Advanced strategies for combating bacterial biofilms
Abstract
Biofilms are communities of microorganisms that are formed on and attached to living or nonliving surfaces and are surrounded by an extracellular polymeric material. Biofilm formation enjoys several advantages over the pathogens in the colonization process of medical devices and patients' organs. Unlike planktonic cells, biofilms have high intrinsic resistance to antibiotics and sanitizers, and overcoming them is a significant problematic challenge in the medical and food industries. There are no approved treatments to specifically target biofilms. Thus, it is required to study and present innovative and effective methods to combat a bacterial biofilm. In this review, several strategies have been discussed for combating bacterial biofilms to improve healthcare, food safety, and industrial process.
1 INTRODUCTION
Bacteria are the cause of evolution of several strategies to deal with problems and challenges existing under hostile environments (Shariati, Azimi et al., 2018). Such challenges may include those triggered by the presence of a host immunity, an antimicrobial agent, and nutrient limitations. One of the significant survival strategies used by bacteria is a biofilm formation (Sharma, Vipra, & Channabasappa, 2018). A biofilm is a multimicrobial community enclosed in a self-produced polymeric matrix, attached to biotic or abiotic surfaces embedded, it can be produced by almost all bacteria under a suitable condition. Reversible and irreversible stages and many conserved and/or species-specific factors are involved in bacterial aggregation and biofilm maturation. In the first step, the microbe is reversibly attached to a surface through a weak interaction (such as the van der Waals forces) with an abiotic or biotic surface. In the second step, irreversible attachment is mediated by flagella, pili, and other surface appendages or particular receptors (Sadekuzzaman, Yang, Mizan, & Ha, 2015). The multilayered cells are accumulated by subdivision, then, they start production of their self-produced extracellular polymeric substances (EPS) matrix, mainly composed of polysaccharides, proteins, and extracellular DNA, which facilitate the maturation of the biofilm (Elbarasi, 2014). A mature biofilm contains water channels, through which nutrients and signaling molecules are effectively distributed within the biofilm. Biofilm cells are detached individually or in clumps because of intrinsic or extrinsic factors; finally, the cells are disseminated, and colonization of other niches is reached (Figure 1; Sadekuzzaman, Yang et al., 2015). Biofilms are ubiquitous, meaning that they can be found in various environments and medical devices relating to public health and in industrial water systems, waste water channels, bathrooms, and hospital labs (Donlan & Costerton, 2002; Miquel, Lagrafeuille, Souweine, & Forestier, 2016). They can be either beneficial or detrimental to humans; in addition, the intestinal microbiota as a bacterial biofilm has a protective and functional role (Azimi, Nasiri, Chirani, Pouriran, & Dabiri, 2018; Shariati, Fallah et al., 2018). Biofilms are usually involved in various microbial infections in the body, and it is estimated that biofilm-related organisms give rise to more than 60% of all microbial infections in humans. Infectious processes with implicated biofilms include common problems such as bacterial vaginitis, urinary tract infections, catheter-related infections, otitis media, dental plaque, gingivitis, coating contact lenses, and cystic fibrosis (Costerton, Stewart, & Greenberg, 1999; Lebeaux, Ghigo, & Beloin, 2014, Parsek & Singh, 2003). They enjoy many characteristics that are conducive to their resistance to antibacterial and immunity, including induction of a persistent state, low metabolic activity, delayed penetration into the biofilm matrix, and expression of specific proteins. A biofilm also facilitates gene transfer among bacteria, leading to an increase in the recombination rate in the strains. In most cases, biofilm-related infections are not responsive to conventional antimicrobials and they persistently reoccur. Antibiofilm agents are of a kind that selectively eradicate the persistent biofilms, thereby facilitating the diffusion of antibiotics into a complex biofilm environment. With respect to the lack of effectiveness and safety of current strategies, the concerns with ways to cope with the biofilm formation are still persisting and have led the search, development, and application of novel approaches to inhibit the formation of biofilms (Srinivasan, Harrington, Xagoraraki, & Goel, 2008). In this review, we have tried to provide a comprehensive picture of current knowledge about antibiofilm agents of different sources. Moreover, several strategies for preventing and inhibiting bacterial biofilms are discussed briefly. In addition, several prospective research lines are proposed to prevent and control biofilms.
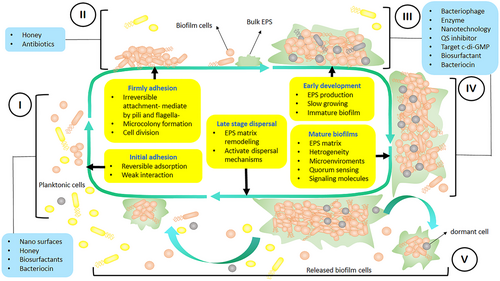
Biofilm formation is an advanced process that consists of 5 distinct stages: (I) initial adhesion, in which microorganisms adhere to surfaces; (II) growth and cell division stage, where microorganisms produce flagella and pili; (III) extracellular polymeric substances (EPS) production; (IV) mature biofilm stage, where microorganisms coexist within polymicrobial interactions and quorum sensing (QS) ensues; (V) dispersal stage, where the cells are separated from the aggregate biofilm and return to planktonic state of the cell. The initial phases of biofilm formation can be disrupted by preventing the attachment of microorganisms. The next steps can be controlled by manipulating the production of exopolysaccharide and signaling molecules [Color figure can be viewed at wileyonlinelibrary.com]
2 ANTIBIOFILM STRATEGIES
2.1 Quorum sensing inhibition
Quorum sensing (QS) is a cell-to-cell communication by bacteria so that individual and group behaviors can be coordinated; in addition, it depends on the accumulation, release, and coordinated detection of extracellular signal molecules, named autoinducers (AIs). By regulating gene expression, AIs drive various processes, including bioluminescence, sporulation, virulence-factor secretion, and biofilm maturation (Rutherford & Bassler, 2012, Verma & Miyashiro, 2013). Resistance of biofilms to antimicrobial agents is attained mainly by a cell-to-cell communication (QS) process. Thus, prevention of the cell-to-cell communication can be a promising approach to inhibit biofilm formation (Lazar, 2011). Several ways are available to disrupt the QS mechanism such as inhibition and direct degradation of signal molecules and mimicking the signal molecules for the inhibition of binding them to their receptor by using synthetic compounds as analogues of signal molecules (Kalia, 2013). There are three types of quorum-quenching enzymes that possess an ability to degrade QS signals: acetyl homoserine lactone (AHL) acylase, AHL lactonases, and oxidoreductases (Dong, Xu, Li, & Zhang, 2000). The results of a published study revealed that AHL-lactonase from endophytic strain of Bacillus cereus VT96 effectively interfered with the production of AHL, thus inhibiting the formation of biofilms (Rajesh & Rai, 2016). On the other hand, coral-associated bacteria (CAB) have the potential to produce bioactive agents with anti-QS and antibiofilm properties. Findings of studies indicated that two CAB isolates, CABs 23 and 41, have been shown to inhibit biofilm formation and the production of QS-dependent virulence factors such as protease, lipase, prodigiosin, and swarming motility in Serratia marcescens (Bakkiyaraj, Sivasankar, & Pandian, 2012). Moreover, quorum sensing inhibitors increase bacterial biofilms' susceptibility to antibiotics (Brackman, Cos, Maes, Nelis, & Coenye, 2011). Some of the oils and plant extracts extracted from some traditional medicinal plants can produce QS-inhibiting compounds that subsequently inhibit biofilms (Mukherji & Prabhune, 2014). For instance, according to Packiavathy , Priya, Pandian, and Ravi (2014), bacterial pathogens' susceptibility to conventional antibiotics as well as the QS-dependent factors, such as exopolysaccharide production, alginate production, and swarming motility of uropathogens, were reported to be enhanced and attenuated, respectively (Packiavathy, Priya, Pandian, & Ravi, 2014). Moreover, in other studies, it is indicated that quercetin can serve as a novel QS-based antibacterial and antibiofilm drug to inhibit food-borne bacteria. clary sage, juniper, lemon, and marjoram essential oils along with their main components restrict biofilm formation and AHL production and result in biofilm reduction in B. cereus. Therefore, the inhibition of QS processes may constitute a suitable strategy for inhibiting biofilm formation (Muras, Mayer et al., 2018). One of the most important QS inhibitors is lactonase. Lactonase is a metalloenzyme produced by certain species of bacteria that target and inactivate AHLs (Fuqua, Winans, & Greenberg, 1996). Results of a recent study showed that more than 70% of biofilm formation in Pseudomonas aeruginosa PAO1 was inhibited by purified AHL-lactonase characterized by a molecular weight of about 30 kDa. Moreover, combined effects of lactonase and the gold and Au nanoparticles have promising anti-biofilm activity. Therefore, it was reported that, in comparison to treatment with AiiA protein alone, treatment with N-acylated homoserine lactonase (AiiA) protein-coated gold nanoparticles (AuNPs) showed the maximum reduction in exopolysaccharide production, metabolic activities, cell surface hydrophobicity, and potent antibiofilm activity against multidrug-resistant Proteus species (Vinoj, Pati, Sonawane, & Vaseeharan, 2015).
