The role of microRNAs in prostate cancer migration, invasion, and metastasis
Abstract
Prostate cancer (PCa) is considered the most prevalent malignancy and the second major cause of cancer-related death in males from Western countries. PCa exhibits variable clinical pictures, ranging from dormant to highly metastatic cancer. PCa suffers from poor prognosis and diagnosis markers, and novel biomarkers are required to define disease stages and to design appropriate therapeutic approach by considering the possible genomic and epigenomic differences. MicroRNAs (miRNAs) comprise a class of small noncoding RNAs, which have remarkable functions in cell formation, differentiation, and cancer development and contribute in these processes through controlling the expressions of protein-coding genes by repressing translation or breaking down the messenger RNA in a sequence-specific method. miRNAs in cancer are able to reflect informative data about the current status of disease and this might benefit PCa prognosis and diagnosis since that is concerned to PCa patients and we intend to highlight it in this paper.
1 INTRODUCTION
Prostate cancer (PCa) is considered as a common nonskin malignancy and the main cause of cancer-associated mortality worldwide (Fabris et al., 2016). Several risk factors are associated with progression of the disease. Based on the most important factor, further steps would be designed. Primarily it is essential to choose an effective diagnostic marker and method. The current standard diagnosis approaches of PCa is based on the prostate-specific antigen (PSA) serum level and the digital rectal examination (DRE; Bunting, 2002). High PSA levels are not always associated with malignancy and its out of range level could be due to inflammation, infection, or benign prostatic hyperplasia (BPH). PSA levels in serum are greater than 2.5–4 ng/ml and aberrances in DRE may allude to the existence of PCa after performing a biopsy-based diagnosis, although the positive predicting levels of these techniques are only 24–37%, relevance (Bunting, 2002; Thompson et al., 2006). Among the common therapeutic methods, choices for the localized PCa treatment are external radiotherapy, prostatectomy, and brachytherapy, whereas active surveillance method has progressed instead of the radical therapy for very low-risk PCa cases (Bangma et al., 2015). In spite of significant advances in routine diagnostic PSA tests clinical and research communities have still doubt of its effective diagnostic application (Schröder et al., 2014) due to the wide range of molecular heterogeneity of PCa patients (Fraser, Berlin, & Bristow, 2015). The determination and clinical interpretation of routinely tested disease and stage-specific molecular biomarkers are practical methods to potentially improve PCa diagnosis, prognosis, and treatment response, flatting the way to personalized medicine. Beyond proteins and messenger RNAs (mRNAs), which have revealed clinical values in different clinical scenarios, there is a rising interest in the possible application of microRNAs (miRNAs) as promising PCa biomarkers.
miRNAs are short noncoding single stranded RNA molecules, composed of approximately 18–22 nucleotides with a wide modulatory activity on molecular signaling pathways (Hemmatzadeh, Mohammadi, Jadidi-Niaragh, Asghari, & Yousefi, 2016; Hemmatzadeh, Mohammadi, Karimi, Musavishenas, & Baradaran, 2016; Mohammadi et al., 2018; Saidi et al., 2017). Stable miRNAs are usually identified in biological fluids, such as serum and plasma. Consequently, the measurement of PCa-related miRNAs is appearing as a noninvasive implement for PCa monitoring.
Accumulative evidence demonstrate that miRNAs can be exerted as possible biomarkers in PCa diagnosis and in the proper selection of patients for active surveillance owing to their association with tumor invasiveness. To date, human PCa has been assigned to demonstrate about 50 miRNAs with dysregulated expression pattern (nearly 40 miRNAs overexpressed and rest underexpressed). Nevertheless, this different miRNAs expression could be exerted as diagnostic biomarkers. Depending on the targeted gene, miRNAs can act as a cancer enhancer (oncomiRs) or a tumor suppressor. miRNAs have also been observed to participate in the resistance against apoptosis in PCa via several mechanisms. MiRNAs also take part in drug resistance. Taxanes are the main group of drugs, widely being used in PCa treatment, resistance to these chemotherapy agents could happen due to the inhibition of apoptosis and activation of ERK/MAPK and PI3-kinase/Akt pathways leading to cell survival (Fitzpatrick & de Wit, 2014). These crucial signaling pathways could be dysregulated by miRNAs, which are the main targets in this review. Therefore, discovering the mechanisms underlying miRNA expression, regulation, and their target site could contribute in designing successful therapeutic tools. To understand the relationship between miRNA expression profile and PCa, we need first to consider that, most of these miRNAs are common among different types of cancers. Key point to remember is that the target genes of these common miRNAs might be variable among different cancer subtypes.
2 MIRNAS AS DISTINCTIVE MARKERS
2.1 Discrimination between PCa and BPH
Available diagnostic markers up to now are not able to distinguish benign hyperplasia from malignant hyperplasia of prostate tissue. PSA is an organ-specific marker, its altered levels are not specific for cancerous tissues, and quantity changes might occur in various conditions in addition to malignancy. This alteration in PSA levels leads to unnecessary prostate biopsy (Cochetti et al., 2016b). miRNAs are reliable biomarkers that help us to have an accurate diagnosis which is first and the most crucial step of the therapeutic process. According to Haj-Ahmad et al. (2014) study, which has been done with 8 PCa and 12 BPH patients and 10 healthy male, miR-1825 and miR-484 have been shown to be potential and valuable diagnostic signatures for discriminating PCa and BPH. In this regard, Let-7c, let-7e, let-7i, miR-26a-5p, miR-26b-5p, miR-18b-5p, and miR-25-3p have been shown by Cochetti et al. (2016a) as valuable signatures to discriminate patients with PCa from BPH while they both showed the higher level of PSA compared with healthy males. In addition, they found miR-25-3p and miR-18b-5p showed the strongest sensitivity and specificity to predict PCa, respectively (Cochetti et al., 2016a). In addition, Al-kafaji et al. (2018) showed significantly reduced expression of miR-15a, miR-126, miR-192, and miR-377 in PCa patients compared with patients with BPH and healthy subjects which are suggesting a promising and noninvasive diagnostic biomarker to discriminate PCa from BPH.
