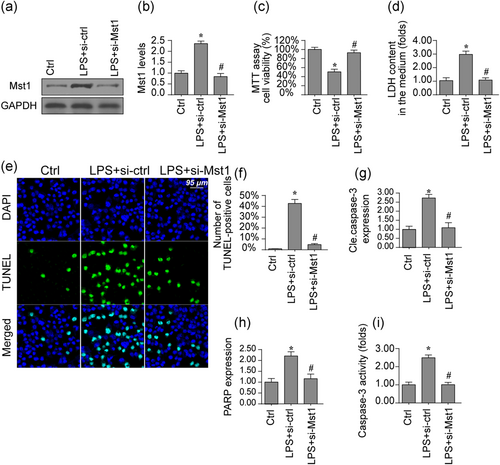
Mst1 deletion attenuates LPS-mediated cell death in BV-2 cells. (a,b) Proteins were isolated from BV-2 cells and then the expression of Mst1 was evaluated via western blots. siRNA against Mst1 was transfected into BV-2 cells. (c) Cell viability was determined via the MTT assay. Mst1 siRNA was transfected into BV-2 cells. (d) LDH release assay was used to detect cell death in response to LPS treatment and/or Mst1 deletion.(e,f) Cell death was determined via TUNEL staining. The number of apoptotic cells were recorded. (g,h) Proteins were isolated from BV-2 cells and then the expression of apoptotic proteins was evaluated via western blots. siRNA against Mst1 was transfected into BV-2 cells. (i) The caspase-3 activity was determined via ELISA. siRNA against Mst1 was transfected into BV-2 cells. Mst1, mammalian Ste20-like kinase 1. *p < .05 vs. control group; #p < .05 vs. LPS + si-ctrl group [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Loss of Mst1 reverses mitochondrial function in the inflammation microenvironment
Mitochondrial damage has been found to be closely associated with neuronal dysfunction via impairing energy supply and activating apoptosis (Montoya-Zegarra et al., 2019). Accordingly, a mitochondrial functional abnormality was detected under LPS-related inflammation stress. In Figure 2a, ATP production was downregulated in BV-2 cell after exposure to LPS. Interestingly, Mst1 knockdown reversed ATP production in BV-2 cells. In addition to energy metabolism, we also found that mitochondrial oxidative stress, as evaluated via observing mitochondrial ROS production through flow cytometry, was elevated due to LPS stress and was reversed to near-normal levels with Mst1 knockdown (Figure 2b). This result suggests mitochondrial dysfunction is modulated by LPS via Mst1 upregulation. Excessive mitochondrial dysfunction would activate mitochondrial apoptosis, as featured by cyt-c release, mPTP opening, and caspase-9 activation. Through immunofluorescence, we found that the nuclear levels of cyt-c were augmented by LPS-related inflammation stress whereas Mst1 knockdown inhibited the rise in the nuclear cyc-t (Figure 2c,d). In addition to cyt-c release, the mPTP opening rate was also positively affected by LPS and was preserved by Mst1 silencing (Figure 2e). Finally, western blots were applied to detect the changes in mitochondria-related apoptotic proteins. In Figure 2f-i, the levels of caspase-9 and Bax were boosted by LPS while the expression of Bcl-2 was downregulated (Li, Cai, et al., 2018). Interestingly, Mst1 knockdown reversed the levels of mitochondrial antiapoptotic factors and inhibited caspase-9/Bax. Herein, this information illuminates that mitochondria function is protected by Mst1 deficiency under LPS stress.