2.2 Targeting the cyclic di-GMP pathway
Signaling molecules of bacteria can help regulate different phenotypes. The regulation of cellular functions by Cyclic di-guanosine monophosphate (c-di-GMP) occurs at multiple levels, including (a) allosteric regulation of an enzyme activity or protein function, (b) regulation of gene expression through modulation of a transcription factor, and (c) regulation of gene expression by direct interaction with noncoding RNA molecules. The molecular bricks, by which c-di-GMP builds these regulatory connections, are constituted by an array of different c-di-GMP-binding receptors or c-di-GMP effector molecules (Ryan, Fouhy et al., 2006). C-di-GMP has emerged as a ubiquitous second messenger and key cytoplasmic signal that controls motility, cell cycle propagation, and virulence as well as other behaviors, including biofilm life cycle in a number of bacterial species. C-di-GMP (e.g., P. aeruginosa biofilms have a 75–110 pmol of c-di-GMP per mg of total cell extract, whereas planktonic cells comprise less than 30 pmol mg−1) moderates biofilm determinants ranging from flagella rotation to Type IV pili retraction, exopolysaccharide production, surface adhesin expression, antimicrobial resistance and other stress responses, secondary metabolite production, and biofilm dispersal (Basu Roy & Sauer, 2014). It appears that cells use c-di-GMP as a checkpoint to go through the diverse stages of biofilm development until they entirely commit to the biofilm lifestyle, although they may still be given the choice to change their decision at any time (Römling, Galperin, & Gomelsky, 2013). In many bacteria, C-di-GMP is synthesized by diguanylate cyclase (DGC) and degraded by phosphodiesterase (PDE) enzymes (Ha & O'Toole, 2015, Römling et al., 2013). Targeting the c-di-GMP pathway is a novel strategy for treating biofilm-associated infections (Opoku-Temeng & Sintim, 2017). Pu, Yang, Xia, and Jin (2017) introduced a functional protein gene PA213332, which led to degradation of c-di-GMP and effective inhibition of biofilm formation of P. aeruginosa. Moreover, results have showed that exogenous nitrate can reduce intracellular levels of the bacterial second messenger c-di-GM and inhibit Burkholderia pseudomallei biofilm formation (Mangalea, Plumley, & Borlee, 2017). LapD, which is a transmembrane receptor for intracellular c-di-GMP, binds c-di-GMP using its cytoplasmic EAL domain and manages biofilm formation through a periplasmic output domain (Navarro, Newell et al., 2011). In case of Pseudomonas fluorescens, biofilm formation begins when an inner membrane effector protein (LapD) binds cellular c-di-GMP to ease the transport of the biofilm adhesion LapA to the cell surface. Results of other studies have shown that the presence of RapA PDE (suggesting decreased cellular c-di-GMP levels) inhibits the secretion of LapA and, therefore, puts an end on biofilm formation (Newell, Monds, & O'Toole, 2009). One of the important environmental signals is the presence of inorganic phosphate (Pi) that controls the biofilm formation by P. fluorescens Pf0–1 through a c-di-GMP-dependent mechanism. When there is a limited amount of Pi, the c-di-GMP PDE RapA is expressed and diminishes cellular c-di-GMP, preventing from biofilm formation (Monds, Newell, Gross, & O'toole, 2007). When there is no binding of c-di-GMP to LapD, LapG is free to cut the N-terminus of LapA, releasing the adhesin from the cell and suppressing biofilm formation. In the absence of Pi, biofilm detachment is facilitated through the LapD-LapG system responding to c-di-GMP depletion by RapA (Navarro, Newell et al., 2011). There exists a protein–protein interaction in Escherichia coli and YcgR protein incorporates cellular c-di- GMP levels and interrelates with components of the flagellar motor to modify motile-to-sessile alteration (Paul, Nieto, Carlquist, Blair, & Harshey, 2010; Ryjenkov, Simm, Römling, & Gomelsky, 2006). According to the results of other studies, a VieS/VieA sensor kinase/response regulator pair acts in a way to decrease the cellular c-di-GMP concentration and to prevent from biofilm formation in Vibrio cholera (Martinez-Wilson, Tamayo, Tischler, Lazinski, & Camilli, 2008). It was observed that VieA PDE negatively affected c-di-GMP when phosphorylated by VieS sensor kinase. There seen an association between the reduced cellular c-di-GMP concentration with a suppressed expression of vps (Vibrio exopolysaccharide synthesis) genes, which are responsible for EPS synthesis and biofilm formation in V. cholerae. Some other studies also reported an increase in vps gene expression and subsequent induction of biofilm formation either through overexpression of putative DGC or inactivation of VieA PDE, both of which are associated with a high cellular c-di-GMP concentration (Tischler & Camilli, 2005).
2.3 sRNAs inhibition
Small noncoding RNAs (sRNAs) also play an important role in the bacterial regulatory networks controlling the switch between planktonic mode and biofilm mode (Chambers & Sauer, 2013). The regulatory functions of sRNAs are achieved by base pairing with other RNAs or binding with proteins. After Romeo and his colleagues found the RNA-binding proteins CsrA and sRNA CsrB and revealed their roles in E. coli biofilm formation and dispersal, more sRNAs have been identified to participate in the biofilm regulatory networks in many bacteria. P. aeruginosa GAC signal transduction network is one of the best examples in this area (Suzuki, Babitzke, Kushner, & Romeo, 2006). GacS is a transmembrane sensor kinase, which induces the phosphorylation of its cognate regulator, GacA. GacA phosphorylation is also promoted by sensor kinase LadS and blocked by RetS. Phosphorylated GacA stimulates the production of two sRNAs: RsmY and RsmZ. RsmY and RsmZ have multiple binding sites for the regulatory protein RsmA. As a translational repressor, RsmA binds to the target mRNAs to promote bacterial motility and impedes the biofilm formation (Wei, Brun-Zinkernagel et al., 2001). Sequestration of RsmA by sRNAs RsmY and RsmZ switches planktonic mode to biofilm formation. The first identified global biofilm regulator is CsrA. The structure of CsrA regulatory network in E. coli is similar to the previously described RsmA system. CsrA's mRNA binding ability is inhibited by two sRNAs, CsrB, and CsrC. In the absence of CsrB and CsrC sRNAs, CsrA inhibits mRNA translation and leads to increased cell motility. CsrB and CsrC sRNAs trap CsrA, enable mRNA translation, and promote biofilm formation. Another master biofilm regulator in E. coli and Salmonella typhimurium is CsgD. CsgD regulation is at transcriptional level that acts by selectively activating curli fimbriae and extracellular polysaccharides genes and repressing flagella genes (Gualdi, Tagliabue, & Landini, 2007, Wei, Brun-Zinkernagel et al., 2001).