2.2 miRNA and PCa
miRNA biogenesis is a multistep procedure, begins with primary miRNA (pri-miRNA) transcript. Pri-miRNA undergoes two levels of cleavage, the first step in the nucleus and the second step in the cytoplasm. Mature miRNA ultimately forms RNA-induced silencing complex by the contribution of RNase III enzyme DICER (Hammond, Bernstein, Beach, & Hannon, 2000; Hemmatzadeh et al., 2018). Variable expression levels of miRNAs regulate mRNA translation process. miRNA transcript profile in PCa was initially introduced by Porkka et al. (2007). They carried out an oligonucleotide array hybridization procedure to assess the transcript profile of 319 human miRNAs in PCa and demonstrated 51 miRNAs aberrantly expressed in PCa (Porkka et al., 2007). A rapidly growing number of platforms have been introduced for miRNA expression profiling. Microarray analysis was the most common procedure performed to recognize cancer-related miRNA signatures. Nevertheless, the progress of next-generation sequencing (NGS) technologies has suggested an advancing technique in the determination of previously unknown miRNAs (Johnson et al., 2005). As recent studies showed miRNAs expression pattern as a powerful tool in categorizing between healthy and cancer samples, its role is being probed as an important clinical biomarker. For example, microarray analysis revealed miR-21 to reduce reversion inducing cysteine-rich protein with kazal motifs (RECK) expression, resulting in an induced expression of matrix metalloproteinase-9 (MMP9) and tumor cell invasion (Leite et al., 2015). In other investigation, intratumoral administration of miR-23b in vivo decreased cancer mass in animals that were ascribed to the repression of protooncogenic signaling pathways in PCa neoplastic cells (Majid et al., 2012).
Some other miRNAs have been assigned to be exceedingly stable in sera; therefore, circulating miRNAs have become important candidates for blood-based biomarkers. Mitchell et al. (2008) revealed that miR-141 serum levels remarkably differentiated PCa patients and healthy controls. Additionally, Taylor and Gercel-Taylor showed upregulation of miR-21, miR-141, miR-200a, miR- 200c, miR-200b, miR-203, miR-205 and miR-214 in circulating cancer exosomes (Taylor and Gercel-Taylor et al., 2008). Likewise, Zhang et al. (2014) discovered 39 new miRNAs candidates as PCa biomarkers analyzing Gene Expression Omnibus datasets for PCa and BPH utilizing bioinformatics methods, considering the modulatory potential of miRNAs.
Typically Let-7c, let-7e, let-7i, miR-26a-5p, miR-26b-5p, miR-18b-5p, and miR-25-3p are confirmed miRNAs as discriminative tools (Cochetti et al., 2016b).
Quantitative reverse transcription polymerase chain reaction confirmed the results, demonstrating that miRNA-648 had the capacity of being a new biomarker of PCa. Therefore, cumulating data offer that miRNAs are able to be exerted as possible biomarkers in diagnosis of PCa.
3 EXPRESSION PROFILE OF miRNA IN PCA
miRNA’s modulatory actions are not restricted to a special mRNA, each miRNA regulates different mRNAs as targets, and each mRNA could act as a target for several miRNAs. Chromosomal translocations, promoter methylation, and other epigenomic actions could alter the function of miRNAs and consequently their target pathways (Cannistraci, Di Pace, De Maria, & Bonci, 2014; Rauhala et al., 2010). Based on the target gene an individual miRNA can either act as a tumor suppressor or oncogene (Cho, 2010). Different miRNA expressions in PCa were scrutinized, utilizing widespread genome-based technologies, which have been capable of differentiating the benign prostate tissue from PCa. Recently, miRNA signatures have been reported, comparing miRNA expressions in benign prostate tissue and castration-resistant prostate cancer (CRPC) clinical specimens taken from the autopsy (Goto et al., 2015). Overexpression of miR-26a, miR-182, miR-182*, miR-183, miR-375, 32, miR-96, miR-181a, miR-93, miR-196a, miR-25, let-7i, and miR-92 and under expression of miR-145, miR-31, miR-125b, miR-16, miR-149, miR-181b, miR-184, miR-205, miR-222, and miR-221 were confirmed in tissues from PCa (Goto et al., 2015; Schaefer et al., 2010). Some other biomarkers have been established utilizing hormone-sensitive PCa (Fuse et al., 2012) or CRPC (Goto et al., 2015; Ottman, Nguyen, Lorch, & Chakrabarti, 2014; Porkka et al., 2007) and serum or urine of PCa cases (Filella & Foj, 2017; Rönnau, Verhaegh, Luna-Velez, & Schalken, 2014). The role of miRNAs in PCa has been discovered by explaining the relationship between miRNAs and genes that they target. Volinia et al. (2006) investigated miRNA expression in PCa tissue by analyzing 228 miRNAs in 56 PCa tissues and six healthy prostate tissues. The authors indicated that 39 miRNAs were overexpressed, while six were underexpressed. Porkka et al. (2007), by evaluating 319 miRNAs in four BPH and nine PCa tissues, demonstrated upregulation in eight miRNAs, while 22 miRNAs were downregulated. Recently, Carlsson et al. (2011), assessing 667 miRNAs, demonstrated nine miRNAs that compatibly disagree between malignant prostate tissues and the normal tissue from each case. Srivastava et al. (2013b) revealed that miR-205, miR-214, miR-221, and miR-99b are significantly upregulated in PCa tissues compared with connecting normal tissue. Areas under the curve obtained by receiver operating characteristics were 0.83, 0.92, 0.75, and 0.86, respectively. Significantly, Hellwinkel et al. (2013) showed that seven miRNAs were aberrantly expressed in healthy prostate tissue from PCa cases compared with healthy prostate tissue from patients with the negative biopsy. Although several investigations found miRNA modification as important diagnostic and prognostic techniques, particular signatures describing the variously expressed miRNAs have not yet been discovered. Diversity between the results from the different groups could be associated with multiple factors. Moreover, different screening approaches as well as diverge characteristics of evaluated specimens (i.e., stroma-to-tumor proportion) can justify the discrepancies in the results. The miRNAs demonstrated as significant ones, were discovered with similar patterns in multiple investigations with various approaches. Despite the diversity reported throughout the publication, promising miRNA signatures are determined and require to be further corroborated in additional large patient cohorts.
