MFGE8 protects against CCl4-induced liver injury by reducing apoptosis and promoting proliferation of hepatocytes
Abstract
Milk fat globule-EGF factor 8 (MFGE8) has been reported to play various roles in acute injury and inflammation response. However, the role of MFGE8 in liver injury is poorly investigated. The present research was designed to clarify the expression and function of MFGE8 in carbon tetrachloride (CCl4)-induced liver injury. Using serum cytokine arrays, we selected a promising cytokine MFGE8 as the candidate in the process of hepatitis-fibrosis-hepatocellular carcinoma (HCC) progression, based on the elevated expression in both hepatic fibrosis and HCC models. We validated the increased expression of MFGE8 in liver tissues and serum samples of acute and chronic CCl4-induced mice. Immunohistochemistry staining of mouse liver tissues indicated that elevated MFGE8 expression was mainly derived from the injured hepatocytes. In addition, MFGE8 expression in the supernatant of primary hepatocytes was accumulated with prolongation of culture time, and CCl4 treatment further increased the expression of MFGE8. Moreover, a strong correlation between serum MFGE8 expression and liver transaminase activities suggested that MFGE8 may be a novel candidate in liver injury. Intriguingly, mice pretreated with MFGE8 were protected from CCl4-induced liver injury through antiapoptosis role in the early stage and proproliferation role in the late stage. MFGE8 reduced apoptosis by inhibiting the activation of IRE1α/ASK1/JNK pathway and promoted proliferation by phosphorylation of ERK and AKT. Moreover, serum MFGE8 expression was increased in hepatitis patients while decreased in liver cirrhosis patients. All the results suggest MFGE8 as a novel marker and promising therapeutic agent of liver injury.
1 INTRODUCTION
Liver injury is essentially involved in liver disease progression including inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC; Luedde, Kaplowitz, & Schwabe, 2014). Normal hepatocytes are vulnerable to be insulted by food-derived toxins, hepatotropic virus infection, and bacteria infection (Chakraborty, Oakley, & Walsh, 2012). Hepatocellular response to cell death, especially resistance to death and rapid regeneration, plays an important role in orchestrating hepatic function (Malato et al., 2011). Cell death in acute liver injury consists of two main mechanisms: necrosis and apoptosis. Apoptosis is a well-synchronized sequence of morphological events requiring cellular ATP, recognized by nuclear condensation, plasma membrane blebbing, and apoptotic bodies emerging (Chakraborty et al., 2012). Persistent apoptosis of injured hepatocytes promoted fibrogenic progression and reduced apoptosis of hepatocytes alleviated hepatic fibrosis (Wang et al., 2017). Apoptosis signaling is frequently interfered in cancer development, thus contributing to the promotion of HCC progression (Lin & Zhang, 2017). Given the significance of apoptosis in liver disease progression and resolution, underlying mechanisms involved in the pathogenesis deserved to be further investigated.
Endoplasmic reticulum (ER) stress usually serves as response to the increased protein folding and degradates the unfolded accumulation of proteins the by activation of three different ER transmembrane proteins: inositol requiring 1α (IRE1α), protein kinase RNA-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6α (ATF6α; Malhi & Kaufman, 2011). Moderate unfolded protein response can be restored by ER stress, while prolonged ER stress sensitizes to apoptosis (Malhotra & Kaufman, 2007). Carbon tetrachloride (CCl4)-induced liver injury is a well-established animal model, which could be metabolized by the cytochrome P450 into trichloromethyl radical (CCl3*). The oxygenation of CCl3* initiates lipid peroxidation and influences mitochondria, ER, and plasma membrane permeability, thus triggering hepatotoxicity (Scholten, Trebicka, Liedtke, & Weiskirchen, 2015). The CCl4 treatment could induce rough ER to swell in hepatocytes and increased expression of molecules related to apoptosis (caspases, Bcl-2/Bax) and ER stress (IRE1α, PERK, GRP78, XBP1, C/EBP homologous protein [CHOP] etc.) (Iracheta-Vellve et al., 2016; Lee, Marahatta, Bhandary, Kim, & Chae, 2016; F. Li et al., 2017; Marumoto et al., 2008; D. G. Zhang et al., 2017). CHOP is increased when eIF2α is phosphorylated by PERK and plays a predamage role in acetaminophen-induced liver injury (Uzi et al., 2013). Prolonged activation of IRE1 and CHOP induces apoptosis in physiological and pathological conditions (Tabas & Ron, 2011). However, CHOP fails to play an essential role in CCl4-induced liver injury since inhibition of CHOP expression did not protect against CCl4-induced damage (Campos et al., 2014). IRE1α activation recruits TRAF2 and activates subsequent molecules including apoptosis signal-regulating kinase 1 (ASK1) and c-Jun N-terminal kinase (JNK; Malhi & Kaufman, 2011). JNK1 activation, rather than JNK2, seems to be directly related to liver injury and apoptosis (Leamy, Egnatchik, & Young, 2013). Hence, the IRE1α/ASK1/JNK pathway may be hypothesized as proapoptotic mechanism in CCl4-induced hepatocyte injury.