2.4 Honey
Honey is an ancient remedy for treating infectious diseases, and van Ketel recognized the antimicrobial features of honey. Honey has several practical advantages, such as being natural, inexpensive, and nontoxic, and has no detrimental effects on the healing of tissue. Honey is produced from many different floral sources and its antimicrobial activity varies with origin and processing (Table 1). The antimicrobial activity of honey is obtained mainly from the following: (a) Hydrogen peroxide produced by honey because of the formation of hydroxyl radicals, which has been proved to induce oxidative damage, restrict bacterial growth, and cause cytotoxic degradation of DNA; (b) honey as a supersaturated solution comprising approximately 80% of sugar, predominantly fructose, and glucose; (c) A contains low-level water, whose molecules are firmly attached to the sugar; (d) honey of low pH (between 3.2 and 4.5) resulting from the presence of several different organic acids; (e) various non-peroxide factors (plant origin; Brudzynski & Lannigan, 2012, Mavric, Wittmann, Barth, & Henle, 2008, Stephens, Schlothauer et al., 2010). Honey has got some antibiofilm molecular mechanisms; however, these mechanisms are not exactly clarified. One of these mechanisms is used to prevent from the formation of biofilms and, therefore, to control the adhesive agents and genes involved in bacterial colonization. Another mechanism is applied in the inhibition of genes involved in quorum sensing (Li, Attila et al., 2007). Results of related published studies have indicated that honey and recombinant bee-derived Defensin-1 (Def-1) exert antibacterial and antibiofilm effects against biofilms formed by bacteria including Staphylococcus aureus, Streptococcus agalactiae, P. aeruginosa, and Enterococcus faecalis. Def-1 is one of the main regular but quantitatively variable antibacterial components of honey (Sojka, Valachova, Bucekova, & Majtan, 2016). Moreover, biofilm inhibition at a honey concentration of 25% was higher than what was obtained at 12.5% concentration, which can be due to its high viscosity that prevented bacterial cells from being attached to the surfaces, this is the most important step in biofilm formation (Lee, Park et al., 2011). Manuka honey has good effects on the topical treatment of wounds containing biofilm formation of Streptococcus pyogenes in the in-vitro condition; accordingly, the possibility of such good effect is achieved by decreasing the expression of proteins that act as adhesions in facilitating bacterial binding to fibronectin (Maddocks, Lopez, Rowlands, & Cooper, 2012). Auto-inducer-2 (AI-2) signaling was reported to have a role in positively controlling biofilm formation, and studies have showed that honey significantly suppresses the expression of QS signaling genes such as AI-2, lsrA, and lsrDBFG. On the other hand, glucose and fructose in honey were identified as the key components for reducing biofilm formation by E. coli O157:H7 (Truchado, Gil-Izquierdo, Tomas-Barberan, & Allende, 2009) through the repression of curli production and AI-2 import. Additionally, honey, glucose, and fructose reduced the colonization of E. coli O157:H7 cells on human HT-29 epithelial cells. According to these results, it can be said that low concentrations of honey, such as in honeyed water, can be a useful means for decreasing the colonization and virulence of pathogenic E. coli O157:H7 (Lee, Park et al., 2011). Polyphenols are one of the most significant natural products derived from several plants and are considered as an essential factor in plant resistance against various pathogens. Manuka honey of floral origin also includes plants polyphenols and several compounds such as flavonoids. As shown by the findings, such compounds have got an antioxidant, antimicrobial, and antibiofilm activity against pathogenic microorganisms (Slobodníková, Fialová, Rendeková, Kováč, & Mučaji, 2016). Honey also has Methylglyoxal (MGO), which is a significant antibacterial component. MGO, as an active ingredient and antibacterial compound of Manuka honey, plays an important role in killing biofilm-embedded wound bacteria in planktonic and biofilm form in several bacteria such as S. aureus and P. aeruginosa (Otto, 2008). Based on the transcriptome analyses, honey considerably repressed 16 LEE virulence genes (including espBDA, the intimin gene eae, and the LEE regulator gene ler), three curli genes (csgBAC), seven quorum-sensing genes (AI-2 import operon lsrA and lsrDBFG and the indole biosynthesis gene tnaA and the indole import gene mtr), and 21 TCA cycle-related genes (Lee, Park et al., 2011). Furthermore, Honey can be used in combination with antibiotics, spices, and plant extracts to increase their antibiotic effects so as to prevent from biofilm formation. Honey plus curcumin (ChC) reduces the QS-mediated production of virulence factors and biofilm formation in P. aeruginosa PAO1. New findings have indicated that ChC decrease the secretion of pyocyanin, pyoverdin, and pyochelin; on the other hand, the inhibition of rhamnolipid, alginate, swimming, and swarming motility results in a decrease in biofilm (Jadaun et al., 2015). Therefore, it can be suggested that honey alone and in combination with other materials has excellent anti-biofilm effects; in addition, because of its minor side effects, it can be a good remedy for inhibiting biofilm infections. Pediatric patients who receive chemotherapy suffer from impaired and prolonged physiologic process of wound healing. Because of intense immunosuppression, wound infection can simply spread and function as the source of sepsis. Based on the results of in vitro studies that revealed the antibacterial potency of special honey preparations against typical isolates of nosocomially acquired wound infections (including Methicillin-resistant S. aureus and Vancomycin-resistant enterococci), and with regard to the promising reports from other groups, Medihoney is now used in wound care (Simon, Sofka et al., 2006). Using honey to clear the wound infection may show more than its just antibacterial properties. In addition, honey at concentrations as low as 0.1% stimulates the proliferation of B- and T-lymphocytes in cell cultures and activates the phagocytes too (Subrahmanyam, 1998). Monocytes in cell cultures are also stimulated by honey at concentration of 1% to release cytokines, tumor necrosis factor (TNF)-alpha, interleukin (IL)-1, and IL-6, which activate immune response against infection (Bangroo, Khatri, & Chauhan, 2005, Tonks, Cooper et al., 2003). Furthermore, the glucose content of honey and the acid pH (typically between pH 3 and 4) may help macrophages in destroying the bacteria. This chemical debridement action of honey not only speeds up the wound healing but it also does not require surgical debridement under general anesthesia (Abuharfeil, Al-Oran, & Abo-Shehada, 1999; Bangroo, Khatri et al., 2005).
| Biofilm forming bacteria | Different type of Honeys | Experimental results and reference |
|---|---|---|
| Staphylococcus aureus, S. pyogenes, Clostridium difficile, P. aeruginosa, E. coli, Proteus mirabilis and Klebsiella spp | Manuka-type honeys |
|
| MRSA, P. aeruginosa and E. coli | Portuguese honeys |
|
| S. aureus, P. aeruginosa and E. coli | Sahara honeys (SHs) and Propolis-Sahara honeys (P-SHs) |
|
| P. mirabilis, P. aeruginosa, P. mirabilis and Klebsiella spp | Egyptian clover honey |
|
| S. aureus | Trigona honey |
|
| Streptococcus mutans | Natural honey (NH) and artificial honey (AH) |
|
| P. aeruginosa | Honey Phytochemicals (HP) |
|
| S. aureus | Costa Rican stingless bee honeys |
|
| P. aeruginosa | Surgihoney RO (SH1), Manuka honey (MH) and Medihoney manuka honey (Med) |
|
2.5 Bacteriophage-biofilm interactions
Bacteriophages, that is, viruses that can specifically infect bacteria, were discovered independently about 100 years ago. Bacteriophages in several bacteria included V. cholera, Yersinia pestis, and Bacillus anthracis encode different proteins that could contribute in virulence and pathogeneses (Parasion, Kwiatek, Gryko, Mizak, & Malm, 2014). In general, bacteriophages have two types of life cycles including lysogenic and lytic phases. The lytic phages could be changed to nonlytic phages using the genetic manipulation. For instance, genetically modifying the P. aeruginosa filamentous phage Pf3 to become a nonlytic, nonreplicative lethal variant (Pf3R) by replacing an export protein gene in the phage genome with the BgIII endonuclease gene. Likewise, according to the results of a study by Matsuda and colleagues converting lyticT4LyD to nonlytic phages is a rewarding strategy to decrease their immunological effects (Hagens, Habel, Von Ahsen, Von Gabain, & Bläsi, 2004). Furthermore, literature shows that changing the lytic P954 phage to a nonlytic phage in S. aureus can be modified by homologous recombination to inactivate the gene coding for the endolysin responsible for bacterial cell lysis (Pires, Cleto, Sillankorva, Azeredo, & Lu, 2016). Like all viruses, bacteriophages can proliferate only in living and susceptible bacterial cells (Matsuzaki, Rashel et al., 2005). Phages replicate within the bacterium after their genome is injected into its cytoplasm (Mc Grath & van Sinderen, 2007). Only lytic phages qualify for therapeutic purposes, because they