4 CIRCULATING miRNA IN PCA
miRNAs originating from PCa tissue can be traced in the blood circulation and therefore can be simply evaluated in the sera from these patients (Moltzahn et al., 2011; Figure 1). Mitchell et al. (2008) showed that miRNAs are stock in a significantly sustained form in the blood and that they are protected from endogenous function of RNase enzymes. Newly performed investigations have confirmed relationship between circulating miRNAs and risk evaluation models. Overexpression of five miRNAs, including miR-141, miR-9*, miR-375, miR-516a-3p, and miR-200b have been observed in sera of patients with metastatic PCa. Along with, expression levels of miR-141 and miR-375 were correlated with metastatic form of the disease in other research (Brase et al., 2011; Watahiki et al., 2013). Overexpression of miR-93 and miR-106a and downregulation of miR-24 were also correlated with metastatic PCa (Moltzahn et al., 2011). Compounding circulating miRNAs correlated with biochemical failures, such as miR-141, miR-146b-3p, and miR-194 with the usual prognostic tools can foretoken disease progression (Das et al., 2017; Selth et al., 2013). Many investigations have recognized particular signatures of miRNAs in circulation for PCa discovery and prognosis utilizing microarrays dissimilarities between panels are remarkable, in which only miR-141, miR-375, and miR-21 appear to be frequent. Therefore, while 74 miRNAs have been offered as circulating biomarker candidates for PCa, only 25 of these miRNAs have been concurrently related to PCa according to several studies in this field. Chen et al. (2012) examined 1,146 miRNA expressions in the plasma from 25 PCa (15 nonmetastatic and 10 metastatic) and 17 BPH patients, discovering a five miRNA panel (miR-622, miR-1285, let-7e, let-7c, and miR-30c) that could discriminate PCa from BPH and from healthy controls. On the other hand, it was discovered that the potential diagnostic application of three particular PCa-related miRNAs, including miR-21, miR-141, and miR-221, indicating that miR-21 and miR-221 were upmodulated in the sera of patients with localized form of cancer than healthy controls (Agaoglu et al., 2011). In contrast, a signature characterized through two miRNAs, namely miR-141 and miR-375, was ascribed by more than one group (Bryant et al., 2012; Kachakova et al., 2015). These miRNAs look to be genuine markers of systemic disease. Both of miR-141 and miR-375 were also correlated with the clinical presentations of the patients, including tumor stage and Gleason score in sera before conducting radical prostatectomy, implying to the fundamental capability of circulating molecules in early identification of PCa. These molecules have also shown a favorable prognostic score. Lately, miR-375 obtained from plasma exosomes was approved as a promising prognostic tool in CRPC (Bryant et al., 2012; Huang et al., 2015). miR-375 was regarded as a potential estimator of survivorship, alone or in combination with miR-1290 (Huang et al., 2015). A shorter total survival rate and increased mortality level was observed in PCa subjects with raised levels of these miRNAs.
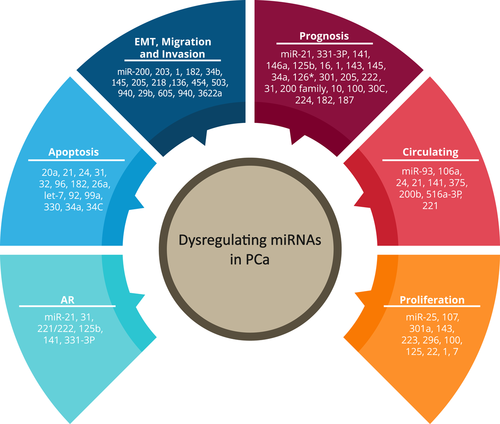
Dysregulating miRNAs in PCa. Recent literature has shown that certain miRNAs are associated with specific steps in PCa pathogenesis, including AR signaling, apoptosis, EMT, prognosis, circulation, and proliferation. AR: androgen receptor; EMT: epithelial-to-mesenchymal transition; PCa: prostate cancer [Color figure can be viewed at wileyonlinelibrary.com]
5 miRNA ASSOCIATED WITH CELL MIGRATION, INVASION, AND EMT
It has been indicated that epithelial-to-mesenchymal transition (EMT) is an important key phase in the metastatic process and involves series of events, during which epithelial cells miss most of their epithelial instructions and augment specifications that are typical of mesenchymal cells (Acloque, Thiery, & Nieto, 2008). Mesenchymal marker overexpression, like vimentin and N-cadherin, and overexpression of E-cadherin transcriptional repressors, composing of zinc finger proteins, namely Snail family transcriptional repressor 1/2 (SNAI1/2), zinc finger E-box-binding homeobox 1/2 (ZEB1/2), twist-related protein 1, and localization of membrane-to-nuclear localization of β-catenin are EMT insignia. Evidence from current studies show that expression alterations in EMT-associated genes are not only attributable to genetic mutations but also to complex networks between miRNAs and related pathways (Miska, 2008). A recent investigation showed that cells with EMT presentation from PCa patients demonstrated stem-like cell features which were connected with diminished expression of miR-200 and the let-7 family (Kong et al., 2010). Epithelial marker loss has been related to transcription suppressors like ZEB1 and ZEB2. miR-182 and miR-203 were discovered to be prevented during EMT. These miRNAs regulate SNAI2 and P-cadherin (Qu et al., 2013). Presence of miR-205 is considered as an obligatory factor for the inhibitory effects of p63, a metastasis suppressor, on EMT markers, ZEB1 and vimentin in cells from PCa (Table 1 and Figure 2).
| miRNAs | Expression | mRNA target | Function | References |
|---|---|---|---|---|
| miR-1 | Downregulated | SNAI2 | EMT | Liu et al., 2013 |
| miR-27b | Downregulated | GOLM1 | tumor cell proliferation, migration, and invasion | Goto et al., 2014 |
| miR-25 | Downregulated | av- and a6-integrin | attenuation of extravasation | Zoni et al., 2015 |
| miR-29b | Downregulated | SNAI1 | Invasion, migration, and EMT | Ru et al., 2012 |
| miR-34b | Downregulated | AKT | Invasion, migration, and EMT | Majid et al., 2013 |
| miR-136 | Downregulated | MAP2K4 | Proliferation and invasion | Zhu, Shao, Pan, Cheng, & Rui, 2018 |
| Cluster of miR-143/145 | Downregulated | GOLM1 | tumor cell proliferation, migration, and invasion | Kojima et al., 2014a |
| miR-145 | Downregulated | ZEB2,FSCN1 and SWAP70 | Invasion, migration, and EMT | Chiyomaru et al., 2011a; Fuse et al., 2011; Ren et al., 2014 |
| miR-200 | Downregulated | SNAI2 | EMT | Liu et al., 2013 |
| miR-203 | Downregulated | ZEB2, Bmi, survivin, Runx2 | EMT | Saini et al., 2010 |
| miR-205 | Downregulated | PKCε, ZEB1/2 | Invasion, migration, and EMT | Gandellini et al., 2009 |
| miR-212 | Downregulated | MAPK1 | Proliferation, invasion, and apoptosis | Hu, Jin, & Wang, 2018 |
| miR-218 | Downregulated | LASP1, LGR4 | Invasion and migration | Li et al., 2016; Nishikawa et al., 2014 |
