LncRNA-MEG3 promotes bovine myoblast differentiation by sponging miR-135
Abstract
Long noncoding RNA maternally expressed gene 3 (lncRNA-MEG3) is an important regulator in multiple biological functions. However, lncRNA-MEG3's function in cattle growth and regulatory mechanism on bovine skeletal muscle development has not yet been well studied. In this project, we first investigated lncRNA-MEG3's expression profile and detected that it was highly expressed in bovine skeletal muscle tissue and its RNA level was kept increasingly during the early phase of bovine primary myoblast differentiation. Using luciferase reporter assays, we identified the lncRNA-MEG3 core promoter containing putative transcription factor binding site for myocyte enhancer factor 2C (MEF2C). Interestingly, we found that LncRNA-MEG3 could significantly upregulate and downregulate myosin heavy chain ( MHC), myogenin ( MyoG), and MEF2C through overexpression and RNAi strategies, respectively. Using luciferase reporter assays, we also verified lncRNA-MEG3 as a miR-135 sponge. Overexpression of miR-135 markedly inhibited the wild type of lncRNA-MEG3, but not the mutant lncRNA-MEG3 reporter. The luciferase activity of miR-135 sensor could be rescued by lncRNA-MEG3 via competing for miRNA-135. In addition, the luciferase activity of MEF2C was significantly upregulated by the wild type of lncRNA-MEG3. This study, for the first time, revealed that lncRNA-MEG3 could promote bovine skeletal muscle differentiation via interacting with miRNA-135 and MEF2C. The results were valuable for further studies and applications of lncRNA related roles in beef molecular breeding.
1 INTRODUCTION
Clarifying the mechanism of skeletal muscle development is essential as improving beef production is one of the most important goals of global agriculture. The development of skeletal muscle is a complex process in mammals. Skeletal muscle is derived from somites during the embryonic development (Buckingham et al., 2003), which is a highly orchestrated process involving several developmental stages of myoblast proliferation, differentiation, and fusion into multinuclear myotubes and myofibers (Bentzinger, Wang, & Rudnicki, 2012). In prenatal and early postnatal periods, the increase in fiber numbers (hyperplasia) is the main form for skeletal muscle growth. After that, an increase in fiber volume (hypertrophy), fiber area, and length occurs in further muscle development (Buckingham et al., 2003; Guo et al., 2015). Long noncoding RNAs (lncRNAs) are transcribed RNA molecules with a length of more than 200 nucleotides that do not encode proteins or micropeptides (less than 100 amino acid open reading frame). Nowadays, lncRNAs have become a hot spot of research due to its important role in multiple biological signs of progress, such as tumorigenesis and development, muscle development, and early embryogenesis (Liz & Esteller, 2016; Neguembor, Jothi, & Gabellini, 2014; Pauli, Rinn, & Schier, 2011).
Using Ribo-Zero RNA-Seq technology, our previous analysis has illustrated the lncRNA landscape of skeletal muscle in Qinchuan cattle, which ranks the first among the Chinese yellow cattle in terms of the meat quality. Notably, the lncRNA located at chr21: 67,377,726-67,395,997 (lncRNA_id: NONBTAT013257, maternally expressed 3 [MEG3 or lncRNA-MEG3], also known as gene trap locus 2 [GTL2]) caught our attention because of its differential expression among three developmental stages (Sun et al., 2016). MEG3 is one of the non-protein-coding regions at the Gtl2-Dio3 locus (da Rocha, Edwards, Ito, Ogata, & Ferguson-Smith, 2008), which is the largest known mammalian microRNA (miRNA) cluster. A subset of the Gtl2-Dio3 miRNAs can repress secreted Frizzled-related proteins (sFRPs), which in turn may affect skeletal muscle development by inhibiting Wnt (wingless/integrated) signaling (Kawano & Kypta, 2003; MacDonald, Tamai, & He, 2009). As a lncRNA, MEG3 does not encode any proteins but functions as an RNA on the embryonic development, muscle development, and hepatocellular cancer. For instance, maternal deletion of MEG3 resulted in perinatal death and skeletal muscle defects. During the development, MEG3 is expressed highly in the paraxial mesoderm, suggesting it might be involved in myogenesis (Schuster-Gossler, Bilinski, Sado, Ferguson-Smith, & Gossler, 1998). Through repressing PTEN by sponging miR-21, downregulation of MEG3 promotes the proliferation and migration of hypoxia-induced human pulmonary artery smooth muscle cells (Zhu et al., 2018). Overexpression of MEG3 in human HCC cells could decrease anchorage-dependent and -independent cell growth and induced apoptosis (Braconi et al., 2011).
However, experiments focused on the effects of lncRNA-MEG3 on cattle skeletal muscle development and underlying molecular mechanisms are still largely lacking. The objectives of this study are to investigate the expression profile of lncRNA-MEG3 in Qinchuan cattle both in tissue and cell levels and to explore whether lncRNA-MEG3 has potential functions on bovine primary myoblast differentiation. We observed that lncRNA-MEG3 could promote myoblast differentiation by sponging miR-135 and might be involved in regulating the MEF2C's expression. This study provides a lncRNA-based strategy to improve the meat production traits for beef cattle breeding.