Milk fat globule-EGF factor 8 (MFGE8), also known as lactadherin, is widely expressed in the liver, kidney, intestine, lung, mammary gland, brain, heart, spleen, and reproductive organs (Aziz, Jacob, Matsuda, & Wang, 2011). It has been reported to play various roles in acute injury and inflammation response. MFGE8 reduces ischemia-reperfusion damages, attenuates organ injury and improves survival in sepsis, alleviates apoptosis and inflammation in early brain injury, ameliorates colon damage in dextran sodium sulfate (DSS)-induced colitis in mice, and attenuates neutrophil infiltration in acute lung injury (Aziz, Matsuda, Yang, Jacob, & Wang, 2012; Liu et al., 2015; Matsuda et al., 2013; Matsuda et al., 2011; R. Wu et al., 2012; Yang et al., 2015; Y. Zhang, Brenner, Yang, & Wang, 2015). However, the role of MFGE8 in liver injury is poorly investigated. Based on previous studies, we hypothesized that MFGE8 may act as antiapoptosis role in CCl4-induced liver injury and wondered whether it functions through inhibiting the activation of IRE1α/ASK1/JNK proapoptotic pathway. We attempted to clarify the expression and function of MFGE8 in damaged hepatocytes and revealed its antiapoptosis and proproliferation roles in liver injury.
2 MATERIALS AND METHODS
2.1 Animals
To induce acute liver injury, 6-week-old male Balb/c mice (Fourth Military Medical University, Xi'an, China) were treated with CCl4 (0.5 ml/kg body weight; diluted in corn oil; 1:9) by intraperitoneal injection at Day 0 (injection once) and killed at either Day 1 or Day 3; or mice were treated with CCl4 at Day 0 and Day 4 (injection twice) and killed at either Day 4 or Day 6. To induce chronic liver injury (hepatic fibrosis), 6-week-old male C3H (Vital River Laboratories, Beijing, China) and Balb/c mice were treated with CCl4 by intraperitoneal injection twice a week until 8 weeks, and the serum and liver tissues were collected at 2, 6 or 8 weeks. N-diethylnitrosamine/phenobarbital (DEN/PB)-induced HCC model in C3H mice was described previously (Lu et al., 2015). Two-month-old male C3H and Balb/c mice were chosen as normal controls. To protect against liver injury, 4 hr before CCl4 injection, 25 μg/kg recombinant mouse MFGE8 protein (2805-MF/CF; rmMFGE8, R&D Systems, Minneapolis, MN) was administered by intraperitoneal injection. The serum and liver tissues were collected at Day 1 and Day 3. All mice were randomly assigned to experimental groups or normal group. All experimental protocols were approved by the Laboratory Animal Ethics Committee of Fourth Military Medical University.
2.2 Human specimens
Human tissue microarrays (LV20812; US Biomax, Rockville, MD) containing 15 normal liver tissues, 24 hepatitis, and 32 liver cirrhosis were detected by immunohistochemistry staining. Human sera including 37 healthy, 67 hepatitis B virus (HBV) hepatitis, and 17 liver cirrhosis were collected in Tangdu Hospital of Fourth Military Medical University and the Second Affiliated Hospital of Xi'an Jiaotong University. Experiments involving human samples were approved by the Ethics Committee of Fourth Military Medical University.
2.3 Mouse cytokine arrays
Custom G-Series Mouse Cytokine Antibody Array Kit (AAM-CUST-G, RayBiotech, Norcross, GA) was purchased from RayBiotech, Inc. A series of cytokines were customized to identify the pivotal cytokine in the progression of liver diseases. The sera of established C3H mouse model were measured and analyzed by the manufacturer using the array. Detailed information was described in Table S1.
2.4 Real-time PCR
Real-time polymerase chain reaction (PCR) was performed as previously described (H. Y. Li et al., 2015). The primer sequences were listed in the Table S2.
2.5 Western blot analysis
Western blot analysis was performed as previously described (J. Wu et al., 2011). The primary antibodies were described in Table S3.
2.6 Haematoxylin & eosin and sirius red staining
Formalin-fixed paraffin-embedded liver tissue sections were deparaffinized by xylene and alcohol. The slides were then counterstained with hematoxylin for 15 min and eosin for 10 min. The slides were stained with 0.1% Sirius red in a saturated aqueous solution of picric acid at 4°C overnight. Then tissue sections were dehydrated and mounted in the resinous medium.