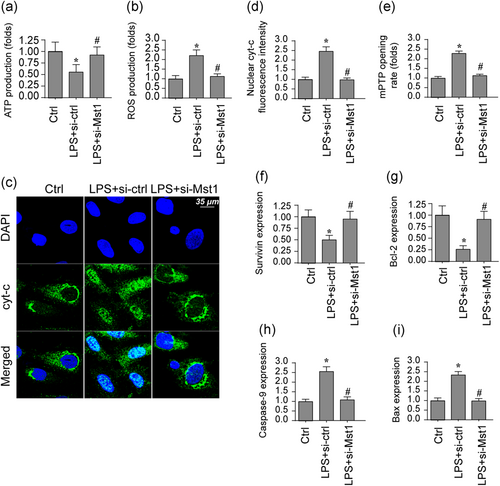
Mst1 inhibition reduces mitochondrial damage induced by LPS. (a) ATP production was evaluated in BV-2 cells in response to LPS treatment. siRNA against Mst1 was transfected into BV-2 cells. (b) ROS production was determined via flow cytometry. BV-2 cells were treated with LPS and transfected with siRNA against Mst1. (c,d) Immunofluorescence assay for cyt-c. The translocation of cyt-c from cytoplasm into the nucleus was determined. BV-2 cells were treated with LPS and transfected with siRNA against Mst1. (e) mPTP opening was evaluated via ELISA. (f-i) Proteins were isolated from BV-2 cells and then the expression of apoptotic proteins was evaluated via western blots. siRNA against Mst1 was transfected into BV-2 cells. Mst1, mammalian Ste20-like kinase 1. *p < .05 vs. control group; #p < .05 vs. LPS + si-ctrl group [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Mst1 activation promotes mitochondrial fission via Drp1
Mitochondrial function and apoptosis have been reported to be governed by mitochondrial fission (Nwadozi et al., 2019). Through analyzing mitochondrial morphology by immunofluorescence (Na et al., 2019), we found that mitochondria were strip and this morphology was converted into fragmentation once treatment with LPS. Interestingly, Mst1 knockdown reversed the mitochondrial network. Previous studies have used the average length of mitochondria to quantify fission. In Figure 3a, the average length of mitochondria was ~9.2 μm under normal conditions (Meyer & Leuschner, 2018). Interestingly, LPS treatment reduced mitochondrial length to~4.2 μm and this parameter was reversed to~8.7 μm after the loss of Mst1. This result indicates that fission is positively affected through LPS due to Mst1 upregulation. Subsequently, protein analysis was used to detect the molecular mechanism underlying LPS-related fission. In Figure 3b-f, LPS upregulated the content of Drp1/Mff, the key factors belonging to pro-fission components. However, Mfn2/ Opa1 were rapidly downregulated by LPS treatment. Mfn2/Opa1 were the key anti-fission factors that correct excessive mitochondrial fission. Interestingly, Mst1 knockdown repressed the levels of Drp1/Mff whereas reversed the content of Mfn2/Opa1. Altogether, the above results illustrate that loss of Mst1 could abolish the mitochondrial fission induced by LPS.
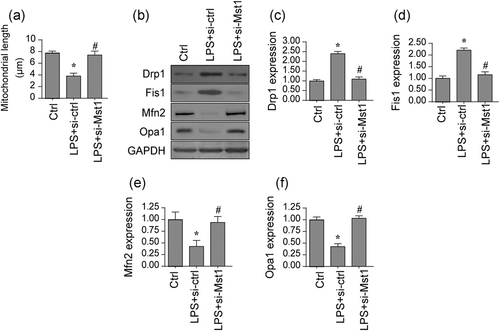
Mitochondrial fission is activated by LPS because of increased Mst1. (a) Immunofluorescence assay for mitochondrial morphology. The average length of mitochondria was evaluated. (b-f) Proteins were isolated from BV-2 cells and then the expression of mitochondrial fission-related proteins was evaluated via western blots. siRNA against Mst1 was transfected into BV-2 cells. Mst1, mammalian Ste20-like kinase 1. *p < .05 vs. control group; #p < .05 vs. LPS + si-ctrl group
3.4 Inhibition of Drp1 abolishes Mst1-induced mitochondrial damage and cell death
Although we found that Drp1-associated fission has been triggered by Mst1 in BV-2 cells, little was known about its role in mitochondrial damage and cell death (Kim et al., 2019). Subsequently, Drp1 adenovirus (ad-Drp1) was transfected into Mst1-silenced cells and mitochondrial function, as well as cell viability, were measured again. As shown in Figure 4a,b, ad-Drp1 significantly elevated the expression of Drp1 in Mst1-deleted cells. Interestingly, cell viability, as assessed via MTT assay, was significantly repressed by ad-Drp1 transfection, when compared with the Mst1-deleted cells (Figure 4c). Besides, the caspase-3 activity, a hallmarker of cellular death, was significantly inactivated by Mst1 silencing in LPS-treated cells (Figure 4d) whereas ad-Drp1 infection-mediated Drp1 overexpression abolished the pro-survival effect of Mst1 silencing. Therefore, these results indicated that Mst1 deletion-mediated cell protection was abolished by Drp1 overexpression. With respect to mitochondrial damage, cyt-c immunofluorescence demonstrated that Mst1 deletion could prevent LPS-mediated cyt-c nucleus migration (Figure 4e,f). However, after Drp1 overexpression via transfecting ad-Drp1 could significantly abolish the inhibitory effects of Mst1 deletion on cyt-c release. Besides, ATP synthesis was maintained by Mst1 silencing because of LPS stress (Figure 4g). Interestingly, Drp1 overexpression repressed ATP generation in Mst1-deleted cells, indicating that Mst1 modulated cellular energy metabolism via Drp1-associated fission. In addition, the caspase-9 activity was boosted by LPS treatment whereas this alteration was abolished because of Mst1 silencing (Figure 4h). However, the caspase-9 activity was re-activated by Drp1 reintroduction induced by ad-Drp1 infection. Herein, our data illuminate that Drp1-associated fission is involved in Mst1-related mitochondrial stress and cell death.