2 MATERIAL AND METHODS
2.1 Animal samples
Animal samples used in this study were approved by the Animal Care and Use Committee of Northwest A&F University. To investigate the expression pattern of lncRNA-MEG3 in cattle, tissue samples from heart, liver, spleen, lungs, kidney, fat, and skeletal muscle (longissimus dorsi) of female Qinchuan cattle at three developmental stages (embryonic 90-day, n = 3; neonatal 3-day-old, n = 3; and adult 24-month-old, n = 3) were collected from Shanxi Kingbull Livestock Co., Ltd. (Baoji, China). All tissues for expression profile were immediately frozen in liquid nitrogen and then were kept at −80℃ until RNA isolation.
2.2 Cell culture and treatment
Primary bovine myoblast cells were isolated from longissimus dorsi muscle tissue and cultured in a sterile environment as previously described (Yu et al., 2018). The HEK 293T cells and C2C12 cells were obtained from Cell Bank of Chinese Academy of Sciences (Shanghai, China). The HEK 293T, C2C12, and myoblasts cells were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM, HyClone) supplemented with fetal bovine serum (FBS, HyClone; 10% and 20% for 293T/C2C12 and myoblasts cells, respectively) and double antibiotics (1% penicillin/streptomycin [P/S, HyClone]). To induce differentiation, cells were switched to differentiation medium (DM; DMEM; 2% heat-inactivated horse serum; 1% P/S) when myoblasts were grown to 90% confluence. All cells described above were cultured at 37℃ in 5% CO2. Myoblasts were transfected with the recombinant vectors, small interfering RNA (siRNA) of lncRNA-MEG3 and miRNA-135 mimic using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's protocol when cell confluence reached approximately 80%.
2.3 Nuclear and cytoplasmic RNA fractionation
Nuclear/cytoplasmic RNA fractionation was performed in primary myoblast cells using a PARIS kit (Carlsbad). Nuclear and cytoplasmic RNA was extracted and transcribed into complementary DNA (cDNA) for further analysis. The locations of lncRNA-MEG3 and GAPDH (cytoplasmic marker gene) were analyzed by PCR.
2.4 Vector construction
Primer information of pGL3-Basic, pcDNA3.1, and psiCHECK2 recombinant vectors was shown in Supporting Information Supplementary Table S1. To identify the active promoter regions of lncRNA-MEG3, four short segments with different ranges of lncRNA-MEG3 promoter were amplified by PCR using specific primers with the restriction enzyme sites and cloned to pGEM-T vector. Then, these vectors were digested and ligated into a luciferase reporter construct on pGL3-Basic vectors and named as −732/+78, −546/+78, −352/+78, and −146/+78, according to their start and end positions in the lncRNA-MEG3 promoter. The full length and fragment of lncRNA-MEG3, containing the sequences complementary to the seed region of miR-135, were amplified and cloned into overexpression vector of pcDNA3.1 + vector (Invitrogen, Carlsbad, CA) using PrimeSTAR Max DNA Polymerase Mix (Takara, Dalian, China; pc-lncRNA-MEG3, pc-lncRNA-MEG3-fragment-WT). To mutate the miR-135 recognition sequences on lncRNA-MEG3, two fragments carrying mutations in the sequences complementary to the seed region of miR-135 were first amplified and then merged based on the complementary sequences using overlapping extension PCR. Finally, the overlapping fragment was cloned into pcDNA3.1 + vector (pc-lncRNA-MEG3-Mut) and psiCHECK2 vector (psiCHECK2-lncRNA-MEG3-Mut) using the method mentioned above, respectively. The fragments of the MEF2C 3′-untranslated regions (3′-UTR) and lncRNA-MEG3, both of which included binding site of miR-135, were amplified and inserted into psiCHECK-2 vector (Promega, Madison, WI) at the 3′ end of Renilla gene using restriction enzymes Xho I and Not I (TaKaRa, Dalian, China) and T4 DNA ligase, to construct the expression plasmids (psiCHECK2-MEF2C-3′-UTR and psiCHECK2-lncRNA-MEG3). For the construction of the miR135 sensor, miRNA-complementary oligonucleotides were annealed and cloned into the psiCHECK2 vector. miR-135 mimics (sense: 5′-UAUGGCUUUUUAUUCCUAUGUGA-3′) and siRNA of lncRNA-MEG3 (sense, 5′-GCACACAA-GCAAUUCAAAUTT-3′; antisense, 5′-AUUUGAAUUGCUUGUGUGCTT-3′) were purchased from Genepharma (Shanghai, China). All constructs were confirmed by sequencing.
2.5 Cell proliferation assay
To gain insights into the effect of lncRNA-MEG3 on myoblast proliferation, EdU incorporation (Ribobio, Guangzhou, China) and CCK-8 (Multisciences, Hangzhou, China) assays were used, as described previously (Li et al., 2018).
2.6 Luciferase reporter assays
To predict the transcription factors interaction with lncRNA-MEG3, we predicted potential transcription factor binding sites in the identified core promoter regions by JASPAR2018 (http://jaspar.genereg.net) and TRANSFAC (www.gene-regulation.com). To identify the possibly targeted relationship between lncRNA-MEG3 and miR-135, we used the RNA target-predicting RNA hybrid (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid/) to find the potential binding sites. Then, we performed the Luciferase reporter assay. HEK293T cells and C2C12 cells were seeded into 48-well plates in triplicates, respectively. For the core promoter analysis, 400 ng of each pGL3-Basic plasmid were cotransfected with pRL-TK normalizing reporter plasmid (Promega, Madison). The pGL3-Basic vector served as a negative control. Two days after transfection, cells were lysed and luciferase activities were measured using a Dual-Luciferase® Reporter Assay System (Promega, Madison).