| miR-454 | Upregulated | NDRG2 | Proliferation and invasion | Fu et al., 2018 |
| miR-466 | Downregulated | RUNX2 | Invasion, migration, and Apoptosis | Colden et al., 2017 |
| miR-503 | Downregulated | TPD52L2 | Migration, proliferation | Chi, Ding, Zhang, & Du, 2018 |
| miR-605 | Downregulated | EN2 | Proliferation and invasion | Zhou et al., 2017 |
| miR-940 | Upregulated | MEIN1 | Migration, invasion, anchorage-independent growth | Rajendiran et al., 2014 |
| miR-3622a | Downregulated | ZEB1, SNAI2 | EMT | Bucay et al., 2017 |
- Note. Bmi: B-cell-specific Moloney murine leukemia virus insertion site 1; EMT: epithelial-to-mesenchymal transition; EN2: engrailed homeobox 2; FSCN1: fascin homolog 1; GOLM1: golgi membrane protein 1; LASP1: LIM and SH3 domain protein 1; LGR4: leucine rich repeat containing G protein-coupled receptor 4; MAPK1; mitogen-activated protein kinase 1; MAP2K4: mitogen-activated protein kinase kinase 4; NDRG2: N-myc downstream-regulated gene 2; PKCε: rotein kinase Cε; RUNX2: runt-related transcription factor 2; SNAI: Snail family transcriptional repressor 1; SWAP70: switching B-cell complex 70 kDa subunit; TPD52L2: tumor protein D52-like 2; ZEB1: zinc finger E-box-binding homeobox 1
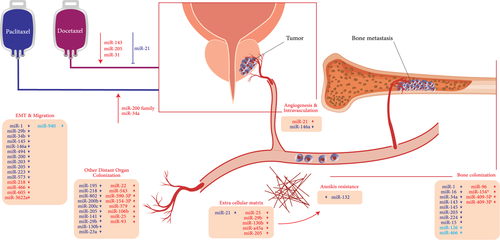
Overview of miRNAs involved in the metastatic cascade. miRNAs revealed to represent a role in inducing or inhibiting different steps of the PCa metastatic process, including the modulation of EMT and cell migration/invasion, the interplay between cancer cells, and the surrounding stroma, the control of angiogenesis, the modulation of the extracellular matrix and anoikis, and the colonization of distant organs, are described. EMT: epithelial-to-mesenchymal transition [Color figure can be viewed at wileyonlinelibrary.com]
5.1 miR-29b
miR-29b was revealed to be downmodulated in human PCa. Overexpression of miR-29b in metastatic tumor cells from PCa upregulated E-cadherin and downmodulated mesenchymal markers, such as N-cadherin, Twist, and Snail, suggesting that the overturn of EMT and the acquisition are less invasive (Ru et al., 2012; Steele, Mott, & Ray, 2010).
5.2 miR-144
The forcefulness of miR-144 to regulate cell expansion, migration, and invasion in cases with nasopharyngeal carcinoma was determined to be a consequence of phosphatase and tensin homolog (PTEN) repression. Mechanistically, miR-144 represses PTEN transcription, resulting in overexpression of pAkt and cyclin D1, promoting G1-phase transition, later on preventing E-cadherin to promote invasion and migration (Zhang et al., 2012). miR-144 cause decreasing the EZH2 expression which stems from the stimulation of Wnt/β-catenin signaling (Guo et al., 2013). EZH2 is an enzymatic part of the polycomb repressive complex 2 expressed in breast and prostate tumors, catalyzing histone H3 trimethylation at lysine 27 (H3K27me3) and repressing the promoter zone (Qi et al., 2012). Forkhead box O3a importantly suppresses the β-catenin expression in PCa and also controls transactivated miR-34b/c that begins a cascade of events, leading to the repression of EMT in PCa (H. Liu et al., 2015).
5.3 miR-145
miR-145 was observed to be regulated by p53, a tumor suppressor gene (Hart et al., 2013; Sachdeva et al., 2009). The loss of miR143/145 induced cancer cell's migration and invasion in the PCa by modulating the golgi membrane protein 1 (Kojima et al., 2014b). miR-145 was overexpressed by wild-type p53 (Ren et al., 2013) and was observed to straightly target the fascin homolog 1 (FSCN1; Fuse et al., 2011) and switching B-cell complex 70 kDa subunit (SWAP70; Chiyomaru et al., 2011b). Knockdown of FSCN1 and SWAP70 suppresses the tumor cell migration and invasion in PCa. The miR-143/145 cluster is downmodulation in PCa cells, affecting the induced cell migration and invasion through downregulating the E-cadherin, hence promoting the EMT phenotype (Huang et al., 2012; Ren et al., 2014). miR-145 targets ZEB2, as an EMT stimulator, and ZEB2 directly downmodulates miR-145 expression. This negative feedback loop plays remarkable roles in repressing the tumor-associated mechanobiology, such as cancer cell invasion, migration, and EMT (Ren et al., 2014).
5.4 miR-205
miR-205 has been observed to be downmodulated in PCa and was revealed to take part in the EMT (Bhatnagar et al., 2010; Verdoodt et al., 2013). It worked as an EMT suppressor, which was connected with metastasis, through repression of ZEB1 and ZEB2 and consequence of high E-cadherin levels (Matsushima et al., 2011; Wu, Zhu, & Mo, 2009). miR-205 downregulation exerted a core role in self-determining androgen expression, correlating with the higher levels of B-cell lymphoma 2 (Bcl2; Verdoodt et al., 2013).
5.5 miR-218
LIM and SH3 domain protein 1 (LASP1) regulates molecular direction and participated in the organization of cytoskeleton and cell migration (Nishikawa et al., 2014). Downmodulation of LASP1 outstandingly interrupted the cell migration and invasion of tumor cells. MiR-218, as a tumor suppressor, was observed to be remarkably downmodulated in PCa and acted through controlling the transcription of the oncogenic protein LASP1 and loss of tumor-repressor miR-218 induced cancer cell migration and invasion in PCa via direct modulation of LASP1. Experiments demonstrated that restoration of miR-218, through direct targeting 3’-untranslated region (3’-UTR) of the LASP1 mRNA, prevented cancer cell migration and invasion (Alajez et al., 2011; Keicher et al., 2004).
5.6 miR-466
Expression analyses demonstrated that miR-466 was downmodulated in PCa relative to normal tissues. Newly Colden et al. (2017) dissected that in vitro upmodulation of miR-466 in metastatic PCa cell lines diminished oncogenic features like cell proliferation, migration/invasion, and increased cell cycle arrest as well as apoptosis of tumor cells than control miRNA. Contrarily, depletion of miR-466 in normal PCa cell lines increased tumorigenic behaviors. miR-466 repressed PCa progression and metastasis by targeting the bone-associated transcription factor RUNX2. Upregulation of miR-466 resulted in an outstanding downmodulation of the target genes in the RUNX2 network, including osteocalcin, osteopontin, ANGPTs, MMP11, Fyn, pAkt, FAK, and vimentin that exert roles in angiogenesis, invasion, migration, metastasis, and EMT. They strongly offered that miR-466-related depletion of RUNX2 as a novel promising therapeutic approach to control PCa growth, especially in bone metastatic cases (Colden et al., 2017).