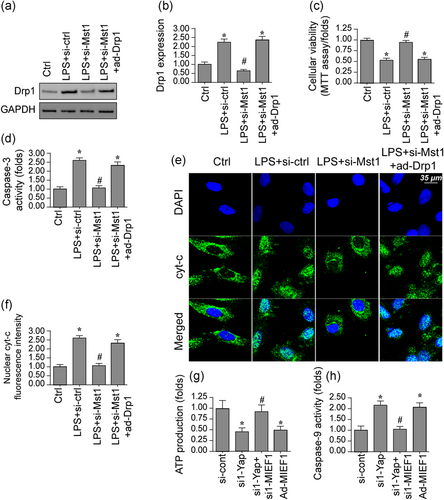
Mst1 modulates mitochondrial fission via Drp1. (a,b) Proteins were isolated from BV-2 cells and then the expression of Drp1 was evaluated via western blots. siRNA against Mst1 was transfected into BV-2 cells. Besides, Drp1 adenovirus were transfected into BV-2 cells to overexpress Drp1. (c) Cell viability was determined via MTT assay in BV-2 cells. siRNA against Mst1 was transfected into BV-2 cells. Besides, Drp1 adenovirus (ad-Drp1) were transfected into BV-2 cells to overexpress Drp1. (d) The Caspase-3 activity was determined via ELISA. (e,f) Immunofluorescence assay for cyt-c. The translocation of cyt-c from the cytoplasm into the nucleus was determined. (g) ATP production was determined in BV-2 cells transfected with Mst1 siRNA and ad-Drp1. (h) ELISA was used to evaluate the caspase-9 activity. Mst1, mammalian Ste20-like kinase 1. *p < .05 vs. control group; #p < .05 vs. LPS + si-ctrl group, @p < .05 vs. LPS + Mst1 group [Color figure can be viewed at wileyonlinelibrary.com]
3.5 Mst1 regulates Drp1-related fission through the JNK axis
Finally, experiments were further conducted to explain how Mst1 affected Drp1-associated fission. The JNK axis has been reported to be a key signaling pathway regulating Drp1-associated fission (Reddy et al., 2018). Protein analysis illuminated that p-JNK expression was elevated through LPS treatment, indicative of JNK activation. Interestingly, Mst1 deletion could reduce the content of p-JNK (Figure 5a-c; Kiel et al., 2018). To demonstrate whether the JNK pathway was required for Drp1-related mitochondrial fission, pathway agonist (Anisomycin, Ani) was applied in the Mst1-deleted cells (Figure 5a-c). Ani treatment reversed the levels of p-JNK and inhibited the content of Drp1, indicative of Mst1 attenuated Drp1 expression via the JNK pathway. To further analyze whether fission is positively controlled by Mst1 via JNK axis, co-immunofluorescence was used. In Figure 5d,e, LPS enhanced the fluorescence intensity of Drp1, leading to a drop in the average length of mitochondria. Interestingly, Mst1 knockdown inhibited Drp1 expression and maintained mitochondrial length; these actions were ineffective after treatment with Ani. Thus, our data indicate that Drp1-associated fission was controlled by Mst1 via the JNK axis (Giatsidis et al., 2018).