2.7 Western blot analysis
To obtain the total protein amounts, cells were harvested and then lysed in protein lysis buffer radioimmunoprecipitation assay (RIPA) supplemented with PMSF (Solarbio, Beijing, China). The extracts were boiled with 4 x sodium dodecyl sulfate (SDS) loading buffer at 98℃ for 10 min, and then 20 mg total proteins were loaded and separated on 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels. After electrophoresis, the proteins were transferred to 0.2-mm polyvinylidene fluoride (PVDF) membrane that was soaked in formaldehyde and then blocked with 5% skim milk in Tris saline with Tween (TBST) buffer for about 2 hr at room temperature. The membrane was then incubated overnight with primary antibodies anti-MyHC (dilution 1:1,000; ab50967; Abcam), anti-MyoG (dilution 1:1,000; ab1835; Abcam), anti-MEF2C (dilution 1:4,000; ab227085; Abcam), anti-PCNA, anti-CyclinD1, anti-Bcl2, anti-Bax, and anti-β-actin (dilution 1:4,000; ab8227; Abcam). The expression of β-actin was used as a loading control. The PVDF membrane was washed three times with TBST buffer and then incubated with secondary antibody for 2 hr at room temperature. Finally, antibody reacting bands were detected using enhanced chemiluminescence (ECL) luminous fluid (Solarbio, China) and visualized using the ChemiDoc XRS + system (Bio-Rad Laboratories, Hercules, CA).
2.8 RNA isolation, cDNA synthesis, and quantitative real-time PCR (qRT-PCR)
Total RNA from tissues or cells was extracted using a Trizol reagent (Takara, Japan) and cDNAs were synthesized using a PrimeScript RT reagent Kit (Takara, Japan), according to the manufacturer's protocol. Primers for quantitative real-time polymerase chain reaction (qRT-PCR) were designed by Prime 5.0 software based on the bovine reference genome UMD3.1 (Supporting Information Supplementary Table S2). The messenger RNA (mRNA) expression levels of target genes were normalized to Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH). The qRT-PCR was performed with SYBR®FAST qPCRMix (Takara, Japan) using a CFX96 Touch Fast Real-Time PCR System (Bio-Rad). Three biological replicates were applied and all the experiments were in triplicates.
2.9 Statistical analysis
The quantitative results were analyzed by the 2−ΔΔCt method and represented as means ± SEM based on replicates. For statistical significance, data were assessed using Student's t-test for normally distributed data by SPSS (Inc., Chicago, IL). The p values of < 0.05 were considered statistically significant differences among groups.
3 RESULTS
3.1 Expression profile of lncRNA-MEG3
Using qRT-PCR, the transcriptional profile of lncRNA-MEG3 in multiple tissues of Qinchuan cattle during three different development periods were investigated (Supporting Information Figure S1A–C). Of note, lncRNA-MEG3 was specifically highly expressed in skeletal muscle at the fetal stage. Compared with adult cattle, the fetal cattle had a remarkably higher lncRNA-MEG3 RNA expression level in skeletal muscle (Supporting Information Figure S1D).
To identify the expression profile of lncRNA-MEG3 in different periods of myogenic differentiation, we collected the bovine myoblasts at Day 0, Day 1, Day 3, and Day 5 after inducing myoblasts differentiation with differentiation medium (DM), respectively. After inducing myoblasts differentiation, the transcriptional levels of three marker genes for myoblasts differentiation, including myogenic differentiation 1 (MyoD), myogenin (MyoG), and myosin heavy chain (MyHC), revealed expected expression trends as previously reported (Sabourin & Rudnicki, 2000; Zammit et al., 2004; Supporting Information Figure S2). Under the microscope, multiple muscle cells were observed to fuse to form the obvious myotubes after Day 3 (Supporting Information Figure S3). These observations indicated that bovine primary myoblast cells were differentiating normally and could be used for subsequent experiments. The mRNA expression levels of lncRNA-MEG3 were detected by both quantitative and semi-quantitative RT-PCR. As shown in Figure 1a, lncRNA-MEG3 was increasingly expressed during bovine myoblasts differentiation. By analyzing the intracellular occurrence of lncRNA-MEG3, we observed that the abundance level of the lncRNA in cytoplasm was obviously more than that in the nucleus, whereas, GAPDH was especially expressed in cytoplasm (Figure 1b).