2.7 Immunohistochemistry staining
Immunohistochemistry staining was performed using a Streptavidin-Peroxidase Kit (Zhongshan Jinqiao Co., Beijing, China) as described previously (Lu et al., 2015). Liver tissue sections were incubated with primary antibodies at 4°C overnight including antibodies against mouse MFGE8 (mMFGE8; 1:200 dilution; sc33546; Santa Cruz Biotechnology, Dallas, TX) and human MFGE8 (hMFGE8; 1:200 dilution; sc8029; Santa Cruz Biotechnology). A score of immunohistochemistry staining was calculated as staining intensity multiply percentage of positive staining (0–100%). Staining intensity was graded as 0 (no staining), 100 (weak), 200 (moderate), and 300 (strong).
2.8 ELISA assay
The concentration of serum MFGE8 of mice and patients was measured using ELISA Kit (mouse MFGE8 ELISA, ELM-MFGE8; human MFGE8 ELISA, ELH-MFGE8, RayBiotech) according to the manufacturer's instructions.
2.9 Alanine aminotransferase and aspartate aminotransferase
Serum alanine aminotransferase (ALT; C009-2, Nanjing JianCheng Co., Nanjing, China) and aspartate aminotransferase (AST; C010-2) assay kits were purchased from Nanjing JianCheng Bioengineering Institute. The activity of ALT and AST were measured according to the manufacturer's instructions.
2.10 Cell lines and primary hepatocytes
The Huh-7 cells were purchased from the JCRB Cell Bank (Tokyo, Japan) and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Waltham, MA) containing 10% fetal bovine serum, 100 U/ml of penicillin, and 100 mg/ml of streptomycin (Gibco). Isolation of mouse primary hepatocytes was performed by tissue perfusion and percoll gradient centrifugation as described previously (J. Wu et al., 2011). Mouse primary hepatocytes were cultured in William's E medium (A1217601; Gibco) as well as primary hepatocyte maintenance supplements (CM4000; Gibco).
2.11 MFGE8 gene silencing
All small interfering RNAs (siRNAs) were designed and synthesized by GenePharma Technology (Shanghai, China). The Huh-7 cells were transfected with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) for 48 hr by silencer-negative control siRNA (snc-RNA) and two siRNAs targeting MFGE8, separately. The siRNAs sequences were listed in Table S4.
2.12 Cell viability
Cell Counting Kit-8 (CCK8; Engreen Biosystem, Auckland, New Zealand) was applied to determine cell viability. Primary hepatocytes were treated with various doses of CCl4 for 2 hr, and incubated with 10% CCK8 reagent for 2 hr. Cell viability of primary hepatocytes was then calculated by absorbance value detected at 510 nm.
2.13 TUNEL assay
The apoptosis of hepatocytes in liver tissue sections was detected by Fluorescein FragEL DNA Fragmentation Detection Kit (QIA39-1EA; Merck Millipore, Darmstadt, Germany). Eight randomized sights (magnification: ×200) of five mouse liver tissues were captured, and ratios of positive cell versus total cell counts were analyzed.
2.14 Ki-67 staining
Ki-67 staining was performed using a Streptavidin-Peroxidase Kit as described previously (Lu et al., 2015). Liver tissue sections were incubated with primary antibodies ki-67 (1:100 dilution; ab16667; Abcam, Cambridge, UK) at 4°C overnight. Positive staining was indicated as a dark brown signal of nucleus rather than nonspecific staining of cytoplasm. Eight randomized sights (magnification: ×200) were captured and mean counts of positive cells were analyzed.
2.15 Statistical analysis
All data were analyzed by GraphPad Prism 5.0 (La Jolla, CA) and presented as mean with standard errors (SEM). Statistical analysis was performed by unpaired two-tailed t test, one-way ANOVA followed by Dunnett's Multiple Comparison Test or Bonferroni's Multiple Comparison Test. Correlations were analyzed by Pearson's correlation test. The p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Identification of elevated cytokines in the process of hepatitis-fibrosis-HCC
To figure out the critical cytokine during the process of hepatitis-fibrosis-HCC, Custom G-Series Mouse Cytokine Antibody Arrays (RayBiotech) were designed by referring to references. Among 40 cytokines, we took the priority to the cytokine with an increased tendency in both fibrosis and HCC models and picked out 4 promising cytokines (Figure 1a,b). The real-time PCR analysis of liver tissues showed that TGFβ1 was increased in hepatic fibrosis model and prostasin increased in HCC model, while MFGE8 was increased in both models (Figure 1c). Therefore, we chose MFGE8 as the candidate that may orchestrate the hepatitis-fibrosis-HCC progression.