lead to lysis of the host cell and do not integrate with the host genome (Parasion, Kwiatek et al., 2014). The phage therapy has several advantages: (a) phage separation is fast, relatively simple, and inexpensive; (b) the resistance to phage is ten times slower than resistance to antibiotics; (c) phage may remain infected in severe environmental conditions and can prolong replicating until the end of the resistance of the bacterial host. Despite their many advantages, phages also have disadvantages. One problem is the use of phages that are recognized by the human immune system as a foreign agent; finally, produced antibody and the use of phage may lead to the transmission of virulence and antibiotic resistance genes (Doss, Culbertson, Hahn, Camacho, & Barekzi, 2017). Phages are currently considered a potential alternative or adjunct to antibiotics for bacterial infections, especially for biofilm inhibition or disruption (Sadekuzzaman, Yang et al., 2015). Results of studies have indicated that phages could be successfully used to reduce food and human pathogens (Cui, Bai, Yuan, Surendhiran, & Lin, 2018). Bacteriophages play an important role in microbial communities, unaffected by antibiotic resistance (unlike many antibiotics), and are able to target bacteria within biofilms (Harper, Parracho et al., 2014). Based on the traditional view of host–phage interaction, the phages mainly cause lysis or mediate the horizontal gene transfer (HGT). However, recent results argue that bacteriophages, through inducing cell death and providing enzymes that can help break down the biofilm matrix, play important roles in biofilm development, especially in the dispersal phases. For instance, bacteriophages D1P12 and CP4-57 have important effects on cell death and dispersal in E. coli biofilms (García-Contreras, Zhang, Kim, & Wood, 2008). In addition, they have some effects on multispecies biofilms having P. fluorescens and Staphylococcus lentus, in which they can induce dispersion of the nontarget S. lentus cells (Sillankorva, Neubauer, & Azeredo, 2010). One of the best studied links between phages and cell death is provided by P. aeruginosa during dispersal. Prophage genes are intensely induced during the later stages of P. aeruginosa biofilm formation, and bacteriophages are voluntarily isolated in the biofilm effluent produced by the laboratory strain P. aeruginosa strain PAO1 and from biofilms of clinical isolates. Actually, cell death of P. aeruginosa strain PAO1 is not induced during biofilm development in the absence of the bacteriophage Pf4, and the biofilms are less stable than those formed by the wild-type strain. A change in the lysogenic to superinfective phage form affects the Pf4-driven dispersal, adding superinfective phages to biofilms of mutant cells that lack Pf4 induces the cell death. The effect of bacteriophages on dispersal of P. aeruginosa strain PA14 provides us with more evidence regarding the role of superinfection. In this strain, lysogeny prevents biofilm formation and swarming whereas removal of the CRISPR (clustered regularly interspaced short palindromic repeats) elements, as host defense systems against phages, restores biofilm formation and swarming (Sillankorva, Neubauer et al., 2010; Zegans, Wagner et al., 2009). In some cases, the particular mechanisms, by which bacteriophages mediate biofilm dispersal, are known. Some bacteriophages make active EPS-degrading enzymes that join the phage particle, probably to allow it to access cell surface receptors (Hughes, Sutherland, & Jones, 1998). Therefore, the combined effects of EPS degradation and lytic activity of the bacteriophage facilitate cell dispersal from the biofilm. In an interesting adaptation of this natural activity, when bacteriophage T7 was engineered to produce dispersing B, biofilm biomass was reduced by 4.5 orders of magnitude compared with the mass of biofilms having non-modified T7 (Lu & Collins, 2007). Besides carrying enzymes, bacteriophages may induce biofilm cell death and dispersal in other ways. In Treponema denticola biofilms, a rise in the expression of genes with homology to a toxin–antitoxin (TA) system is associated with dissolution of the biofilm and release of dispersal cells 93. In case of E. coli, the induction of genes with homology to certain TA genes involved in bacteriophage-mediated lysis also leads to biofilm cell death and dispersal. In this case, the response regulator Hha negatively regulates the expression of genes that encodes rare tRNAs, and this results in the activation of the toxin proteins in the TA pairs through the activity of proteases. Hha induction may happen due to the nutrient limitation or in the presence of uncharged tRNAs, and cellular physiology and growth status would, therefore, be closely linked to cell death via this pathway (McDougald, Rice, Barraud, Steinberg, & Kjelleberg, 2012). Phage therapy is also used to treat some human infectious diseases (e.g., dysentery, skin infections, lung infections, meningitis, wound infections, and osteomyelitis) caused by a wide range of organisms. Using such therapy for device-associated infections may decrease the use of antimicrobial agents and hence limit the spread of antimicrobial resistant organisms. For instance, phage T4 has the ability to infect and replicate within E. coli biofilms and interrupts biofilm morphology by destroying the bacterial cells. Phage-based proteins can be useful antimicrobials because they dynamically target and break down the cell wall of bacteria, resulting in bacterial cell death (Corbin, McLean, & Aron, 2001). Phages can affect the bacterial quorum quenching and direct destruction leads to biofilm control. Interference with QS, that is, quorum quenching, via enzymes that degrade AHLs has been shown to modulate biofilm formation. For instance, AHL lactonases encoded by the aiiA genes of Bacillus species have broad-range specificity for cleaving the lactone rings of diverse AHLs, resulting in prevention of formation of P. aeruginosa biofilms (Pei & Lamas-Samanamud, 2014). The findings of studies have revealed that an engineered T7 phage incorporating the AHL lactonase aiiA gene from B. anthracis degraded AHLs from diverse bacteria and caused inhibition of biofilm formation in a mixed-species biofilm composed of P. aeruginosa and E. coli. This enables the engineered quorum-quenching T7 phage to be a promising antibiofilm agent. The polysaccharide depolymerases produced by phages have the potential to destroy the biofilm EPS matrix. Endolysins are a class of peptidoglycan hydrolases that are encoded by bacteriophage. During the life cycle of a bacteriophage, endolysins can digest the bacterial cell wall aiming to release progeny bacteriophage. If applied externally to vulnerable gram-positive bacteria, endolysins bind to and cleave the cell wall, usually in a species-specific manner, leading to hypotonic lysis and bacterial death. In the past decade, recombinant endolysins, sometimes called enzybiotics, have been studied as therapeutic agents against gram-positive pathogens because of their ability to lyse in vitro and in vivo. It has also been shown that Endolysins can work on strains that display resistance to multiple antibiotics (Donlan, 2009). Narrow host range, bacterial resistance to phage, and phage-encoded virulence genes that can integrate into the host bacterial genome are some of the shortcomings of phage. Phage might be inactivated by immune system and impure phage preparations could have endotoxin. Phages are likely to change into lysogenic conditions inside the human body and can cause everlasting infection in the body. To overcome such obstacles, we can use phage mixtures or engineered phages (Pei & Lamas-Samanamud, 2014). Destruction of a biofilm by bacteriophage is done through the following methods: (a) bacteriophages replicate within their host cells, spreading through the biofilm and destroying the EPS produced by bacteria; (b) they produce several enzymes that disrupt the biofilm matrix, degrade the EPS, and increase their susceptibility to antibiotics; (c) bacteriophages can inject persister cells; even though bacteriophages are not able to replicate within and damage inactive cells, they may remain in these bacteria only to reactivate and, then, initiate a productive infection so as to destroy the cells at some point (Miquel, Lagrafeuille, Souweine, & Forestier, 2016). Moreover, the result of a published study stated that bacteriophage tubular proteins include A-TTPAgp31 and TTPAgp44 as unique enzymes with anti-biofilm activities as well as KP32 and KP34 bacteriophages that produce several depolymerizes with a very high anti-biofilm activity (Azeredo & Sutherland, 2008). One of the important challenges in hospitals and health care centers is the prevention of catheter-related infections. Catheter-associated urinary tract infections (CAUTI) are too difficult to be treated because these bacteria have the ability of biofilm formation and drug resistance. The recent investigation has shown that anti-P. mirabilis phage cocktail has antibiofilm effects and can be a good remedy for the fight against CAUTI because of the high antibiotic resistance in this bacterium phage (Maszewska, Zygmunt, Grzejdziak, & Różalski, 2018). Some bacteriophages that inhibit biofilm formation are listed in Table 2.