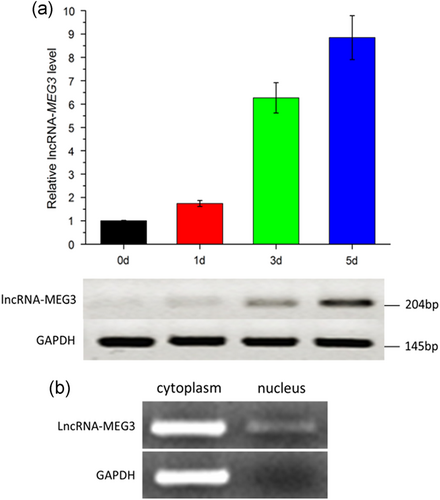
Expression profile of lncRNA-MEG3 in bovine primary myoblast cells. (a) Expression level of lncRNA-MEG3 during bovine primary myoblast differentiation detected by qRT-PCR (upper panel) and semi-quantitative RT-PCR (lower panel). Cells were harvested and total RNA was extracted at Day 0 (0 d), Day 1 (1 d), Day 3 (3 d), and Day 5 (5 d). Error bars represented mean ± SEM (n = 3). (b) Expression level of lncRNA-MEG3 and GAPDH in cytoplasm and nucleus by semi-quantitative RT-PCR. Cells were harvested and total RNA was extracted at Day 4. lncRNA: long noncoding RNA; qRT-PCR: quantitative real-time polymerase chain reaction [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Identifying the core promoter of bovine lncRNA-MEG3
To further understand the regulation mechanism of lncRNA-MEG3, we constructed the bovine lncRNA-MEG3 promoter vectors containing 5′ unidirectional deletions of the promoter region. The relative activities of the 5′ flanking region fragments expressed in 293T (green) and C2C12 cells (gray) are shown in Figure 2. From the luciferase assays, we noted that promoter activities of the fragments −732/+78 and −546/+78 were significantly higher than the pGL3-basic control vector in the two cell types (p < 0.01). Promoter activity increased from −732 to −546 bp but markedly decreased when the ranges of promoter sequences narrowed from −546 to −352 bp. These results indicated that the proximal minimal promoter of the lncRNA-MEG3 was located within the region between −546 and −352 bp relative to TSS. By predicting the potential transcription factor interacted with lncRNA-MEG3, we observed that the bovine lncRNA-MEG3 core promoter contained a series of putative binding sites for transcription factors related to the muscle development, such as Myod1, MEF2C, and MEF2A.
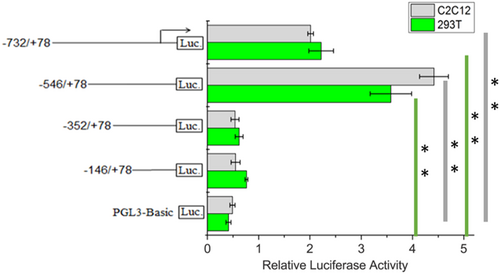
Luciferase activities in the bovine promoter LncRNA-MEG3 construct in C2C12 cells and 293T cells. The plasmids containing 5′ unidirectional deletions of the promoter region of the LncRNA-MEG3 were ranged from −732, −546, − 352, and −146 bp to +78 bp related to TSS, respectively. Cells were lysed and luciferase activities were measured at 48 hr after transfection. Error bars were the mean ± SEM (n = 3) based on firefly luciferase activity normalized to the Renilla luciferase activity. (**) p < 0.01. lncRNA: long noncoding RNA [Color figure can be viewed at wileyonlinelibrary.com]
3.3 LncRNA-MEG3 promotes skeletal muscle cell differentiation
We modulated expression levels of lncRNA-MEG3 through overexpression and RNA interference (RNAi) strategies. We transfected pc-lncRNA-MEG3 and siRNA of lncRNA-MEG3 into bovine primary myoblasts in growth medium (GM; Day 0) and then induced to cell differentiation. The cells were harvested at Day 4 and then analyzed by qRT-PCR and western blot. The transfection efficiency of pcDNA-lncRNA-MEG3 and siRNA were measured. Overexpression of lncRNA-MEG3 led to > 9,000-fold induction of lncRNA-MEG3 (Figure 3a, p < 0.01) and siRNA specific to lncRNA-MEG3 reduced its RNA level by 44% (Figure 3b, p < 0.05). Meanwhile, we analyzed the expressions of two established myogenic markers, MyoG and MyHC, at the mRNA and protein levels, respectively. The results showed that the expressions of the two marker genes were significantly increased after overexpression of lncRNA-MEG3 and were remarkably decreased by transfection of siRNA (Figure 3c–e, p < 0.05). Collectively, these results revealed that lncRNA-MEG3 could specifically accelerate bovine myoblast differentiation.