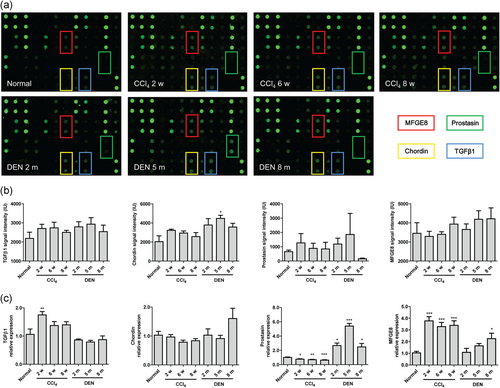
Screen of the pivotal cytokine in hepatitis-fibrosis-HCC progression. (a) Representative images of cytokine arrays. (b) Signal intensity of cytokine arrays (n = 5). (c) The mRNA expression of four potential cytokine expressions in liver tissues by real-time PCR (n = 5). Data were presented as mean ± SEM. Fibrosis model and HCC model was analyzed versus normal group using one-way ANOVA followed by Dunnett's Multiple Comparison Test. *p < 0.05, **p < 0.01, ***p < 0.001. HCC: hepatocellular carcinoma; mRNA: messenger RNA; PCR: polymerase chain reaction; SEM: standard error of mean [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Elevated expression of MFGE8 in acute and chronic CCl4-induced mouse models
First, we confirmed the establishment of acute and chronic CCl4-induced mouse models by haematoxylin & eosin (H&E) and Sirius red staining (Figure 2a,b). Liver injury was most serious at Day 1 and gradually attenuated along with chronic inflammatory and regeneration. No obvious collagen deposition was observed in acute liver injury, while collagen deposition was increased with prolongation of exposure time since Week 2. The expression of MFGE8 increased significantly in both acute and chronic CCl4-induced mouse models, mainly expressed in hepatocytes of the injured areas (Figure 2b). Increased MFGE8 expression was also validated by Western blot analysis and real-time PCR in total liver tissues (Figure 2c,d). Serum MFGE8 expression jumped in acute liver injury and climbed in chronic fibrosis progress (Figure 2e). Intriguingly, serum ALT and AST showed similar trends with serum MFGE8 expression (Figure 2f). Pearson's correlation test indicated strong correlations between MFGE8 expression and each liver injury parameter (Figure 2g).
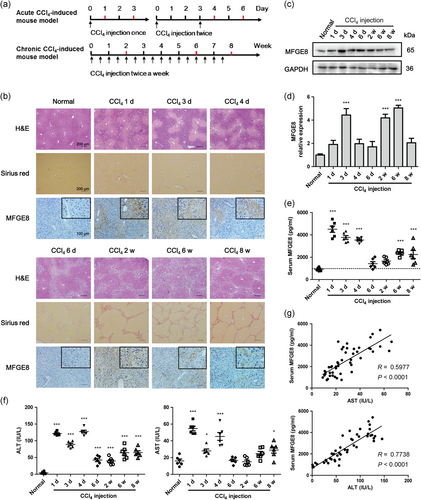
Increased expression of MFGE8 in CCl4-induced liver injury in vivo. (a) Study design of acute and chronic CCl4-induced mouse models. The sera and liver tissues were collected at the indicated time highlighted by red color. (b) H&E staining, Sirius red staining and immunohistochemistry staining of acute and chronic CCl4-induced mouse models. (c) Protein expressions of MFGE8 in liver tissues were analyzed by western blot. (d) The mRNA expressions of MFGE8 in liver tissues were analyzed by real-time PCR (n = 6). GAPDH was used as the internal control gene. (e) Serum MFGE8 expression was determined by ELISA (n = 6). (f) Liver injury was assessed by serum ALT and AST (n = 6). (g) Correlation of liver injury parameters with serum MFGE8 expression (Correlations were analyzed by Pearson's correlation test). Data were presented as mean ± SEM. *p < 0.05, ***p < 0.001 versus normal group using one-way ANOVA followed by Dunnett's Multiple Comparison Test. ALT: alanine aminotransferase; AST: aspartate aminotransferase; CCl4: carbon tetrachloride; GADPH: glyceraldehyde 3-phosphate dehydrogenase; mRNA: messenger RNA; PCR: polymerase chain reaction; SEM: standard error of mean [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Elevated expression of MFGE8 in primary mouse hepatocytes
To identify the secretion of MFGE8 in CCl4-induced hepatocyte injury, we isolated the primary mouse hepatocytes and treated them with different concentration of CCl4. As shown in Figure 3a,b, the viability of primary mouse hepatocytes was preserved with the stimulation of CCl4 at concentrations of 400 and 800 μM, while the hepatocyte injury parameter was elevated. The expression of MFGE8 in the supernatant was accumulated with prolongation of culture time in vitro, and CCl4 treatment further increased the secretion of MFGE8 as determined by ELISA (Figure 3c). The increased MFGE8 expression was confirmed using Western blot analysis and real-time PCR analyses (Figure 3d,e).