5.7 miR-605
miR-605 has been shown to take part in the progression of tumors types (Chen, Cao, Rong, Wang, & Cao, 2017; Poltronieri-Oliveira et al., 2017). Newly, miR-605 has been assigned as a molecular mark for differentiation of the indolent and metastatic forms of PCa (Alhasan et al., 2016). miR-605 was revealed to be downmodulated in tissue and cells from PCa patients than normal tissues and cells. Zhou et al. (2017) demonstrated that engrailed homeobox 2 (EN2) is inversely modulated through miR-605, and miR-605 downregulation which stimulates the expansion and invasion of cells from PCa patients by overexpressing EN2, subsequently eventuates in PCa progression.
5.8 miR-940
miR-940 is upmodulated in PCa cells and is regarded as the main modulator of migration and invasion enhancer 1 (MIEN1), which is directly targeted by miR-940. It was observed that miR-940 prevented migratory and invasive cells, diminished their anchorage-independent growth and E-cadherin overexpression by involving in MET. Experiments demonstrated that miR-940 downmodulation in PCa cells culminated in MIEN1 overexpression, which in turn led to PCa development (Rajendiran et al., 2014).
5.9 miR-3622a
Evaluation of miRNA transcript profile in clinical tissues from human PCa cases revealed that miR-3622a was outstandingly downmodulated and is remarkably associated with tumor development and poor survival of patients. Newly, Bucay et al. (2017) demonstrated that miR-3622a exerted a critical function in EMT of PCa patients. Their study showed that endogenous expression of miR-3622a is mandatory to maintain the epithelial condition in normal and non-neoplastic cells from PCa cases. Both in vitro and in vivo experiments demonstrated that miR-3622a expression prevents EMT, progression, and metastasis of PCa cells. Further, they discovered that EMT effector molecules, namely ZEB1 and SNAI2, were directly targeted by miR-3622a. This data offered that loss of miR-3622a, which frequently occurs at chr8p21 region, resulted in stimulation of EMT, which in turn caused PCa development and metastasis.
6 STEMNESS PROPERTIES OF CANCER STEM CELLS
Properties of cancer stem cells are similar to other stem cell populations in the body. They have self-renewal potential and are able to proliferate despite concomitant maintenance of stem cell pool. Flow cytometry is a common method used for cancer stem cells (CSCs) identification. Special surface markers for CSCs are CD44 and CD133. High levels of CD44 expression as an adhesion molecule is associated with increased metastasis potential (Patrawala et al., 2006). miR-143 and miR-145 have a crucial role in progression and metastatic states of disease by regulation of stemness properties of these cells.
miR-34a inhibits PCa stem cells by directly targeting CD44, metastasis would be repressed in this manner (Liu et al., 2011b).
CSCs are able to enter blood circulation by the common process of EMT, which has been discussed in previous sections. EMT is regulated by different transcription factors such as Twist, Slug, and Snail these transcription factors are specialized targets for several miRNAs. miR-200 inhibits TGF induced EMT in human breast CSCs (Shimono et al., 2009). The equivalent of this miRNA might control EMT in different cancer subtypes. CSCs are important targets in cancer therapy due to their distinct differences with normal cells in the body and could be considered as highly effective targets in cancer therapy.
7 THE CLINICAL APPLICATION OF miRNA AS PCA BIOMARKERS
Owing to their key role in cancer pathogenesis, miRNAs have demonstrated a wide spectrum of possible utility as diagnostic, prognostic, or predictive biomarkers for both primary and metastatic tumors (Cortez et al., 2011; Walter, Valera, Pinto, & Merino, 2013). miRNAs possess many traits that make them significant biomarkers. The basis of this application is to suppress oncogenic miRNAs and delivery of miRNAs with tumor suppressor properties.
7.1 miRNA as diagnostic markers in PCa
Clinical stage, Gleason score, and PSA level provide the prevalent parameters for PCa diagnosis. miRNAs confer beneficial information beyond these parameters, and by combining them into these parameters miRNAs can improve these clinicopathological parameters and diagnostic and prognostic effectiveness. A study by Haj-Ahmad et al. (2014) discovers six overexpressed miRNAs (miR-234, miR-1238, miR-1913, miR-486-5p, miR-1825, and miR-484) in urine that was capable to distinguish between PCa patients and BPH. miR-222-3p* miR24-3p/miR30c-5p are three miRNA model useful for discrimination between BPH and PCa. These are detectable in urine samples of patients (Fredsøe et al., 2017). Nevertheless, this specific field requires further development to ascertain a better plasma/serum/urine signature, providing an insight to determine men with potential PCa risk through a fast, easy, and noninvasive test. A remarkable potential obstacle is a pollution from bladder and kidney epithelial material; the potential application of plasma miRNA test looks to be more developed. Searching for miRNAs that are expressed in common between extracellular and intracellular contexts a group of 29 miRNAs, including let-7a/b/c/i, miR-15b, miR-17, miR-20a, miR-21, miR-24, miR-25, miR-26a/b, miR-31, miR-32, miR-34b, miR-93, miR-106a, miR-141, miR-143, miR-145, miR-148a, miR-155, miR-182, miR-187, miR-200b, miR-218, miR-221, miR-223, and miR-375. Among these miRNAs, some of them have been related with a functional mechanism in the PC onset; For example, the let-7 family and miR-145 exert a paramount role in modulating the tumor cell proliferation, either via modulation of both CCND2 and c-Myc mRNAs (Sachdeva et al., 2009; Wagner, Ngezahayo, Murua Escobar, & Nolte, 2014). miR-205, miR-214, miR-221, and miR-99b were the purpose of another study. All these four miRNAs were available in detectable concentrations in urine. Amount of miR-205 and miR-214 were remarkably lower compared with healthy control cases (Srivastava et al., 2013a). Not only underexpressed miRNAs are being used as diagnostic markers but also overexpressed miRNAs are important for diagnosis. According to a study, miR-148a and miR-375 are two important overexpressed miRNAs being used for detection of the disease (Stuopelyte et al., 2016).
By progression of knowledge about miRNA profile in PCa, a number of distinct miRNAs should be selected as indicators to get used in diagnosis.