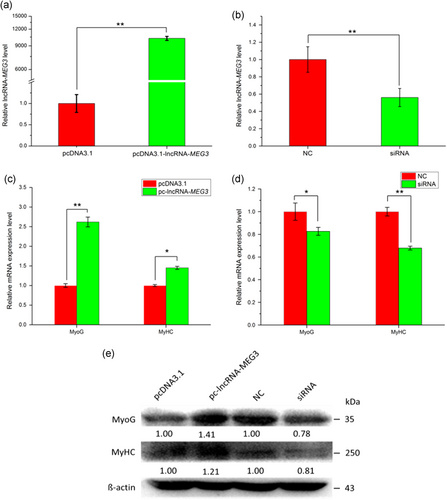
LncRNA-MEG3 promotes myoblast differentiation. Overexpression of lncRNA-MEG3 promotes skeletal muscle differentiation (a,c, and e), while silencing lncRNA-MEG3 decreases myoblast differentiation (b,d, and e). PcDNA-lncRNA-MEG3 or siRNA specific for lncRNA-MEG3 were transfected to bovine myoblast cells in growth medium (GM) and changed to differentiation medium (DM) after 12 hr. Cells were harvested on DM 3 day and used for qRT-PCR and western blot analysis. The relative mRNA expressions (a–d) were shown as mean ± SEM (n = 3). (*) p < 0.05; (**) p < 0.01. Western blot images were quantified using the ImageJ software (National Institutes of Health). The numbers below the western blots (e) mean the fold change of target genes’ proteins quantities related to the control group (pcDNA 3.1 or NC). lncRNA: long noncoding RNA; qRT-PCR: quantitative real-time polymerase chain reaction [Color figure can be viewed at wileyonlinelibrary.com]
3.4 LncRNA-MEG3 does not affect myoblast cell proliferation and apoptosis
To investigate the role of lncRNA-MEG3 in myoblast proliferation, cell counting kit-8 (CCK-8), 5-ethynyl-20-deoxyuridine (EdU), qPCR, and western blot analysis assays were conducted. The CCK-8 assay revealed that neither overexpression nor RNAi of lncRNA-MEG3 could significantly affect cell proliferation (p > 0.05; Figure 4a,b). The EdU assay had similar results (Figure 4c–e). Also, we detected the effects of lncRNA-MEG3 on cell-proliferation-related genes, like proliferating cell nuclear antigen (PCNA) and cyclin D1, and found that lncRNA-MEG3 did not significantly increase or decrease these genes’ expressions at both mRNA and protein levels (Figure 4f–h). These results reveal that lncRNA-MEG3 does not affect cell proliferation.
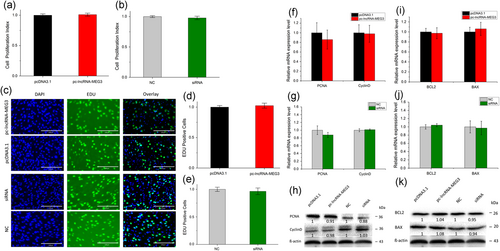
LncRNA-MEG3 does not affect myoblast cell proliferation and apoptosis. (a and b) Cell proliferation index was detected by cell counting kit-8 (CCK-8) assay after overexpression (a) and RNAi (b) of lncRNA-MEG3. (c) Cell proliferation was detected with 5-ethynyl-20-deoxyuridine (EdU); the scale bar represents 200 mm. (d and e) EdU-positive cell index statistics are shown after overexpression (d) and RNAi (e) of lncRNA-MEG3. (f–h) The expressions of proliferation marker genes, PCNA and cyclin D1, were detected by real-time qPCR (f and g) and western blot analysis (h) by overexpression and RNAi of lncRNA-MEG3. (i–k) Overexpression and silence of lncRNA-MEG3 have no effects on the apoptosis of bovine myoblast cells. The mRNA of apoptosis marker genes (Bcl-2, Bax) was detected using real-time qPCR (i and j) and western blot analysis (k). pcDNA-lncRNA-MEG3 or siRNA specific for lncRNA-MEG3 was transfected to bovine myoblast cells in GM. The cells were harvested 24-48 hr later and used for qRT-PCR or western blot analysis. Error bars were means ± SEM for three individuals. Western blot images were quantified using the ImageJ software (National Institutes of Health). The numbers below the western blots (h and k) mean the fold change of target genes’ proteins quantities related to the control group (pcDNA 3.1 or NC). lncRNA: long noncoding RNA; qRT-PCR: quantitative real-time polymerase chain reaction [Color figure can be viewed at wileyonlinelibrary.com]
Bcl-2 has been described as an antiapoptotic protein and plays important roles in regulating proliferation and apoptosis in myoblasts (Schöneich, Dremina, Galeva, & Sharov, 2014). To explore whether antiapoptotic protein Bcl-2 and proapoptotic protein Bax can be affected by lncRNA-MEG3 in myoblast, we quantified the expression of Bcl-2 and Bax pretreated with pc-lncRNA-MEG3 and siRNA of lncRNA-MEG3 using qPCR or western blot analysis. No significant differences were observed at both mRNA and protein levels (Figures 4i–k). These results suggest that lncRNA-MEG3 does not regulate myoblast apoptosis.
3.5 LncRNA-MEG3 acts as a competing endogenous RNA for miR135
To explore the regulatory mechanism of lncRNA-MEG3, the recognition sequences of miR-135 targeting for lncRNA-MEG3 were predicted using bioinformatics analysis (Supporting Information Figure S4A). The recognition sequences of miR-135 were found to be conserved across different mammalian genomes such as human, mouse, and domesticated animals (Supporting Information Figure S4B). To test the correlation between lncRNA-MEG3 and miR-135, on the one hand, we constructed the wild type of reporter containing the miR-135 binding site (psiCHECK2-lncRNA-MEG3-WT) and the mutant reporter (psiCHECK2-lncRNA-MEG3-Mut), of which the miR-135 recognition sequences were mutated. We then cotransfected them with mimics for miR-135 to HEK293T cells, respectively. Compared with the control NC group, we observed a notable inhibition in psiCHECK2-lncRNA-MEG3-WT containing the bovine lncRNA-MEG3 fragment at the 3′-UTR of the Rluc (Figure 5a, p < 0.05). However, no significant difference for the relative Rluc activity was detected in psiCHECK2-lncRNA-MEG3-Mut lacking miR-135 binding site. On the other hand, we constructed a miR-135 sensor by introducing its perfect complement into the psiCHECK2 vector, thus specifically silencing by RNAi in the presence of the miR-135. Then, the psiCHECK2-miR-135 sensor was cotransfected to HEK293T cells with miR-135 mimics and different quantity (0, 0.1, and 0.2 μg for each treatment) of pc-lncRNA-MEG3-fragment-WT and pc-lncRNA-MEG3-Mut plasmids. The results showed that the relative luciferase activities of miR-135 sensor were significantly rescued by the wild type of pc-lncRNA-MEG3 plasmid but were not markedly influenced by the mutant type (Figure 5b,c). These findings validate the role of LncRNA-MEG3 in sponging miR-135.