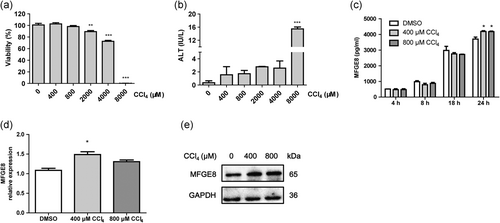
Increased expression of MFGE8 in CCl4-induced liver injury in vitro. (a) Cell viability of primary mouse hepatocytes induced by different concentrations of CCl4. (b) ALT activity of primary mouse hepatocytes induced by different concentrations of CCl4. (c) Elevated MFGE8 expression in the supernatant of CCl4-treated primary mouse hepatocytes was determined by ELISA. The mRNA (d) and protein expression (e) of MFGE8 in primary mouse hepatocytes treated with CCl4 for 24 hr. Data were presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 versus normal group using one-way ANOVA followed by Dunnett's Multiple Comparison Test. ALT: alanine aminotransferase; CCl4: carbon tetrachloride; mRNA: messenger RNA; SEM: standard error of mean
3.4 MFGE8 attenuated apoptosis in CCl4-induced primary mouse hepatocytes
Knockdown of MFGE8 expression by siRNA in huh-7 cells increased the expression of Bax and decreased the expression of Bcl-2, which indicated the antiapoptosis role of MFGE8 (Figure 4a,b). Isolated primary hepatocytes were then induced by CCl4 in the presence of rmMFGE8 pretreatment. After CCl4 treatment, Bcl-2 expression was reduced and Bax expression was increased, while rmMFGE8 pretreatment reversed the tendency (Figure 4c).
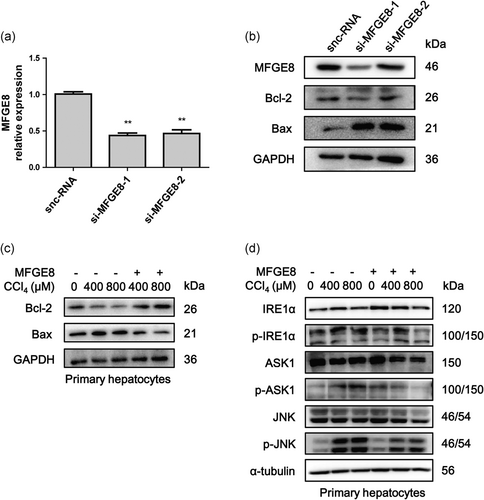
MFGE8 reduced apoptosis of hepatocytes in vitro. (a) The expression of MFGE8 in Huh-7 cells treated by siRNA was determined by real-time PCR. (b) Protein levels of MFGE8, Bcl-2, and Bax were determined by Western blot analysis. (c) Primary mouse hepatocytes were pretreated with 500 ng/ml rmMFGE8 for 4 hr and then treated with CCl4 for 2 hr. Protein levels of Bcl-2 and Bax were determined by Western blot analysis. (d) Protein levels of IRE1α, p-IRE1α, ASK1, p-ASK1, JNK, and p-JNK in primary mouse hepatocytes induced by CCl4 with or without rmMFGE8 pretreatment. Data were presented as mean ± SEM. **p < 0.01 versus control group by unpaired two-tailed t test. ASK1: apoptosis signal-regulating kinase 1; CCl4: carbon tetrachloride; IRE1α: inositol requiring kinase enzyme 1 α; JNK: c-Jun N-terminal kinase; PCR: polymerase chain reaction; SEM: standard error of mean; siRNA: small interfering RNA
3.5 MFGE8 pretreatment reduced apoptosis in CCl4-induced acute mouse model
We next evaluated the antiapoptosis role of MFGE8 pretreatment in CCl4-induced acute mouse model (Figure 5a). TUNEL analysis showed that liver apoptosis occurred mainly at 24 hr after CCl4 injection (Figure 5b), while rarely occurred at 72 hr (data not shown). Exogenous rmMFGE8 pretreatment significantly reduced the apoptotic cell counts in liver tissues of CCl4-induced acute mouse model (Figure 5b,c). Caspase-3 and Bax expressions were also decreased by rmMFGE8 pretreatment (Figure 5d).