7.2 miRNAs as prognostic markers in PCa
To date, expression profile evaluations showed potential miRNAs as prognostic factors with some stable outcomes, even if dissimilarities in methodologies and patient choice are the crucial problems to reach a ubiquitous expression pattern. Assessment of PCa prognosis is a challenging issue and now is contingent upon histopathological parameters (such as Gleason score) alongside with PSA levels, which do not precisely reverberate disease status invariably. A bulk of studies has shown a critical utility of particular miRNA expression patterns to help in connecting PCa with its aggressive symptoms (Lichner et al., 2013; Shen et al., 2012; Spahn et al., 2010; Walter et al., 2013), either alone or alongside with prevalent prognostic features (Selth et al., 2013). The expression of miR-125b (Shi et al., 2007), miR-21 (Ribas et al., 2009), and miR-141 (Catto et al., 2011) are regulated through androgen responsive element, which modifies transcription of these miRNAs and, therefore, the blockage of their targets. miR-331-3p also associates with modulation of androgen receptor (AR) pathway, since overexpression of its target, erb-b2 receptor tyrosine kinase 2, has been related to disease development and AR signaling (Epis, Giles, Barker, Kendrick, & Leedman, 2009). Other miRNAs, such as miR-141, miR-143, and miR-145 have been discovered in relation to tumor cell migration. miR-141 is upmodulated in metastatic cells from PCa patients and its transcript level was related to Gleason score (Brase et al., 2011; DeVere White, Vinall, Tepper, & Shi, 2009). Downregulation of miR-143 and miR-145 was correlated with progression and development of PCa (Zaman, Deng, & Dahiya, 2010) and metastasis (Peng et al., 2011; Saini, Majid, & Dahiya, 2010; Szczyrba et al., 2010) and it was also related with Gleason score (Peng et al., 2011). PCa metastasis has been also connected with the downregulation of miR-16, miR-34a, miR-126*, miR-205, and miR-146a and upregulation of miR-301 and miR-125b. The miR-200 family regulates the EMT and was its loss of expression was recognized in cancer tissues (Ambs et al., 2008; Porkka et al., 2007; Tong et al., 2009). Various miRNAs like miR-1, miR-31, and miR-205 were also connected with Gleason scores; miR-125b, miR-205 and miR-222 with tumor stage; miR-1 with pT stage; miR-1, miR-10, miR-30c, miR-100, miR-125b, and miR-224 with perineural invasions status; and miR-96 with biochemical progression. An investigation discovered that miR-182 transcription was potentially related to the prognostic factors of biochemical progression-free survival and progression-free survival. A relation was observed between miR-182 transcript level and Gleason score, in which patients with Gleason score < 7 and downregulation of miR-182 were regarded at lower risk of cancer development relative to those with Gleason score > 7 and miR-182 upmodulation (Casanova-Salas et al., 2014). Recently, a tool named miQ has been introduced as the prognostic approach, which involves a combination of miR-96-5p, miR-221-5p, miR-183-5p, and miR-145-5p, with the prognostic ability to forecast cancer aggressiveness, metastatic status, and overall survival in a Dutch cohort (Larne et al., 2013). Recently, upregulation of let-7a and miR-141 was discovered to be related to PCa incidence (Westermann et al., 2014). Looking for miRNA signature that is expressed in both extracellular and intracellular conditions, resulted in the identification of a category of seven miRNAs, including let-7a, miR-141, miR-145, miR-195, miR-221, miR-375, and miR-451. A functional mechanism in PC onset has been introduced for some of these miRNAs, including miR-141, miR-195, and miR-375, which have been related to PC metastasis (C. Liu et al., 2015; Szczyrba et al., 2010; Zhang et al., 2013) while miR-145 and miR-221 may regulate proliferation of PC cells (Ozen, Creighton, Ozdemir, & Ittmann, 2008; Wang et al., 2015a).
7.3 miRNA as predictive markers in PCa
As a substitute indicator, miRNAs have the potential to be fascinating biomarkers because they are comparatively compatible with biological fluids, easy to consider and sustain during storage handling. The abnormal miRNA profile in cancer tissue, serum or plasma, and urine can be a critical biomarker for the diagnosis of PCa, prognostic prediction or therapy efficiency (Cannistraci et al., 2014). A study evaluating the miR-21 levels in sera revealed upregulation in CRPC patients, particularly in cases who did not respond to chemotherapy through docetaxel-based approach. Therefore, miR-21 can be a beneficial marker to reveal the transformation to hormone-resistant disease, and a critical predictor of the influence of docetaxel-based chemotherapy (Zhang et al., 2011). Other investigation showed that miR-141 as a critical biomarker for indication of therapeutic response in PCa. Serum miR-141 can be a new predictive biomarker for PCa progression when compared with ratified biomarkers such as PSA and circulating tumor cells. Nevertheless, miR-141 was less specific than PSA (Gonzales et al., 2011). Therefore, miRNAs should be compounded with other ratified biomarkers to increase their success. The transcript levels of miRNAs derived from prostate tissue have been analyzed and showed from numerous laboratories. Utilizing radical prostatectomy samples, miR-21, miR-200a, miR-145, miR-30d, miR-301a, miR-449b, and miR-182 were identified as biomarkers to forecast biochemical recurrence after prostatectomy (Casanova-Salas et al., 2014; Li et al., 2012; Mortensen et al., 2014). Additionally, Goto et al., (2014; 2015; 2016) demonstrated that miR-27b, miR-222, and miR-452 could be advantageous biomarkers predicting progression time to CRPC. Serum levels of miR-21, miR-375, miR-378*, miR-141, miR-201, miR-200c, miR-423-3p, and miR-210 were upmodulated in CRPC patients (Brase et al., 2011; Cheng et al., 2013; Nguyen et al., 2013; Shen et al., 2012; Watahiki et al., 2013). miRNA levels in urine have been investigated in several cancers such as bladder cancer, renal cell carcinoma, and PCa (Huang, Liang, Dittmar, & Wang, 2013). Recently, it has been indicated that miRNA levels in urine samples from PCa patients are importantly altered compared with that from benign prostate hypertrophy patients. They found that raised levels of urinary miR-100 and miR-200b were competent parameters to find the presence of PCa with increased PSA levels found in the gray zone of the prostate (Salido-Guadarrama et al., 2016).
8 miRNA INVOLVEMENT IN DRUG RESISTANCE
To date, aberrant expression of 50 miRNAs has been established in human PCa (about 40 overexpressed and remaining underexpressed), though, the knowledge about the influence of miRNAs on the circumstance of anticancer drug responses is still confining.