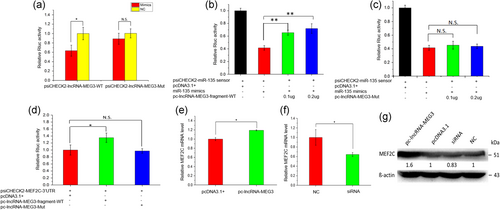
LncRNA-MEG3 acts as a competing endogenous RNA for miR135. (a–c) lncRNA-MEG3 could sponge miR-135. (a) Overexpression of miR-135 significantly inhibited Rluc expression of lncRNA-MEG3-WT, but had no obvious effects on lncRNA-MEG3-Mut. The mimics of miR-135 were transfected into HEK293 cells, together with psiCHECK2-lncRNA-MEG3-WT and psiCHECK2-lncRNA-MEG3-Mut, respectively. (b) Wild type of lncRNA-MEG3 fragments rescued the relative luciferase activities of miR-135 sensor by competitively sponging miR-135. (c) Mutant lncRNA-MEG3 could not sponge miR-135. The psiCHECK2-miR-135-sensor was transfected into 293T cells together with miR-135 mimics and wild type lncRNA-MEG3 (pcDNA-lncRNA-MEG3-fragment-WT, b) or mutant lncRNA-MEG3 (pcDNA-lncRNA-MEG3-Mut, c). (d-g) LncRNA-MEG3 affected the expression of MEF2C. (d) Overexpression of lncRNA-MEG3 fragment significantly promoted Rluc expression of MEF2C, but lncRNA-MEG3-Mut had no obvious effects on MEF2C. psiCHECK2-MEF2C-3′-UTR plasmid was cotransfected into HEK293 cells, together with pc-lncRNA-MEG3-fragment-WT or pc-lncRNA-MEG3-Mut. Cells were lysed and luciferase activities were measured at 48 hr after the transfection. Overexpression of lncRNA-MEG3 significantly promoted the expression of MEF2C at mRNA (e) and protein level (g), whereas silencing lncRNA-MEG3 decreased the expression of MEF2C at mRNA (f) and protein level (g). pcDNA-lncRNA-MEG3 or siRNA specific for lncRNA-MEG3 was transfected to bovine myoblast cells in GM and changed to DM after 12 hr. The cells were harvested on DM 3 day and used for qRT-PCR or western blot analysis. Error bars (a–f) were mean ± SEM (n = 3). (*) p < 0.05; (**) p < 0.01. N.S. means there was no significant difference. Western blot images were quantified using the ImageJ software (National Institutes of Health). The numbers below the western blots (g) mean the fold change of target genes’ proteins quantities related to the control group (pcDNA 3.1 or NC). lncRNA: long noncoding RNA; qRT-PCR: quantitative real-time polymerase chain reaction [Color figure can be viewed at wileyonlinelibrary.com]
Previously, it has been demonstrated that miR-135 can target MEF2C gene and affect myoblast differentiation (Cesana et al., 2011). To explore the impacts of LncRNA-MEG3 on the target gene of miR-135, we then constructed a psiCHECK2-MEF2C-3′-UTR containing miR-135 binding site and transfected into HEK293T cells together with either pcDNA3.1-lncRNA-MEG3-fragment-WT or pc-lncRNA-MEG3-Mut. Notably, the pcDNA3.1-lncRNA-MEG3-fragment-WT clearly increased the relative Rluc activity of psiCHECK2-MEF2C, whereas no significant change was induced by pc-lncRNA-MEG3-Mut (Figure 5d). These results indicate that lncRNA-MEG3 could relieve the inhibitory effect of miR-135 on MEF2C. Thus, our results confirmed that MEF2C, as a potential target, could be affected by lncRNA-MEG3. In the bovine myoblasts, we found that both the mRNA and protein expressions of MEF2C were upregulated with overexpression of lncRNA-MEG3 but were remarkably decreased by suppressing lncRNA-MEG3 with specific siRNA (Figure 5e–g, p < 0.05). These results suggest that LncRNA-MEG3 may act as a competing endogenous RNA for miR135 and thus affect the target gene MEF2C.