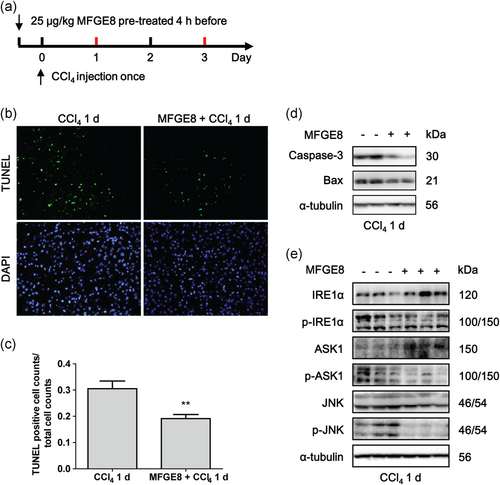
MFGE8 pretreatment decreased apoptosis of hepatocytes in vivo. (a) Study design of CCl4-induced acute mouse model pretreated with 25 μg/kg rmMFGE8 protein 4 hr before. (b) Representative images of TUNEL staining of liver tissues induced by CCl4 with or without rmMFGE8 pretreatment. (c) Quantification of TUNEL staining (mean counts of eight different fields from five different mice). (d) Protein levels of caspase-3 and Bax in mouse liver tissues induced by CCl4 for 1 day with or without rmMFGE8 pretreatment. (e) Protein levels of IRE1α, p-IRE1α, ASK1, p-ASK1, JNK, and p-JNK in mouse liver tissues. Data were presented as mean ± SEM. **p < 0.01 versus CCl4 group by unpaired two-tailed t test. ASK1: apoptosis signal-regulating kinase 1; CCl4: carbon tetrachloride; IRE1α: inositol requiring kinase enzyme 1 α; JNK: c-Jun N-terminal kinase; SEM: standard error of mean; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling [Color figure can be viewed at wileyonlinelibrary.com]
3.6 MFGE8 pretreatment reduced apoptosis through inhibiting IRE1α/ASK1/JNK pathway
We next detected whether the antiapoptosis role of MFGE8 performed through inhibiting the activation of IRE1α/ASK1/JNK proapoptotic pathway. As shown in primary hepatocytes induced by CCl4 in the presence of rmMFGE8 pretreatment, IRE1α/ASK1/JNK pathway was activated after CCl4 treatment, while rmMFGE8 pretreatment inhibited the activation of this proapoptotic pathway (Figure 4d). Moreover, rmMFGE8 pretreatment in CCl4-induced acute mouse model indicated that total IRE1α and ASK1 were upregulated after MFGE8 pretreatment, while phosphorylated IRE1α, ASK1, and JNK were downregulated (Figure 5e). Both of results in vivo and in vitro suggested that MFGE8 pretreatment reduced apoptosis through inhibiting IRE1α/ASK1/JNK proapoptotic pathway.
3.7 MFGE8 pretreatment promoted proliferation in CCl4-induced acute mouse model
Positive staining of ki-67 in hepatocyte nuclei (red arrow) indicated the proliferation of hepatocytes after CCl4 injection, while the rest of brown area was the nonspecific staining of necrotic tissues (Figure 6a). With the prolongation of CCl4 exposure, the increased proliferation of hepatocytes in the nonnecrotic area represented the repair of the natural process against exogenous insults. When rmMFGE8 pretreatment was applied, the ki-67 positive hepatocytes were rarely increased at Day 1 and dramatically increased at Day 3 after CCl4 injection (Figure 6a,b). Phosphorylation of ERK and AKT was slightly increased by MFGE8 pretreatment at Day 1 and dramatically increased at Day 3 (Figure 6c), which was in accordance with ki-67 staining.
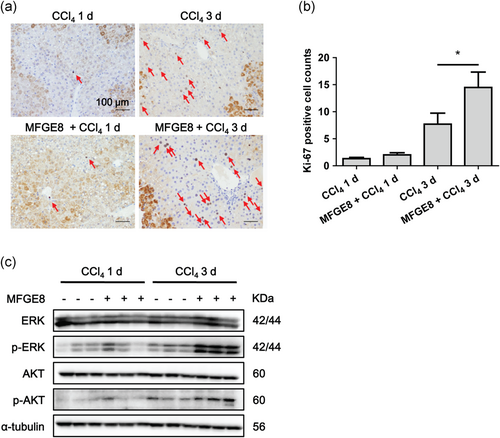
MFGE8 pretreatment promoted proliferation of hepatocytes. (a) Representative images of ki-67 staining of liver tissues induced by CCl4 with or without rmMFGE8 pretreatment. The red arrow indicated the proliferation of hepatocytes. (b) Quantification of ki-67 staining (mean counts of eight different fields from five different mice). (c) Protein levels of ERK, p-ERK, AKT, and p-AKT in mice liver tissues induced by CCl4 with or without rmMFGE8 pretreatment. Data were presented as mean ± SEM. *p < 0.05 versus CCl4 group using one-way ANOVA followed by Bonferroni's Multiple Comparison Test. CCl4: carbon tetrachloride; SEM: standard error of mean [Color figure can be viewed at wileyonlinelibrary.com]
3.8 MFGE8 expression in hepatitis and liver cirrhosis patients
To further clarify the clinical significance of MFGE8 in liver, we examined the MFGE8 expression in both liver tissues and serum samples of patients with hepatitis and liver cirrhosis. Immunohistochemical staining validated that MFGE8 was expressed in both cytoplasm and cell membrane of hepatocytes (Figure 7a). The expression of MFGE8 was slightly decreased in patients with hepatitis and liver cirrhosis (Figure 7b). Intriguingly, serum MFGE8 was increased in patients with hepatitis while decreased in patients with liver cirrhosis (Figure 7c).