| Biofilm forming bacteria | Phages | Experimental results and reference |
|---|---|---|
| S. aureus | ϕ11, ϕ12, vB_SauM-LM12 (LM12), Sb-1, SATA 8505, Phage K, DRA88 and SAP-26 |
|
| Vibrio alginolyticus | VP01 and pVa-21 |
|
| S. mutans, S. aureus and Staphylococcus epidermidis | Phi-IPLA7, Phage K and Protein (Dpo7)from Phage vB_SepiS-phiIPLA7 |
|
| S. epidermidis | vB_SepiS-phiIPLA5 and vBSepiS phiIPLA7 | Virions of these phages are provided of pectin lyase-like domains, which may be regarded as promising antibiofilm tools (Gutiérrez, Martínez, Rodríguez, & García, 2012) |
| Staphylococcal species | Phage-derived peptidase, phiIPLA-RODI and phiIPLA-C1C |
|
| S. pyogenes | PlyC | PlyC directly lyses Group A streptococcus (GAS) cells within the biofilm matrix (Shen, Köller, Kreikemeyer, & Nelson, 2013) |
| E. coli | E. coli O157:H7 phage, vB_EcoM_ECOO78 (Dpo42), EC3a and Engineered T7, |
|
| E. coli and P. aeruginosa | T7 |
|
| P. aeruginosa | Phiibb-PAA2, phiIBB-PAP21 and Phage cocktail (CT-PA) |
|
| P. mirabilis | vB_PmiS-TH |
|
2.6 Nanotechnology
Over recent decades, nanotechnology has gained widespread attention in the field of medicine because of its exceptional properties derived from the nanosize effect and has proved its superiority to other traditional formulations in local topical therapy (Li, Zhang et al., 2013). Nanoparticles (Nps) have a high surface area and, therefore, have high interaction with biological targets. It has been documented that metal Nps have antimicrobial effects on bacteria, fungi, and viruses. NPs function through having a direct contact with the bacterial cell wall, without a need to penetrate the cell; this raises the hope that NPs would be less prone to promoting resistance in bacteria than antibiotics. Various NPs are often reported to have an inhibitory effect against planktonic and biofilm cells. This activity is related to ATP-associated metabolism, permeability of the outer membrane, and the generation of hydroxyl radicals that are induced by bactericidal compounds. NPs can act on the ion channels in bacterial biofilms, thereby regulating the metabolic activity of bacteria (Algburi, Comito, Kashtanov, Dicks, & Chikindas, 2017). Some Nps can be used as promising agents to effectively impede biofilm formation by foodborne pathogens (Khiralla & El-Deeb, 2015). Silver is an antimicrobial nontoxic metal, and silver nanoparticles (AgNPs) are used in many disinfectant agents to disinfect medical devices because they show antimicrobial characteristics as well as anti-biofilm effects. AgNPs show multiple antibacterial mechanisms, such as adherence and penetration into the bacterial cell wall, and cause disintegration and increased permeability of bacterial cell membrane (Afkhami, Pourhashemi, Sadegh, Salehi, & Fard, 2015). According to the results of several studies, AgNPs demonstrated the antibiofilm activity against the selected five strong biofilms that form multidrug-resistant gram-negative bacterial strains (Ramachandran & Sangeetha, 2017). Moreover, results of a published study revealed that the photocatalytic activity of titanium dioxide reduced Listeria monocytogenes biofilm formation (Chorianopoulos, Tsoukleris, Panagou, Falaras, & Nychas, 2011). On the other hand, selenium nanoparticles (SeNPs) inhibited the biofilm formation of S. aureus, P. aeruginosa, and P. mirabilis up to 42%, 34.3%, and 53.4%, respectively (Shakibaie, Forootanfar, Golkari, Mohammadi-Khorsand, & Shakibaie, 2015). Antibiofilm property of β1–3 glucan-binding protein-based silver nanoparticles (Ppβ-GBP-AgNPs) was assessed, and related findings demonstrated that it inhibited 85% and 80% of immature biofilms by E. faecalis and P. aeruginosa, respectively (Anjugam, Vaseeharan et al., 2018). The surface modification of catheters with magnesium fluoride (MgF2) NPs is effective in blocking the path toward bacterial colonization, keeping them sterile for a long time, and providing catheters with enduring self-sterilizing properties. Recent studies have demonstrated that stable colloidal bismuth nanoparticles have had 69% antimicrobial activity against S. mutans growth and completely inhibited biofilm formation (Hernandez-Delgadillo, Velasco-Arias et al., 2012). Lee, Kim, Cho, and Lee (2014) reported that effects of Zinc oxide (ZnO) nanoparticles on the control of pyocyanin production and biofilm formation required czc regulator (CzcR), and ZnO nanoparticles were potential antivirulence materials against recalcitrant P. aeruginosa infections. Nanostructuration technology of some medicinal plants shows significant results against biofilm. Therefore, results of a study showed that the activity of Melaleuca alternifolia oil or “tree of tee” oil (TTO) nanoparticles on P. aeruginosa biofilms demonstrated that the nanostructuration of TTO could be a feasible option against mature biofilm of bacteria (Comin, Lopes et al., 2016). On the other hand, green synthesized Nps (silver and gold), bimetallic (Ag/Au) Nps, and lectin-coated AgNPs reduced the biofilm formation and interfered with cell adhesion and polysaccharide matrix (Gopinath, Kumaraguru et al., 2016, Jayanthi, Shanthi et al., 2017). According to the recent studies, Crataeva nurvala -AgNPs (CN-AgNPs) efficiently suppressed the production of QS-mediated virulence factors, such as pyocyanin, protease, hemolysin, and biofilm formation in P. aeruginosa (Ali, Ansari et al., 2017). The interaction of AgNPs with the bacterial cell membrane disrupts the membrane permeability and inhibits the respiratory enzymes as well as the production of reactive oxygen species (ROS). Results of some studies have shown that cellular membranes suffer from more damage at higher production levels of ROS, leading to increased ampicillin and vancomycin uptake (Algburi, Comito et al., 2017). Finally, NPs have been used for the delivery of therapeutic agents, and nanoliposomes are one of the most important factors used to perform as the mediating delivery of antibiotics. Li et al. (2013) investigated the flexible nanoliposomes for mediating topical delivery of daptomycin and documenting permeation rates and antibiofilm activity towards skin infections. These results showed that the daptomycin-loaded flexible nanoliposomes (DAP-FL) could improve daptomycin's ability to penetrate into the skin efficiently to counteract biofilm-related infections with its powerful antibacterial action and activity (Li, Zhang et al., 2013, Martinez-Gutierrez, Boegli et al., 2013). In general, the antimicrobial potential of NPs compounds may depend on their sizes, charges, and stability to develop antibiotics and control biofilm (Algburi, Comito et al., 2017).
2.7 Enzymes
Antimicrobial enzymes are abundant in nature and play a significant role in counteracting bacterial attack. These enzymes are currently used remarkably against microbial systems and, usually, to target biofilm matrix. They possess certain abilities to directly attack the microorganism, interfere with biofilm formation, and destroy the biofilms that lead to their widespread use in the industry. Enzymes can directly attack the biofilm components, degrade them, induce cellular lysis, and interfere with the QS system, or even catalyze the formation of antimicrobials (Lequette, Boels, Clarisse, & Faille, 2010; Meireles, Borges, Giaouris, & Simões, 2016; Thallinger, Prasetyo, Nyanhongo, & Guebitz, 2013). Several studies have evaluated anti-biofilm activity of enzymes against different bacterial biofilms, this study reviewed some of the most important of these enzymes.
2.8 Lysostaphin
Lysostaphin (LS) is a Staphylococcus simulans metalloendopeptidase that can be served as an antimicrobial against S. aureus (Landini, Antoniani, Burgess, & Nijland, 2010). LS is an antibacterial enzyme that is specifically capable of cleaving the cross-linking pentaglycine bridges in the bacteria cell walls and can effectively suppress biofilm formation. This enzyme not only kills bacteria in biofilms, but also disrupts the extracellular matrix of biofilms under an in vitro condition. Moreover, results of scanning electron microscope confirmed that LS eradicated the extracellular matrix of the biofilm (Wu, Kusuma, Mond, & Kokai-Kun, 2003). On the other hand, a combination of enzymes and antibiotics may extend therapeutic options to treat biofilm-associated infections. Kokai-Kun, Chanturiya, and Mond (2009) evaluated the effect of LS separately and also in combination with nafcillin on the eradication of S. aureus biofilms in mouse jugular vein catheters. The obtained results showed that LS can be represented as an effective treatment and a prophylaxis against S. aureus biofilms on indwelling catheters. Finally, investigations have revealed that the LS-coated surfaces kill hospital strains of S. aureus in less than 15 min and prevent from biofilm formation (Yeroslavsky, Girshevitz, Foster-Frey, Donovan, & Rahimipour, 2015).