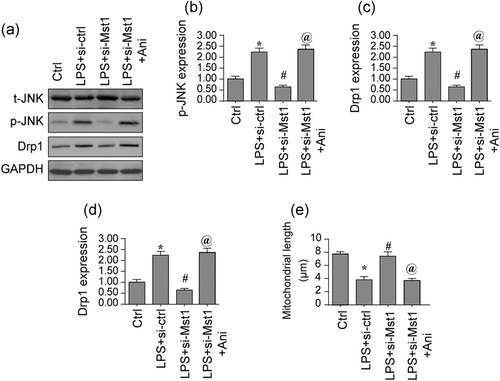
Mst1 affects mitochondrial fission via the JNK pathway. (a-c) Proteins were isolated from BV-2 cells and then the expression of Drp1 was evaluated via western blots. siRNA against Mst1 was transfected into BV-2 cells. Besides, anisomycin (Ani) was used to activate the JNK pathway in Mst1-deleted cells. (d-e) Double immunofluorescence assay for Drp1 and mitochondria. The expression of Drp1 was determined and the average length of mitochondria was recorded. Mst1, mammalian Ste20-like kinase 1. *p < .05 vs. control group; #p < .05 vs. LPS + si-ctrl group, @p < .05 vs. LPS + Mst1 group
3.6 Blockade of JNK pathway promotes cell survival and maintains mitochondrial homeostasis
Finally, we asked whether the JNK axis was implicated into Mst1-induced mitochondrial damage and cell death (Staudacher et al., 2018). In Figure 6a, LDH release was augmented by LPS treatment whereas Mst1 silencing inhibited LPS-mediated LDH release. Interestingly, activation of JNK pathway could re-caused LDH release in Mst1-deleted cells. These data were further validated through analyzing cell death rate using TUNEL assay (Hobson et al., 2018). In Figure 6b,c, the number of TUNEL-positive cells was elevated by LPS and was suppressed because of Mst1 silencing. Interestingly, JNK activation abolished the antiapoptotic action exerted by Mst1 silencing in BV-2 cells, as evidenced by the increased number of TUNEL+ cells. Therefore, our data suggest a necessary action exerted by the JNK axis in controlling the Mst1-induced cell death. With respect to mitochondrial damage, cell oxidative stress, and the caspase-9 activity were evaluated (Fernandez Vazquez, Reiter, & Agil, 2018). In Figure 6d-f, the levels of antioxidant were downregulated by LPS treatment. However, Mst1 silencing restored the content of antioxidants in BV-2 cells, and this action was ineffective because of JNK activation (Serrato et al., 2018). Besides, the LPS-activated caspase-9 activity could be suppressed through silencing Mst1 (Figure 6g), this action was also abolished through JNK activation. Altogether, the above data illustrated that JNK axis was involved in Mst1-triggered mitochondrial damage and cell death.
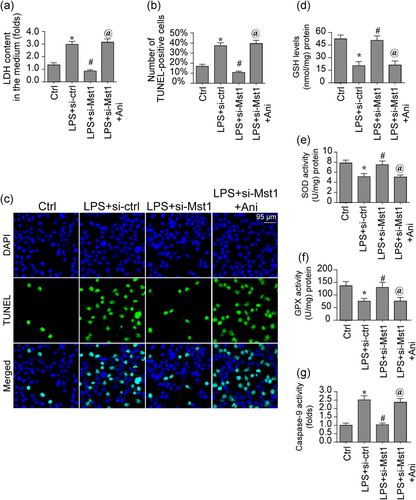
The JNK pathway is also involved in the regulation of BV-2 cell viability. (a) The LDH release assay was used to detect cell death in response to LPS treatment and/or Mst1 deletion. Besides, anisomycin (Ani) was used to activate JNK pathway in Mst1-deleted cells. (b,c) Cell death was determined via TUNEL staining. The number of apoptotic cells was recorded. (d-f) ELISA was used to determine the levels of cellular antioxidative factors. (g) The Caspase-9 activity was determined via ELISA. Mst1, mammalian Ste20-like kinase 1. *p < .05 vs. control group; #p < .05 vs. LPS + si-ctrl group, @p < .05 vs. LPS + Mst1 group [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Neuroinflammation has been acknowledged as primary factors for the development of several CNS diseases, such as Alzheimer's Disease and PD. At the molecular levels, moderate inflammation response promotes the tissue repair (Angelova et al., 2018; Zhou, Du et al., 2018). Unfortunately, uncontrolled inflammation seems to be a kind of pathophysiological features of neurodegenerative disease since pro-inflammatory factors would shape a neurotoxic environment that mediates neuronal dysfunction and cell death (Zhou, Li et al., 2018; Zhu et al., 2019). Accordingly, it is critically important to gain a better understanding of the mechanism underlying inflammation-initiated neuronal dysfunction (Bocci et al., 2019; Zhu, Hu et al., 2018). In the current study, Mst1 protein was activated through LPS in BV-2 cells and increased Mst1 was closely associated with BV-2 cell death via activating mitochondrial apoptosis (Cuadrado, Kugler, & Lastres-Becker, 2018; Zhao, Lu et al., 2018). Besides, we found that Mst1 upregulation also promoted mitochondrial fission via upregulating Drp1 and augmenting the JNK pathway (Ter Horst et al., 2018; Zhang, Zou, et al., 2019). These observations collectively indicate that Mst1 critically augments inflammation signals and that loss of Mst1 could be recognized as a critical approach to control the inflammation-triggered neuronal injury (Chrifi et al., 2019; Zhou, Shi et al., 2018).