4 DISCUSSION
The cattle breed resources are quite abundant in China, whereas, a considerable part of them have such shortcomings as poor beef quality and low beef productivity (Huai, Zhiyong, & Zhijie, 1993). Among the Chinese indigenous cattle, Qinchuan is famous for its remarkably high meat quality and the underlying genetic mechanisms are waiting for clarification. In recent decades, much attention has been paid on identifying candidate genes underlying favorable economic traits, to improve the efficiency and accuracy of cattle breeding (Chen & Zhang, 2008). Majority of mammalian genomes is transcribed to abundant noncoding RNAs (ncRNAs), such as microRNAs, circRNAs, and lncRNAs. Among them, the lncRNAs is one of the most functionally versatile classes, playing important roles in the regulation of development, differentiation, and maintenance of cell identity (Neguembor et al., 2014). To our knowledge, LncRNA-MEG3 has been reported to interact with crucial signaling pathways, including angiogenesis, cell proliferation and differentiation, and survival regulation, and has been found deregulated in several types of tumors (Benetatos, Vartholomatos, & Hatzimichael, 2011). Also, MEG3 could regulate muscle differentiation by the interaction with PRC2 and TGF-β pathway genes (Mondal et al., 2015; Neguembor et al., 2014). In Large White pigs, lncRNA MEG3 has been found to be associated with corrected back fat thickness and age to reach 100 kg value (Yu et al., 2018). In cattle, this study provided a comprehensive report on lncRNA-MEG3's expression profile, core promoter region, effects on bovine myoblasts differentiation and the functional mechanisms.
We found lncRNA-MEG3 RNA level in skeletal muscle was much higher than other tissues at fetal stage, which is perfectly accordant with our previous study on the lncRNA landscape during the cattle muscle development (Sun et al., 2016) and the expression pattern in Longissimus dorsal muscles of embryonic pigs (Yu et al., 2018). Similar to the tissue expressions of postnatal Day 160 in Large White pigs, lncRNA-MEG3 was also highly expressed in skeletal muscle and kidney in the calf and adult cattle. Besides, lncRNA-MEG3 was observed to highly expressed in the liver in calf and spleen and lung of adult cattle. These observations support the previous study showing lncRNA-MEG3 was conserved and expressed in many tissues in human (Zhang et al., 2010). Moreover, we observed that lncRNA-MEG3 was increasingly expressed during the early phase of bovine primary myoblast differentiation. These results implied the important roles of lncRNA-MEG3 in myoblast differentiation. We further investigated the localization of lncRNA-MEG3 in primary bovine myoblast cells and found high RNA levels in cell cytoplasm but low levels in the nucleus. The previous study reported that some MEG3 transcripts were localized to the nucleus in some cell types and stages during the development (Schuster-Gossler et al., 1998). Herein, our finding indicated that lncRNA-MEG3 may crucial roles in the gene regulations in the cell cytoplasm.
To explore the effects of lncRNA-MEG3 on myogenic differentiation, we enforced overexpression and silencing of lncRNA-MEG3 in bovine differentiated myoblasts. Our results demonstrated that lncRNA-MEG3 could affect myogenic differentiation of skeletal muscle cell by significant upregulation or downregulation of myogenic markers, MyoG and MyHC. This observation indicates the influence of lncRNA-MEG3 on skeletal muscle development and thus lead to significant production trait differences, which has been shown in the preliminary study on porcine lncRNA-MEG3′ SNPs effects on meat productions (Yu et al., 2018). In this study, we further demonstrated that overexpression and RNAi of lncRNA-MEG3 had marked impacts on expression of MEF2C, a known regulator of muscle differentiation and growth (Cesana et al., 2011; Honardoost, Soleimani, Arefian, & Sarookhani, 2015; McKinsey, Zhang, & Olson, 2002). MEF2C belongs to the myocyte enhancer factor 2 (MEF2) families, encoding key transcription factors that mediate gene expression in myocytes (Black & Olson, 1998). As known, MEF2C is a transcription factor mediating cell differentiation by multiple signaling pathways, some of which are known to perturb the myogenic program. For example, the cyclin D-cdk4 activity could block the association of MEF2C with the coactivator GRIP-1, thereby, represses skeletal muscle differentiation (Lazaro, Bailey, & Lassar, 2002). Transforming growth factor β (TGF-β) suppresses myogenic differentiation via its effector, Smad3, which is in part capable of disrupting MEF2C and GRIP1 association (Liu, Kang, & Derynck, 2004). MAML1 coactivates MEF2C to promote the transcription of muscle-specific genes (Shen et al., 2006). In this study, lncRNA-MEG3 significantly upregulated the MEF2C mRNA and protein expression and the luciferase activity of MEF2C 3′-UTR reporter could be rescued by the wild type of lncRNA-MEG3 plasmids, indicating that lncRNA-MEG3 might be a key regulator of MEF2C. In addition, the binding site for MEF2C transcription factor was predicted in the lncRNA-MEG3 core promoter regions, suggesting the co-operative interaction between lncRNA-MEG3 and MEF2C. In this case, further validation of the interaction is needed in the following study.
To unravel the underlying regulatory mechanism, we predicted the possible miRNAs that may interact with lncRNA-MEG3 and found the promising miR-135, which has been previously proved to play key roles in skeletal myogenic differentiation by regulating the IRS/AKT/PI3K pathway (Honardoost et al., 2015; Li, Deng, Li, & Li, 2015). The inhibitors of miR-135 and miR-143 could induce skeletal myogenic differentiation of human adult dental pulp stem cells (Li et al., 2015). In our study, overexpression of miR-135 was found to markedly inhibit the wild type of lncRNA-MEG3, whereas the mutation of the seed region of the predicted miR-135 target site abolished sponging lncRNA-MEG3. In addition, the luciferase activity of miR-135 sensor could be rescued by lncRNA-MEG3 via competing for miRNA-135. These observations, for the first time, provided the evidence that lncRNA-MEG3 could sponge miR-135.