2.9 α-Amylase
Marine bacterial-derived amylase enzyme can be developed as a potential antibiofilm agent, and this enzyme exhibits excellent antibiofilm activity against the marine-derived biofilm forming bacteria including P. aeruginosa and S.aureus in the in vitro condition. Moreover, the confocal laser scanning microscopic analysis showed the inhibition of complete biofilm formation on amylase enzyme treated glass surface (Vaikundamoorthy, Rajendran, Selvaraju, Moorthy, & Perumal, 2018). However, several studies approved that α-amylase enzyme could be singly used to inhibit and disrupt the biofilms of P. aeruginosa, methicillin resistance S. aurous (MRSA), and V. cholera strains. In addition, it has the potential to fulfill many objectives in clinical applications (Kalpana, Aarthy, & Pandian, 2012). Other studies have demonstrated that α-amylase compounds could rapidly detach biofilms of S. aureus and inhibit biofilm formation from different biological sources. Furthermore, these results demonstrated that α-amylase compounds had the ability to reduce and disassociate S. aureus cell-aggregates grown in liquid suspension (Craigen, Dashiff, & Kadouri, 2011).
2.10 Deoxyribonuclease 1
Deoxyribonuclease 1 (DNase1) degrades the extracellular DNA (e-DNA) present in the matrix, rendering the matrix weak and susceptible to antimicrobials. Several findings have shown that the DNase I can reduce biofilm formation in P. aeruginosa infections. Nguyen and colleagues reported that the addition of DNase I during biofilm formation reduced attachment of P. aeruginosa to polystyrene. Extracellular polymeric matrix synthesized in biofilm formation could hold bacterial cells together and attaches them to the different abiotic and biotic surfaces. Extracellular DNA that is a constituent of the bacterial biofilm matrix has several roles including cation chelation, biocide resistance, biofilm cohesion, and genetic exchange. Several studies that utilized laboratory grade DNase I have shown that exogenously-added deoxyribonucleases can detach pre-formed biofilms, sensitize biofilm bacteria to biocide killing, and finally inhibit biofilm formation (Tetz, Artemenko, & Tetz, 2009). Moreover, other studies evaluated the antibiofilm activity of pharmaceutical grade of recombinant human DNase I (rhDNase) against S. aureus and S. epidermidis, and results indicated that biofilm formation by S. aureus was efficiently inhibited by rhDNase. According to the results of published studies, in several cases such as those associated with cystic fibrosis, chronic wounds and medical implants, rhDNase, either alone or in combination with antimicrobial agents including dispersin B, may have potential applications in developing products for the prevention and treatment of staphylococcal biofilm infections (Kaplan, LoVetri et al., 2012). Furthermore, DNase-coated nanoparticles enhanced antibiotic delivery in biofilm infections, and investigations demonstrated that DNase I activated nanoparticles not only were able to prevent from biofilm formation of P. aeruginosa, but also successfully reduced established biofilm's mass, size, and living cell density. On the other hand, the administration of DNase I-coated nanoparticles encapsulating ciprofloxacin, repeated over three days, was able to reduce up to 95% and eradicate more than 99.8% of the established biofilm (Baelo, Levato et al., 2015). On the other hand, another research reported that DNase I coating of titanium was shown to have a significant role in preventing S. mutans and S. aureus adhesion and biofilm formation (Ye, Shao et al., 2017).
2.11 Lyase
Many studies stated that treatment with the liquid crystals containing alginate lyase (AlgL) and gentamicin resulted in a greater 2-log reduction in mucoid P. aeruginosa (clinical isolate) biofilms (Thorn, Prestidge, Boyd, & Thomas, 2018). Furthermore, a combination of AlgL and other antibiotics, such as Piperacillin/Tazobactam, Tobramycin, and Clarithromycin, enhanced the disruption of Ciprofloxacin-resistant P. aeruginosa (CRPA) biofilms and other bacterial biofilms significantly (Bugli, Palmieri et al., 2016, Cho, Huang et al., 2016, Jang, Piao et al., 2016, Lamppa & Griswold, 2013).
2.12 Lactonase
Lactonase is a metalloenzyme, produced by certain species of bacteria that targets and inactivates AHLs (Fuqua, Winans et al., 1996). Results of a recent study showed that more than 70% of biofilm formation in P. aeruginosa PAO1 was inhibited by purified AHL-lactonase characterized by a molecular weight about 30 kDa. Moreover, combined effects of the lactonase and the gold and Au nanoparticles have promising anti-biofilm activity. Therefore, it was reported that treatment with N-acylated homoserine lactonase (AiiA) protein-coated gold nanoparticles (AuNPs) showed maximum reduction in exopolysaccharide production, metabolic activities, cell surface hydrophobicity, and potent anti-biofilm activity against multidrug resistant Proteus species compared to treatment with AiiA protein alone (Vinoj, Pati et al., 2015).
2.13 Papain
Papain is a complex mixture of proteolytic enzymes and peroxidases extracted from the latex of Carica papaya. It is recognized by the accelerating feature in the healing wounds. Papain effects on functional groups, such as hydroxyl, amino, carbonyl, and phosphoryl reduced biofilm formation. Several studies evaluated the ability of papain to inhibit the formation of Methicillin-resistant S. epidermidis (MRSE) and methicillin-resistant S. haemolyticus (MRSHa) biofilms; in addition, papain was able to disrupt mature biofilms made by MRSE (Oliveira, Fleming et al., 2014). On the other hand, proteases reduced oral biofilm in elderly subjects, suggesting that protease digests fimbriae and inhibits biofilm formation; thus, papain and trypsin reduce multispecies biofilm (Mugita, Nambu, Takahashi, Wang, & Komasa, 2017). Mohamed, Mohamed, Khalil, Mohamed, and Mabrouk (2018) evaluated the effect of papain enzyme on Klebsiella pneumoniae planktonic cells as well as on the formation, eradication, and cells' viability of biofilms. These results revealed that the biofilm inhibition of different Klebsiella pneumonia strains ranged from (10.6–56.2%) at a concentration of 50 mg/mL and increased to (21.4–59.0%) at 100 mg/mL of papain. Therefore, papain and other proteases, including trypsin, prevented the initial binding of bacteria to various surfaces and, also, hindered the formation of biofilms by destroying protein structures of bacteria such as Fimbria, although their role in degradation of mature biofilms was also reported.
2.14 DispersinB
The periodontal pathogen, Aggregatibacter actinomycetemcomitans (Blackledge, Worthington, & Melander, 2013, Kaplan, Ragunath, Ramasubbu, & Fine, 2003) produces Dispersin B (DspB) as a glycoside hydrolase. DspB hydrolyzes β − 1,6-N-acetyl-d-glucosamine, which is an important polymer required for biofilm attachment onto surfaces (Thallinger, Prasetyo et al., 2013). Findings of published articles revealed that the stability and anti-biofilm activity of DspB were improved through immobilization on carboxymethyl chitosan nanoparticles. Moreover, these results demonstrated that the antibiofilm activity was increased due to the dual mechanism, including the improvement of the enzyme stability and the antibiofilm activity of modified carboxymethyl chitosan nanoparticles (Tan, Ma, Liu, Yu, & Han, 2015). Moreover, DspB can prevent biofilm formation and trigger biofilm detachment in any PNAG-producing bacterial species. Exposure to DspB in the presence of antibiotics or disinfectants such as Triclosan results in synergistic biofilm removal and bacterial killing. DspB in combination with Triclosan is now marketed in gel preparations for the treatment of wound and skin infections and for disinfection of medical devices, suggesting that combinations of antimicrobials and EPS-degrading enzymes can represent a powerful tool for biofilm eradication in these settings (Donelli, Francolini et al., 2007; Eckhart, Fischer, Barken, Tolker-Nielsen, & Tschachler, 2007). The novel synthetic antimicrobial peptide, KSL-W, is a Mammalian cationic antimicrobial peptide that has a prokaryotic selectivity and decreases the risk of microbial resistance. The findings have shown that the combination of DispersinB and KSL-W peptide showed synergistic anti-biofilm and antimicrobial activity against chronic wound infection associated with biofilm-embedded bacteria such as MRSA, S. epidermidis, Coagulase-negative Staphylococci (CoNS), and Acinetobacter baumannii (Gawande, Leung, & Madhyastha, 2014). Finally, a combination of triclosan and DspB showed synergistic anti-biofilm activity against S. aureus, S. epidermidis , and E. coli (Darouiche, Mansouri, Gawande, & Madhyastha, 2009).