8.1 miR-21 and role in docetaxel-resistance
The miR-21 is considered as one of the frequently implicated miRNAs in cancer. In PCa, miR-21 takes part in tumorigenesis and invasiveness of the tumor through targeting the mRNA of PTEN, programmed cell death 4 (PDCD4), BTG2, and RECK. A study showed that miR-21 can downregulate PDCD4 expression directly and, therefore, suppresses the tumorigenesis in PC3 cells (Lu et al., 2008). Utilizing thePDCD4 microarray technique, Shi et al. (2010) demonstrate a significant alteration in the transcription of several miRNAs in the docetaxel-resistant PC3 cells (PC3R). Among these differentially expressed miRNAs, miR-21 was overexpressed in PC3R cells, eventuated in raised resistance of PC3 against docetaxel in the wild-type cells. In contrast, using transient transfection to target miR-21, the cells became sensitive to docetaxel. miR-21 functions as an outstanding regulator of drug resistance to docetaxel in PCa patients, providing novel insights that miRNAs may exert a role in the tumor resistance to chemotherapy. In other investigation (Folini et al., 2010), miR-21 was overexpressed in docetaxel-resistant PC3R cells as well. Likewise, Shi et al. (2010) demonstrated that dysregulation of miR-21 expression enhanced the resistance against docetaxel in PC3 wild-type cells, whereas inhibition of miR-21 expression in PC3R cells eventuated insensitivity of these cells toward docetaxel. On the other side, Zhang et al. (2011) identified that miR-21 levels in sera were upmodulated in hormone-resistant prostate cancer (HRPC) patients than those with androgen-dependent prostate cancer and localized PCa. Increased levels of miR-21 in sera of HRPC patients resulted in resistant to docetaxel-based chemotherapy. Level of miR-21 in sera was related to the serum level of PSA in metastatic PCa patients. Their results showed that miR-21 might be a crucial biomarker for PCa patients during disease progression.
8.2 miR-143 and role in docetaxel-sensitivity
Ahmad et al. (2013) findings demonstrated a marked relation between low miR-143 and augmented ERK5 levels in primary human PCa. miR-143 participates in contributing to suppressing tumor development. Upregulation of miR-143 suppresses migration of PCa cells in vitro. miR-143-treated PCa cells demonstrated promoted sensitivity to chemotherapy by docetaxel, implying to a beneficial function of miR-143 in chemotherapy (Xu et al., 2011).
8.3 miR-205 and miR-31 and playing role in docetaxel-resistance
Bhatnagar et al. (2010) reported downmodulation of miR-205 and miR-31 in progressed PCa cells. Upmodulation of miR-205 and miR-31 downregulated Bcl-w and E2F6. In contrast, transfecting to inhibit miR-205 and miR-31 in WPE1-NA22 cells culminated raised protein levels of Bcl-w and E2F6, respectively. Moreover, the anti-miR-205 and anti-miR-31 also inhibited apoptosis in WPE1-NA22 cells due to docetaxel. Cells with stable expression of E2F6 demonstrated refractory to cisplatin and docetaxel.
8.4 miR-34a and its role in paclitaxel and camptothecin-resistance
It was observed that miR-34a was downregulated in androgen-resistant Du145 and PC3 cells and differed from androgen-sensitive LNPCa and healthy prostate epithelial cells. Additionally, transcription of miR-34a is contingent upon the p53 function in PCa cells and seems not to be expressed in p53-null PCa cell lines. Kojima et al. (2010) showed that miR-34 directly and indirectly targets the 3’-UTR of SIRT1 and Bcl2 mRNAs and suppresses their expression by modifying HuR expression (Kojima, Fujita, Nozawa, Deguchi, & Ito, 2010). Downregulation of miR-34a leads to upregulation of SIRT1 and Bcl2, consequence in resistance to apoptosis due to paclitaxel. Singh et al. (2012) showed diminished expression of miR-34a in DU145-TXR and PC3-TXR cells and PCa tissue. Experimental replenishment of miR-34a in cultured cells prevented metastasis and invasion. They concluded that paclitaxel-based chemoresistance in both PC3-TXR and DU145-TXR cells was probably regulated through miRNAs.
8.5 miR-148a and its role in paclitaxel-resistance
Fujita et al. (2010) demonstrated downregulation of miR-148a in PC3 and DU145 hormone-resistant cells. On the other hand, overexpression of miR-148a led to downregulation of MSK1 and prevented progression, migration, and invasion of PC3 cells. Moreover, paclitaxel-resistant cell line from PC3 cells (PC3PR) demonstrated declined resistance to paclitaxel mediated through miR-148a.
8.6 miR-200 family and role in docetaxel- and paclitaxel-resistance
miR-200 family members could prevent EMT and suppress tumor invasion through repression of the translation factors ZEB1 and ZEB2 directly. Upmodulation of miR-200b causes repression of PCa cell expansion and migration and augmented sensitivity to docetaxel-based chemotherapy through targeting mRNA of B-cell-specific Moloney murine leukemia virus insertion site 1 (Yu et al., 2014). Screening for critical modulators of epithelial phenotype showed a significantly diminished expression of miR-200c in docetaxel-refractory cells (Puhr et al., 2012).
8.7 Let-7c
Nadiminty et al. (2012a; 2012c) data revealed that let-7c level was declined in the castration-refractory cell lines C4-2B, LN-IL6, and LNCaP-s17 compared with controls. Downregulation of let-7 eventuated in the raised synthesis of inner membrane protease-1, which in turn culminated in raised levels of MDR1 protein in tumor cells. MDR1 functions in transporting of microtubule-targeting drugs from intra to extracellular spaces and its role have been implicated in chemoresistance (Pekarik, Gumulec, Masarik, Kizek, & Adam, 2013). On the other hand, miR-let-7c represses AR through targeting c-myc, patients with downregulated let-7c would be more sensitive to androgen depletion as a therapeutic approach (Nadiminty et al., 2012b).
9 MIRNAS AND APOPTOSIS
Research done by Shi et al. (2011) demonstrated that miR-125b leads to downregulation of three important proapoptotic genes, p53, p53 upregulated modulator of apoptosis (PUMA), and Bak1 (Li et al., 2011) in which increased levels of oncomiR-125b are followed by decreased levels of p53 and it is followed by tumor cells survival.
miR-15a and miR-16-1 are located at fragile sites of DNA on ch13q14. Partial or whole genomic loss in these sites is associated with tumorigenesis and metastasis. Downregulation of these two miRNAs is related to advanced levels of PCa.
Several oncogenes such as BCL2, CCND1 and WNT3A are regulated by the cluster of miR-15a and miR-16-1, downregulation of this cluster increases the survival rate of PCa cells under androgen depletion circumstance (Li et al., 2011). Above all, it seems miR-16 could be considered as a therapeutic tool.
miR-34a, miR-34c, miR-15a, and miR-204-5p in PCa directly target BCL-2, which has antiapoptotic properties, and inhibits its function and subsequently leads to apoptosis initiation and increased sensitivity to chemotherapy agents. Antiapoptotic effects of bcl-2 are due to the inhibition of proapoptotic genes such as PUMA and NOXA (L Jackson, Grabowska, & L Ratan, 2014). Apoptosis could be controlled either by intrinsic and extrinsic pathways each by different miRNAs and different signaling pathways.
miR-19b, miR-23b, miR-26a, and miR-92a have been reported to target PTEN and induce prostate cell proliferation. In contrast downregulation of PTEN (which is a tumor suppressor) is followed by activation of the PI3K-AKT pathway helping tumor progression first by decreased apoptosis and secondly by increased survival signals (Di Cristofano & Pandolfi, 2000).
miR-21 is common onco-miRNA among different cancer subtypes. In PCa it targets PTEN and PDCD4 leading to the presentation of CRPC. Overexpression of miR-21 leads to androgen-dependent PCa growth and downregulation of transforming growth factor β receptor II (TGFBR2) expression in PCa (Mishra et al., 2013). miR-21 has a wide range of activities in different cellular processes, with this in mind; we mentioned its role in PCa, which is due to its different signal transduction pathway in PCa.