In recent years, increasing evidence has demonstrated that lncRNAs, as competing endogenous RNA (ceRNAs) for binding to miRNAs and circRNAs, were involved in the muscle and adipose development and differentiation via modulating their target mRNAs (Li et al., 2016; Song et al., 2018; Sun et al., 2016). This crosstalk between lncRNA–miRNA–mRNA plays a critical role in multiple biological processes in development and disease (Cesana et al., 2011; Liu et al., 2016; Tay, Rinn, & Pandolfi, 2014), and has been recognized as a common mode of action in lncRNA functional regulation process (Ballantyne, McDonald, & Baker, 2016; Paraskevopoulou & Hatzigeorgiou, 2016). In mouse and human myoblast differentiation, it has been demonstrated that MEF2C can be repressed by miRNA-135, which can be inhibited by Linc-MD1 acting as a molecular sponge for miR-135 (Cesana et al., 2011; Neguembor et al., 2014). In bovine primary myoblasts, this study identified another functional lncRNA and illustrated the mechanism of lncRNA-MEG3′s regulation on bovine myogenic differentiation, in which lncRNA-MEG3 might modulate MEF2C expression by competing for miR-135 in the ceRNA regulatory network (lncRNA-MEG3/miR-135/MEF2C; Figure 6). In this model, lncRNA-MEG3 could increase the expression of MEF2C by sponging miR-135 and influencing the MEF2C-mediated signaling pathways that promote the myogenic differentiation. However, this could be just one strategy of lncRNA-MEG3′s regulation mechanisms. We observed that the fold changes in HEK293 cells were statistically significant but smaller than in primary bovine myoblast cells, suggesting there might be some other functional genes or pathways related to lncRNA-MEG3. We also observed the potential binding site for MEF2C within the core promoter of lncRNA-MEG3, indicating the MEF2C may interact directly with lncRNA-MEG3. In addition, the previous study has demonstrated that lncRNA-MEG3 repress the protein-coding gene Dlk1 and the lncRNA Gtl2-as by binding to PRC2 recruitment to the Dlk1-Dio3 locus (Benetatos et al., 2013). Dlk1 is involve in skeletal muscle development (Abdallah et al., 2004) and skeletal muscle remodeling (Andersen et al., 2009). Previous studies have shown that lncRNA-MEG3 is reciprocally imprinted with the paternally expressed gene DLK1, constituting an imprinting domain on mouse chromosome 12 and on human chromosome 14q32 (Lin et al., 2003). This indicates that the interactions of lncRNAs-MEG3 with the target genes in muscle differentiation is complicated and further study is still necessary for the future.
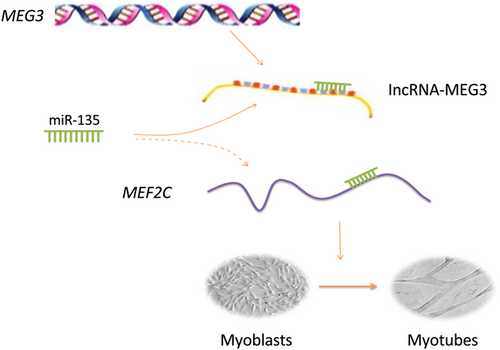
Model of the possible lncRNA-MEG3-miR-135-MEF2C pathway in bovine skeletal muscle differentiation. LncRNA-MEG3 sponged miR-135 and indirectly promoted MEF2C expression, resulting in myoblast differentiation [Color figure can be viewed at wileyonlinelibrary.com]
In brief, we found that lncRNA-MEG3 could promote bovine skeletal muscle differentiation and provided evidence on the interactions of lncRNA-MEG3, miRNA-135, and MEF2C, in which LncRNA-MEG3 could sponge miRNA-135 and affect MEF2C expressions. Our results unraveled one of the possible underlying molecular mechanisms and indicated that the modulation of lncRNA-MEG3 expression in muscle tissue may emerge as a promising tool in beef cattle breeding.
ACKNOWLEDGMENTS
We acknowledge the members of the Shanxi Kingbull Livestock Co., Ltd. for their help on sampling the tissues of Qinchuan cattle. We acknowledge Hui Li, Chengchuang Song, Yueyu Bai, and Yun Ma for the advice and guidance for this project.
AUTHORS' CONTRIBUTIONS
Bo Li and Mei Liu designed the study, performed the experiments and drafted the manuscript. Wenwen Peng, Yilei Ma, Yongzhen Huang, Xianyong Lan, Chuzhao Lei, and Xinglei Qi helped to perform the experiments and analyze the data. George E. Liu helped to write the manuscript. Hong Chen helped to design the experiments and write the manuscript.
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
FUNDING
This study was supported by the National Natural Science Foundation of China (31772574), Program of National Beef Cattle and Yak Industrial Technology System (CARS-37), Bio-breeding capacity-building and industry specific from National Development and Reform Commission (2014–2573), and Specific Projects of Science and Technology in Henan Province (141100110200).
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.