2.15 Biosurfactants
Microbial surfactants or biosurfactants (BSs) are low-molecular-weight, amphipathic and surface-active compounds, which are produced by microorganisms. These molecules tend to accumulate at the interface between phases (liquid–liquid/air–liquid), showing varying degrees of polarity and hydrogen bonding. Most BSs are diverse, complex molecules, composed of different structures that include glycolipids, lipoproteins, lipopeptides, fatty acids, phospholipids, neutral lipids, and polymeric and particulate BSs. The main classes of BSs consist of glycolipids, phospholipids, lipopeptides (surfactin), and polymeric compounds (Banat, Franzetti et al., 2010; De Rienzo & Banat, 2015; Díaz De Rienzo, Stevenson, Marchant, & Banat, 2015; Makkar & Rockne, 2003, Md, 2012; Padmavathi & Pandian, 2014). The high antimicrobial, antiadhesive and strong dispersal properties of BSs make them hopeful agents for eradicating biofilms (some BSs that inhibit biofilm formation are listed in Table 3) (Krasowska, 2010). Considering BSs' role as anti-adhesive agents against several pathogens, suitable anti-adhesive coating agents are suitable for medical insertional materials that can reduce a large number of hospital infections without a need for synthetic drugs and chemicals. Several studies reported that surfactin and pseudofactin II inhibit biofilm formation of S. enterica, S. typhimurium, E. coli, and P. mirabilis in polyvinyl chloride microtiter wells or in urethral catheters (Mireles, Toguchi, & Harshey, 2001). Studies on oral disease showed that BSs from Lactobacillus casei strains exhibited anti-biofilm activity against oral S. aureus strains (Merghni, Dallel et al., 2017). Moreover, the application of BSs can help prevent biofilms and biofouling in industrial plants; in a wider context, it can also be applied to metal medical devices. On the other hand, researchers indicated that the glycolipid derived from a tropical marine strain of S. marcescens could thus serve as a potential anti-biofilm agent. This surfactant prevents adhesion and disrupts preformed biofilms of P. aeruginosa and Bacillus pumilus (Dusane, Pawar et al., 2011). In addition, Walencka, Różalska, Sadowska, and Różalska (2008) reported that the Lactobacillus acidophilus derived surfactants inhibited bacterial deposition rate and biofilm development (and also its maturation) because of the effect exerted on the cell-surface hydrophobicity of Staphylococci. Sophorolipids and rhamnolipids are promising compounds for the inhibition/disruption of biofilms formed by gram-positive and gram-negative microorganisms. The antibiofilm effect of these in combination with booster compounds can increase significantly; findings have shown that combinations of rhamnolipids and caprylic acid led to 90% destruction of biofilms. In a similar study, results showed that the ability of attaching surfaces and biofilm formation of P. aeruginosa, E. coli, and B. subtilis was inhibited by sophorolipids in the presence of caprylic acid (De Rienzo, Stevenson, Marchant, & Banat, 2015, 2016). On the other hand, BSs in combination with antibiotics lead to a synergistic increase in the efficacy of antibiotics in biofilm killing, and suggested that the effect of antibiotics in combination with the BSs was significantly increased and, finally, reduced biofilm formation (Rivardo, Martinotti, Turner, & Ceri, 2011). Furthermore, gtfB and gtfC play an important role in the sucrose dependent attachment of S. mutans cells to hard surfaces. Thus, these genes have been considered a potential target for protection against dental caries. GtfB and gtfC gene expression was significantly reduced by tested BSs in the biofilm environment (Tahmourespour, Salehi, & Kermanshahi, 2011).
| Source | Biosurfactant class | Name | Effectiveness |
|---|---|---|---|
| Pontibacter korlensis | Lipopeptide | Pontifactin | Anti-biofilm activity showed against S. aureus, B. subtilis, Salmonella typhi and V. cholera (Balan, Kumar, & Jayalakshmi, 2016) |
| Candida bombicola | Glycolipid | Sophorolipid | Disrupt biofilms formed by single and mixed cultures of S. aureus and B. subtilis (De Rienzo et al. 2015) |
| Burkholderia thailandensis | Glycolipid | Rhamnolipid | Anti-biofilm activity reported against Neisseria mucosa, Streptococcus oralis, Actinomyces naeslundii, and Streptococcus sanguinis (Elshikh, Funston et al., 2017) |
| B. cereus | Lipopeptide | NS | Reduced the ability of biofilm formation of P. aeruginosa, S. aureus and K. pneumoniae (Hassan & Mohammad, 2015) |
| Acinetobacter indicus | NS | NS | Treatment of biofilms for seven days at 500 μg/ml resulted in up to 82.5% biofilm disruption (Karlapudi et al., 2018) |
| B. subtilis | Lipopeptide | surfactin, iturin and fengycin | Biofilm formation on uropathogenic bacteria reduced (Moryl, Spętana et al., 2015) |
| Corynebacterium xerosis | Lipopeptide | Coryxin | Disrupted preformed biofilms of E. coli (66%), S. mutans (80%), S. aureus (82.5%), and P. aeruginosa (30%). (Dalili, Amini et al., 2015) |
| Fasciospongia cavernosa | Lipopeptide | NS | 125 mg/ml of lipopeptide was effective in reducing the biofilm formation activity of pathogenic MDR S. aureus (Kiran, Priyadharsini et al., 2017) |
| Bacillus licheniformis | NS | NS | Inhibited P. aeruginosa and Vibrio harveyi biofilms on polystyrene surfaces up to around 78% and 80% respectively (Hamza, Kumar, & Zinjarde, 2016) |
| S. lentus | Glycolipid | NS | At a concentration of 20 μg was able to disrupt mature biofilms of V. harveyi (78.7 ± 1.93%) and P. aeruginosa (81.7 ± 0.59%; (Hamza, Satpute, Banpurkar, Kumar, & Zinjarde, 2017) |
| Lactobacillus casei | Glycolipid | NS | Potentially disrupted biofilm formation under dynamic conditions (Kiran, Sabarathnam, & Selvin, 2010) |
| Lactobacillus jensenii and Lactobacillus gasseri | NS | NS | Disrupted biofilms of E. coli, Enterobacter aerogenes and Staphylococcus saprophyticus (Morais, Cordeiro et al., 2017) |
- Note. NS: not specified.