In fact, neuroinflammation is always seen in both human patients and in animal models of PD. However, the molecular mechanism by which inflammation mediates neuronal damage has not been fully explored (Ba & Boldogh, 2018; Zhao, Wang et al., 2018). Herein, after exposure to LPS-mediated inflammation environment, mitochondrial fission was activated, as characterized by the formation of mitochondrial fragmentation and reduced mitochondrial length (Riehle & Bauersachs, 2018; Zhang, Zhang et al., 2019). Besides, our studies validated a necessary action of Drp1 in initiating mitochondrial fission in the LPS context. Drp1 deficiency sustained mitochondrial function and attenuated LPS-mediated cell death. These data confirm that Drp1-related mitochondrial fission seems to be a potential target of inflammation-mediated neuronal damage (Darden, Payne, Zhao, & Chappell, 2019; Zhang et al., 2016). This observation was in agreement with several previous research. For example, in oxygen glucose deprivation-induced neuroinflammation, neuronal dysfunction, and cell death are highly regulated by the TLR4/Myd88/Drp1-related mitochondrial fission. In metabolic syndrome-mediated neuroinflammation, mitochondrial fission is activated by Sirt3 downregulation (Li, Xin et al., 2018; Zhang, Jin et al., 2019). In manganese-treated astrocytes, neuroinflammation is also handled by mitochondrial fission. On the basis of this information, modulation of mitochondrial fission is vital to sustain neuronal viability.
Mst1, a key factor of Hipoo-pathway, is closely associated with the cell death via modulating the stability of Bcl-2 (Abukar et al., 2018; Yao et al., 2019). Recent studies have found a link between Mst1 and brain damage. For example, Mst1/Hippo pathway dysregulation has been noted in human Huntington's disease. Besides, Mst1 suppression attenuates early brain injury via repressing the NF-κB/MMP9 signaling pathway after subarachnoid hemorrhage in mice. Besides, cerebral reperfusion injury is also controlled by Mst1 (Ding et al., 2018; Zhou, Liu et al., 2018). Interestingly, Mst1 is also the upstream mediator of mitochondrial fission in various disease models. For example, sepsis-triggered myocardial damage is augmented by Mst1 activation and mitochondrial fission initiation. In gastric cancer, Mst1 knockdown promotes cancer survival via modulating AMPK-Sirt3-mitochondrial fission pathway (Shi et al., 2018). In renal ischemia reperfusion injury, Mst1 deletion attenuates kidney oxidative stress via suppressing mitochondrial fission (Zarfati, Avivi, Brenner, Katz, & Aharon, 2019). Herein, we found that Mst1 is involved in inflammation-mediated neuronal dysfunction via activating mitochondrial fission, and this finding was in accordance with the previous conclusions. Besides, we further found that JNK axis was governed by Mst1 and activated mitochondrial fission via upregulating Drp1 at the posttranscription levels (Meyer & Leuschner, 2018). Actually, the role of JNK pathway in mitochondrial fission has been widely discussed. In thyroid cancer tongue cancer, non-small cell lung cancer, and cerebral ischemia reperfusion attack (Anderton et al., 2019), the JNK axis is a key upstream signaling pathway regulating mitochondrial fission activation and augmentation (Erland, Shukla, Singh, Murch, & Saxena, 2018). These findings were in agreement with our results. Therefore, an approach to modulate the activity of JNK to control the mitochondrial fission would provide more benefits for patients with neuroinflammation (Cameron et al., 2018).
Overall, our data offer a novel insight into the mechanism underlying neuroinflammation. Increased Mst1 promotes neuronal dysfunction and cell death via activating Drp1-associated fission through the JNK axis. However, more clinical studies are required to validate our findings in the furture (Jin et al., 2018).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS CONTRIBUTIONS
H.T. and K.W. conceived the research. M.J., J.T.L., and Y.B.Y. performed the experiments. All authors participated in discussing and revising the manuscript.
AVAILABILITY OF DATA
All data generated or analyzed during this study are included in this published article.