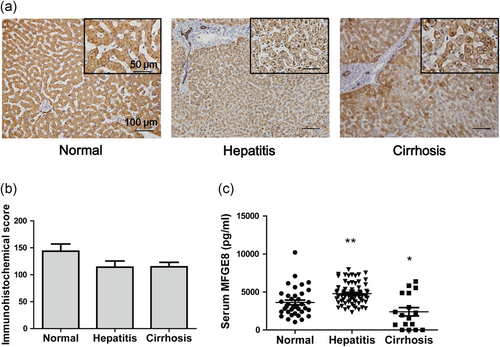
Expression of MFGE8 in hepatitis and liver cirrhosis patients. (a) Representative images of immunohistochemistry staining of MFGE8 in hepatitis and liver cirrhosis patients. (b) Quantification of immunohistochemistry staining (15 healthy donors, 24 HBV hepatitis patients, and 32 liver cirrhosis patients). (c) Serum MFGE8 expression of hepatitis and liver cirrhosis patients was detected by ELISA assays (37 healthy donors, 67 HBV hepatitis patients, and 17 liver cirrhosis patients). Data were presented as mean ± SEM. *p < 0.05, **p < 0.01 versus normal group using one-way ANOVA followed by Dunnett's Multiple Comparison Test. HBV: hepatitis B virus; SEM: standard error of mean [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Hepatitis, hepatic fibrosis, and HCC were recognized as different stages of the same disease, namely chronic liver diseases along with chronic liver injury and repair. To clarify the critical cytokine in hepatitis-fibrosis-HCC progression in a mouse model, serum cytokine arrays were designed as a semi-quantitative high-through method to give us some hints to carry out further studies. Based on the increased tendency in serum cytokine arrays and increased expression in real-time PCR analysis, we supposed that MFGE8 might play a pivotal role in hepatitis-fibrosis-HCC progression. The previous study reported that MFGE8 secreted by mesenchymal stem cells could attenuate liver fibrosis (An et al., 2017). Then, we focused on the role of MFGE8 in acute and chronic liver injury induced by CCl4. We confirmed the increased expression of MFGE8 in acute and chronic liver injury mouse models and identified the elevated secretion of MFGE8 in the supernatant of primary hepatocytes. Moreover, a strong correlation between serum MFGE8 and transaminases indicated MFGE8 may be a novel biomarker in liver injury.
In our study, we observed that the expression pattern of MFGE8 was different between human and mouse. The expression of MFGE8 in normal mouse liver tissues was relatively low and elevated after CCl4 treatment, while MFGE8 expression was relatively high in healthy liver tissues. We assumed that human healthy liver tissues were not the real state of normal condition, as the liver was exposed to various insults all the time including food-derived toxins, alcohol consumption, drugs etc.
Our results indicated that physiological concentration of serum MFGE8 is about 1–5 ng/ml in both human and mouse. In acute CCl4-induced mouse models, the most serious liver injury appeared at Day 1, followed by an acute liver repairing process. The reduced apoptosis and increased proliferation of hepatocytes at Day 3 represent the natural process of liver injury and repair against exogenous insults. Meanwhile, MFGE8 expression in liver tissues gradually climbed (peak at Day 3) and decreased later, which suggested MFGE8 participated in the repairing process of acute liver injury. Although MFGE8 expression is elevated under a pathological condition, the protective effect of MFGE8 seems to be far from enough. When exogenous MFGE8 pretreatment was applied, the apoptosis of hepatocytes was further reduced in the early stage (Day 1), and the proliferation was further promoted in the late stage (Day 3).
MFGE8 indicated the antiapoptosis role in various acute injury models including ischemia-reperfusion damages, brain injury, DSS-induced colitis (Liu et al., 2015; Matsuda et al., 2013; Matsuda et al., 2011; R. Wu et al., 2012; Y. Zhang et al., 2015). However, the underlying mechanism is poorly investigated. Previous studies reported that CCl4-induced hepatocyte apoptosis through ER stress pathway in vivo and in vitro (Lee et al., 2017; Lee et al., 2016; F. Li et al., 2017; San-Miguel et al., 2015; D. G. Zhang et al., 2017), while there is no study concerning the role of MFGE8 in regulating ER stress pathway. Here, we first reported that MFGE8 reduced apoptosis in CCl4-induced hepatocytes injury by inhibiting the activation of IRE1α/ASK1/JNK pathway. Detailed mechanism of MFGE8 regulating IRE1α proapoptotic pathway will be further investigated.