2.16 Bacteriocins
Bacteriocins are ribosomally synthesized by all prokaryotic lineages and are defined as proteins or small chains of amino acids with antibacterial activity that kills or prevents the growth of closely related bacteria by various mechanisms such as inhibiting cell wall synthesis and biofilm formation, increasing cell membrane permeability, and inhibiting DNAse or RNAse activity (some bacteriocins that inhibit biofilm formation are listed in Table 4) (Klaenhammer, 1993). Through different methods of actions, bacteriocins are normally active against both antibiotic-sensitive and antibiotic-resistant pathogens. The colicins of E. coli are the mostly studied bacteriocins, and Nisin is approved for use in over 40 countries and has been in use as a food preservative for over 50 years. Nisin A is known to possess a dual mode of action: Inhibition of cell wall synthesis by masking lipid II (bacteriostatic) and membrane insertion followed by pore formation that rapidly kills cells (bactericidal; Mathur, Field et al., 2018). Inhibition of the gram-positive foodborne pathogens, such as S. aureus, B. cereus, and L. monocytogenes, by the bacteriocins, including nisin and bovicin HC5, has been reported in some studies (Antolinos, Muñoz et al., 2011; Pimentel-Filho, Mantovani, de Carvalho, Dias, & Vanetti, 2014). Nisin has the potential as an adjunctive endodontic therapeutic agent, and bacterial biofilms treated by ≥10 mg/mL of Nisin for 10 min show a reduction in biofilm biomass (Kajwadkar, Shin et al., 2017). An important step in the formation of biofilms is attachment to abiotic surfaces such as polystyrene. Moreover, Pimentel-Filho et al. (2014) reported that using nisin and bovicin rendered the surfaces more hydrophilic and changes in the free energy of adhesion between the polystyrene surfaces and bacterial cells prevented adhesion to the surface. Therefore, bacteriocins may bring about some changes in the hydrophobicity of abiotic surfaces as well as bacterial cell surfaces, thus this can impede this critical adhesion stage in biofilm formation. Significantly, it was shown that nisin and bovicin also had an effect on the transcription of certain genes in S. aureus, primarily affecting clfB, fmnbA and icaD, which are involved in biofilm formation (Mathur, Field et al., 2018). Besides, bacteriocins could affect AI-2 and AHL and contribute to inhibition of QS and biofilm formation. The results have shown that nisin may reduce RNAIII-activating protein (TRAP) phosphorylation, reduce RNAIII expression and cell attachment in vitro and decrease biofilm formation and pathogenicity in vivo through downregulating the opuCABCD operon in S. aureus (Zhao, Meng et al., 2016). A novel plasmid-encoded bacteriocin BMP11 produced by Lactobacillus crustorum MN047 exhibited anti-biofilm formation activity. In addition, BMP11 inhibited the growth of L. monocytogenes in milk (Yi, Li et al., 2018). Results of a study that evaluated the effect of bacteriocin from P. putida FStm2 on biofilm-forming bacteria isolated from urinary catheter showed that treatment of urinary catheter infections with this bacteriocin can be a powerful strategy to prevent biofilms in the urinary tract infections (Ahmad, Hamid, & Usup, 2014). Findings of another study suggested that hyicin 4244 not only prevented biofilm formation by planktonic cells, but also penetrated into the biofilm matrix and forced bactericidal activity against Staphylococcal sessile cells (Duarte, Ceotto-Vigoder et al., 2018). On the other hand, Sharma, Dang, Gupta, & Gabrani, (2018) reported that bacteriocin produced by B. subtilis GAS101 not only inhibited the biofilm formation, but also disrupted the preformed biofilm established by S. epidermidis. The interaction of bacteriocin and essential oils has significant effects on bacterial biofilms, which is a promising source for development of more potent broad-spectrum antimicrobial blend for food preservation. Studies indicated that synergistic effects of a bacteriocin and antibiotics resulted in a reduction in biofilm formation. Furthermore, several research studies proposed that the concentrations of polymyxins could effectively inhibit biofilm formation, which in turn significantly reduced it when combined with nisin (Field, Seisling, Cotter, Ross, & Hill, 2016). The anti-adhesion and antimicrobial effects of nisin in combination with the physical properties of carbon nanotubes pave the way for generating effective anti-biofilm formation surfaces, and many studies demonstrated that nisin coating on multi-walled carbon nanotube sheets decreased surface hydrophobicity and inhibited the biofilm formation up to 94.6% (Dong, McCoy, Zhang, & Yang, 2014). On the other hand, the immobilization of nisin with poly (ethylene glycol) as a linker significantly enhanced the antimicrobial and anti-biofilm properties of MWNT against E. coli, P. aeruginosa, S. aureus, and B. subtilis (Qi, Poernomo et al., 2011). Moreover, nanofibers containing nisin and 2,3-dihydroxybenzoic acid (DHBA) led to significant effects on bacterial biofilms, and findings revealed that the biofilm formation decreased up to 88% after 24 hr of exposure to nanofibers containing Nisin and DHBA (NDF; Ahire & Dicks, 2015). Bacteriocins forming stable pores on biofilm cells are potent enough to treat MRSA biofilm infections. Therefore, activities and action modes of the three bacteriocins characterized with different structures (Nisin A, Lacticin Q, and Nukacin ISK-1) on the MRSA biofilms were evaluated. Of all the tested bacteriocins, Nisin A exhibited the highest bactericidal activity against biofilm cells (Okuda, Zendo et al., 2013).
| Source | Name | Effectiveness |
|---|---|---|
| Bacillus sonorensis | Sonorensin | Inhibition activity against biofilm of S. aureus (Chopra, Singh, Jena, & Sahoo, 2015) |
| Citrobacter freundii | colA-43864 | Highly effective in killing Citrobacter species, E. coli, and K. pneumoniae cells in a planktonic and biofilm state (Shanks, Dashiff, Alster, & Kadouri, 2012) |
| Burkholderia cenocepacia | LlpA | Result in 52% reduction in biofilm formation at 5.8 nmol/L of LlpA and 36% reduction at 58 nmol/L of LlpA (Ghequire, De Canck et al., 2013) |
| L. crustorum MN047 | BM1157 and BM1300 | Killed L. monocytogenes by biofilm destruction and pore formation (Yi, Luo, & Lü, 2018) |
| LAB | NS | Significant reduction in listeria biofilm formation (Camargo, de Paula, Todorov, & Nero, 2016) |
| LAB | Enterocin-B, A | Antibacterial and anti-biofilm activity against, L. monocytogenes, S. aureus, E. coli and S. enterica (Ankaiah, Palanichamy et al., 2018) |
| B. licheniformis and Lactococcus lactis | Licheniocin 50.2 and NS | Inhibit biofilm formation of CoNS and L. monocytogenes (Cirkovic, Bozic et al., 2016) |
| E. faecalis | Enterocin B3A-B3B | Inhibit about 2 log of biofilm formation by L. monocytogenes (Al-Seraih, Belguesmia et al., 2017) |
| Lactobacillus plantarum | NS | Inhibitory effects on biofilm formation by S. sanguis, P. aeruginosa, and S. aureus (Ming, Zhang, Yang, & Huang, 2015) |
| Lactobacillus sakei CRL1862 | NS | Reduction of biofilm formation by L. monocytogenes (Pérez-Ibarreche, Castellano, Leclercq, & Vignolo, 2016) |
| L. acidophilus and L. plantarum | NS | Inhibitory activity against planktonic and biofilm forms of S. marcescens strains (Shahandashti, Kermanshahi, & Ghadam, 2016) |
| L. lactis | Nicin | Inhibitory activity against planktonic and biofilm cells at 25 pg/ml of nicin (Sudagidan & Yemenicioğlu, 2012). |
| Enterococcus faecium | Enterocin-A | Effective antibacterial and anti-biofilm activity against food born as well as human bacterial pathogens (Ankaiah, Esakkiraj, Perumal, Ayyanna, & Venkatesan, 2017). |
- Note. LAB: lactic acid bacteria; NS: not specified.
3 CONCLUSION
The emergence of severe biofilm infections and, consequently, high resistance to antibiotic therapy and antimicrobial agents can be a major challenge in the field of medicine and global health. The formation of biofilms on food products, aquatic products, and medical equipment relative to health is one of the simplest problems of human society. Current therapies are limited to inhibiting biofilms and, more often, to coping strategies which depend on a broad spectrum of antibiotics and disinfectants. Considering the high rate of resistance to antibiotics among bacteria, the development and use of appropriate agents and factors is one of the basic requirements of dealing with biofilm-related diseases. Biofilms are really important in human infections such as bacterial vaginosis, urinary tract and middle ear infections, tooth or gum plaque formation, catheter and prosthetic joints infections, and contact lenses infections. In addition, considering their importance in even less prevalent but more important infections, which can be fatal such as endocarditis, cystic fibrosis infection, heart valve infection and its association with antibiotic resistance; therefore, it seems vital to find new and effective strategies for controlling it. With regard to the ongoing studies, vaccination, as an effective vision, can be very useful and effective in the near future. Because of the diversity of antigens and bacterial proteins, finding an antigen, protein, and target site is one of the barriers for designing vaccine against biofilms. Therefore, it is suggested to design vaccines which target specific molecular pathways in the biofilm formation process. Vaccines can also be used in combination with antibiotics and other substances introduced in this study. Moreover, some of the new methods, which can be used to prevent from biofilm formation, include designing and manufacturing of surfaces to inhibit biofilm formation; using bacteriocins along with food preservatives; using anti-biofilms in urinary catheters surfaces; using compounds, such as MGO in honey, in food products and cosmetics; using nanoparticles for drug delivery in dangerous infections, such as burn infections for which the absorption of antibiotics is weak; using genetically engineered phages that can specifically target multidrug resistant infections (MDR and XDR) and do not enter the lysogenic phase; and, finally, using plant extracts, which have been widely studied worldwide.
AUTHOR CONTRIBUTION
J.Y.S. and A.H. conceived and designed the study. T.A. and A.S. contributed in Comprehensive research. M.K and A.S designed the Figure. J.Y.S and A.H wrote the paper. T.A and H.S participated in manuscript editing.