10 miRNA AS TARGETS FOR TREATMENT OF PCA
Identification of miRNA roles in PCa development could lead to the generation of novel miRNA-based treatments. The basic strategies in miRNA development as therapeutic tools would consist: (a) targeting the 3’-UTR of the oncogenes regulated by PCa-downregulated miRNA, utilizing miRNA mimic synthetic products; (b) returning physiological levels of intracellularly overexpressed miRNAs, by utilizing antago-oligonucleotide (antagomiR) or synthetic constructs that reduce the cells of particular miRNAs (called miRNA sponge); and (c) compounding miRNA-based tools with existing antitumor medicines. A diagnostic, prognostic, and therapeutically suitable molecule might be a contributing factor in forecasting disease strength and establishing advantageous therapy against PCa. Treatments for the human disorder by RNA inhibition consist of drug arrangement against mRNA participating in the mechanobiology of the disease and direct targeting of noncoding RNAs participating in the disease pathogenesis. The primary RNA inhibitory agents utilized in clinical studies are antisense oligonucleotides (ASOs), ribozymes, DNAzymes, small interfering RNAs (siRNAs), short hairpin RNAs, and anti-miRNA agents, such as ASO-anti-miRNAs, locked nucleic acids-anti-miRNAs, or antagomiRs, class of bicyclic high-affinity RNA targeting miRNA. Recently an investigation has demonstrated competent miRNA delivery techniques utilizing PCa-targeted nanoparticles (R11-SSPEI; Zhang, Xue, He, & Hsieh, 2015). They demonstrated that R11-SSPEI/miR-145 peptide prevents intraperitoneal immunized PC3 cancer progression in vivo. miR-16-conjugated atelocollagen was revealed to prevent bone metastatic human prostate xenograft proliferation in the mouse bone in vivo (Takeshita et al., 2010). miR-34 exerts a tumor suppressor function in PCa (Bader, 2012); consequently, this miR-34 delivery system will be fruitful for PCa treatment or, at least, amelioration of clinical symptoms in future (Bahrami et al., 2017; Najafi-Hajivar et al., 2016; Siahmansouri et al., 2016). Several therapeutics based on miRNAs have already well-passed Phase II clinical examinations (Frankel & Lund, 2012). Inhibitions of tumor cell expansion and migration with genistein, a small biologically active flavonoid, has been discovered to act by silencing oncogenic miRNAs like miR-21, miR-151, miR-221, and miR-222, which mediates their function through silencing the Notch signaling, RAC1/VEGF-associated angiogenesis, and upregulation of cancer suppressor gene aplysia ras homology member I. On the contrary, genistein suppresses cell expansion via cancer suppressor miR574-3p overexpression (Chiyomaru et al., 2013). A natural agent, isoflavone, was discovered to modify methylation sites of DNA encoding miR-29a and miR-1256, resulting in their overexpression and downregulation of TRIM68 and PGK-1, which prevents PCa cell expansion and invasion (Frankel & Lund, 2012). The first observation of a miRNA signature aberrantly expressed in response to radiation treatment (RT) was reported in 2008 (Josson, Sung, Lao, Chung, & Johnstone, 2008), showing six miRs (miR-512, miR-196a, miR-133b, miR-143, miR-145b, and miR-218) as outstandingly downmodulated in PCa cell lines, both in androgen interdependent or refractory models. Specifically, miR-521 was discovered to be downmodulated significantly, and its forced overexpression was capable to sensitize cells in vitro to RT, opening new horizons in the possible application of miRNAs as therapeutic agents. Li et al. (2011) and Shi et al. (2011) showed that miR-106b could also be utilized for targeted therapy because its overexpression was enough to override cell cycle stop in PCa cells following RT, by the adaptation of its ratified target p21 (CDKN1A). To note, this miRNA was previously discovered to be upregulated in PCa (Ambs et al., 2008). A miRNA that looks to be a promising molecule for PCa treatment is miR-34a, owing to its known association with p53 mRNA. The gene encoding miR-34 is hypermethylated in a tumor, leading to prevention of miR-34a expression (Cheng et al., 2014). Upregulation of both miR-34a and miR-34c eventuates in increased p53-mediated apoptosis in response to doxorubicin therapy in PCa cell lines (Rokhlin et al., 2008). Nevertheless, miR-34a looks to be the more promising molecule owing to its function has been observed in xenograft experiments, where its reintroduction appears to increase the prostate xenograft expansion (Yamamura et al., 2012). In addition, xenograft mouse models of PCa constructed via PC3 cell administration validated the capability of both miR-200b and let-7a to prevent PCa growth in vivo (Dong et al., 2010; Williams, Veliceasa, Vinokour, & Volpert, 2013). An obvious miR-221/222 action on cell growth and progression control has been established in vitro (Wang et al., 2015b) and in vivo in PC xenografted mouse models (Mercatelli et al., 2008). Therefore, let-7a, miR-15/16, miR-21, miR-141, miR-143, miR-145, miR-199a-3p, miR-200b, and miR-221/222 could be offered as a crucial therapeutic and diagnostic approach in PCa. miR-34, as one of the prognostic miRNAs in intracellular space related to increased Gleason score in PCa, has formerly been evidenced as an important miRNA-based therapeutic tool (Liu et al., 2011a).
11 CONCLUSION
The important application of miRNAs in the clinic as diagnostic or prognostic tools for PCa is underlying cumulative evidence obtained through examinations over the last decade. Several research demonstrated the clinical application of miRNAs in the monitoring of PCa. However, results differ regarding their correctness and the association with the tumor aggressiveness. Multiparametric approaches using diverse types of molecules hold the promise of increasing the specificity and sensitivity of molecular markers as diagnostic tests. Additionally, considering their values as disease and therapeutic biomarkers, miRNAs have great importance as therapeutic targets. Even though miRNAs seem as inherently stable molecules, optimization and standardization of approaches are needed to catch high quality and reproducible results. The best published results suggest the application of miR-141, miR-34, and miR-21. Further investigations on developing the standards regarding the reports and assay are still required. Although miRNA arena looks to be interesting, however, it is not straightforward that much, implying to the potential diagnostic, prognostic, and therapeutic utility of miRNAs in clinical practice.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.