MFGE8 increased the phosphorylation of ERK and AKT in various diseases, thus triggering various downstream reactions. MFGE8 increased phosphorylation of monocyte chemoattractant protein-1 (MCP-1) and ERK1/2 in the early arterial damages of diabetes, which promoted the proliferation of vascular smooth cells and aortic remodeling (Yu et al., 2012). MFGE8 promoted the phosphorylation of p38 and ERK in dHL-60 cells and inhibited neutrophil migration in αvβ3-integrin-dependent way (Aziz et al., 2015). Similarly, we also observed the increased proliferation of hepatocytes as well as increased phosphorylation of ERK in MFGE8 pretreatment group. The role of MFGE8 in regulating AKT is controversial in different diseases. The effect of MFGE8 on AKT signaling pathway was involved in promoting epithelial-to-mesenchymal, migration, invasion, and survival of colorectal cancer cells and melanoma cells (Jinushi et al., 2008; Zhao et al., 2017). In addition, MFGE8 was reported to coordinate fatty acid uptake through phosphorylation of AKT in αvβ3/αvβ5 integrin-dependent way (Khalifeh-Soltani et al., 2014). On the other hand, MFGE8 was reported to inhibit AKT pathway to ameliorate cardiac hypertrophy (Deng et al., 2017). In our study, we observed that MFGE8 promoted the proliferation of hepatocytes by phosphorylation of AKT.
In conclusion, our findings indicated the protective role of MFGE8 in acute liver injury in two aspects. In the early stage, elevated MFGE8 expression, derived from damaged hepatocytes, reduced apoptosis of hepatocytes through inhibiting IRE1α/ASK1/JNK proapoptotic pathway. JNK performs some proapoptotic effects, including activation of proapoptotic protein Bim and inactivation of antiapoptotic protein Bcl-2, thus inducing Bax-dependent apoptosis (Lei & Davis, 2003; Yamamoto, Ichijo, & Korsmeyer, 1999). In the late stage, elevated MFGE8 promoted proliferation of hepatocytes by phosphorylation of AKT and ERK. Both sides worked together to fight against CCl4-induced liver injury (Figure 8).
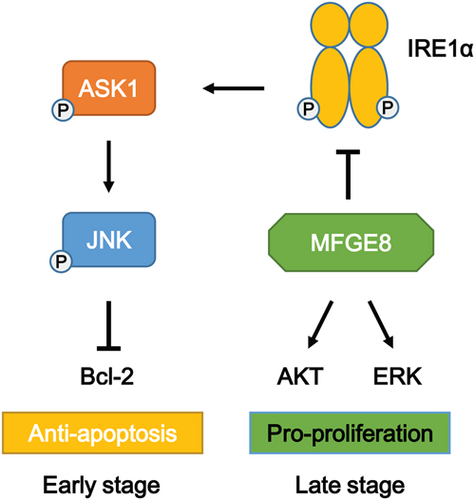
Protective role of MFGE8 in acute liver injury by regulation of apoptosis and proliferation. In the early stage, MFGE8 reduced apoptosis of hepatocytes through inhibiting IRE1α/ASK1/JNK proapoptotic pathway. In the late stage, MFGE8 promoted proliferation of hepatocytes by phosphorylation of AKT and ERK [Color figure can be viewed at wileyonlinelibrary.com]
In the clinical settings, our data indicated that serum MFGE8 expression of HBV hepatitis patients was higher than healthy donors. Increased serum MFGE8 in hepatitis represents the balance between liver injury and repair in the early stage. With prolongation of chronic liver injury, the expression of MFGE8 is decreased and the protective role of MFGE8 fails to maintain homeostasis, eventually promoting the progression of chronic liver diseases. Decreased expression of MFGE8 was also reported in liver tissues of liver cirrhosis patients, and MFGE8 derived from mesenchymal stem cells attenuated hepatic fibrosis by inhibiting TGF-β signaling in αvβ3-integrin-dependent way (An et al., 2017). The therapeutic role of MFGE8 in liver injury and fibrosis makes it promising to develop a novel therapy to attenuate liver injury and hinder the progression of chronic liver diseases including cirrhosis and HCC.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (31571434) and National Basic Research Program of China (2015CB553701).
CONFLICT OF INTEREST
The authors declare that there are no conflict of interest.
AUTHOR CONTRIBUTIONS
The authors contributed in the following ways: H. L., Z.-N. C., and H. J. B. conceived the study. H. L., T. Z., and K. W. designed and performed the experiments. H. L., T. Z., K. W., M. L., Y.-H. G., Y. Z. acquired and analyzed the data. H. L. and T. Z. drafted the original manuscript. H. L., T. Z., Z.-N. C., and H. J. B. revised the manuscript. Z.-N. C. and H. J. B. were involved in acquiring funding and supervised the study. All authors have reviewed and approved the final manuscript.