The chemokine system and its role in obesity
Abstract
The chemokine system is a complex arrangement of molecules that attract leukocytes to the site of injury or inflammation. This chemotactic behavior gives the system the name “Chemokine.” The intricate and redundant nature of the chemokine system has made it a subject of ongoing scientific investigation. Obesity is characterized as low-grade systemic or chronic inflammation that is responsible for the release of cytokines, adipokines, and chemokines. Excessive tissue fat expansion triggers the release of chemokines, which in turn attract various leukocytes and activate the resident immune surveillance system, eventually leading to worsening of obesity and other related comorbidities. To date, 50 chemokines and 20 chemokine receptors that belong to the G-protein-coupled receptor family have been discovered, and over the past two decades, the physiological and pathological roles of many of these chemokines and their receptors have been elucidated. The objective of this review is to present an update on the link between chemokines and obesity under the light of recent knowledge.
1 INTRODUCTION
Chemokines are low molecular weight (8–13 kD), small heparin-binding proteins and chemotactic factors that are released from various cells within the body (Baggiolini, Dewald, & Moser, 1997). Chemokines are also known as chemotactic cytokines that direct the movement of circulating leukocytes to the sites of inflammation or injury. In addition, chemokines activate the production and secretion of inflammatory mediators (Charo & Ransohoff, 2006). A great expansion in chemokine biology has occurred in recent times (Acker, Voss, & Timmerman, 1996). According to a recent update, there are approximately 50 chemokines acting on 23 discrete receptors (Edderkaoui, 2017).
2 MECHANISMS OF REGULATION OF THE CHEMOKINE-RECEPTOR NETWORK
The interaction of chemokines with G-protein-coupled receptors (GPCRs) promotes the migration of leukocytes, thus producing an inflammatory response to injury and infection. There are various biochemical and cellular mechanisms by which the interactions of chemokines with chemokine receptors are regulated: selective and competitive binding interactions; genetic polymorphisms; messenger RNA splice variation; variation of expression, degradation, and localization; downregulation by atypical receptors; interactions with cell-surface glycosaminoglycans; posttranslational modifications; oligomerization; alternative signaling responses; and binding to natural or pharmacological inhibitors (Stone, Hayward, Huang, Huma, & Sanchez, 2017). It has long been recognized that a hallmark feature of the inflammatory response is the accumulation of leukocytes (white blood cells) in injured or infected tissues, where they remove pathogens and necrotic tissue by phagocytosis and proteolytic degradation. A major advance in the understanding of the molecular mechanisms underlying leukocyte migration (trafficking) was the discovery of chemokines and chemokine receptors (Baggiolini, 2001; Moser, Wolf, Walz, & Loetscher, 2004). Chemokines are small proteins expressed in tissues during normal immune surveillance or in response to injury or infection. They subsequently bind and activate chemokine receptors, GPCRs embedded in the cell membranes of leukocytes, thereby inducing leukocyte adhesion to the vessel wall, morphological changes, extravasation into the inflamed tissue, and chemotaxis along the chemokine gradient to the site of injury or infection (Moser et al., 2004). In addition to their roles in leukocyte trafficking, chemokine activation of chemokine receptors can give rise to a variety of additional cellular and tissue responses, including proliferation, activation, differentiation, extracellular matrix remodeling, angiogenesis, and tumor metastasis (Ben-Baruch, 2006; Luther & Cyster, 2001; Salcedo & Oppenheim, 2003; Speyer & Ward, 2011; Szekanecz & Koch, 2001). For example, Rezaeeyan, Shirzad, McKee, and Saki (2018) recently concluded in a review that chemokine receptors can lead to cancer metastasis in different organs based on the nature of cancers. Due to their central roles in inflammation, many chemokine receptors (and, to a lesser extent, chemokines) have been identified as potential therapeutic targets in a wide range of inflammatory diseases (Proudfoot, 2002).
3 CHEMOKINES AND OBESITY
Obesity in humans, which is triggered by consumption of a high-calorie diet, is associated with increased incidence of, severity of, and mortality from cardiovascular disease, metabolic syndrome, and different types of cancer. However, adipose tissue expansion also depends on the interactions between white adipose tissue (WAT)—which is the primary site of energy storage serving as the body's largest endocrine organ secreting hormones, cytokines, and growth factors that affect cell function on the local and/or systemic level—and brown adipose tissue (BAT), which is rich in mitochondria that burn energy through activity of uncoupling protein 1 (UCP1) factors (Yao, Heuser-Baker, & Barlic-Dicen, 2014). CCL2 seems to play a role in obesity-associated infiltration of macrophages into adipose tissue in mouse models and in humans (Weisberg et al., 2003; Xu et al., 2003). Obesity is associated with macrophage accumulation in adipose tissue, but functional data are not clear (Chow et al., 2007; Inouye et al., 2007). Within the CC chemokine family, different ligands can bind to different receptors with overlapping specificities (Bacon et al., 2001; Charo & Ransohoff, 2006). Therefore, chemokines and receptors responsible for monocyte infiltration have to be analyzed in a comprehensive manner to understand better the regulation of the inflammatory process in the adipose tissue of obese patients (Huber et al., 2008). Chemokines and chemokine receptors facilitate the development of obesity by promoting and/or supporting inflammatory leukocyte influx, especially, recruitment of proinflammatory monocytes, into hypertrophic fat tissue (Yao, Heuser-Baker, & Barlic-Dicen, 2014). One of the earliest studies that shed light on any kind of involvement of chemokines in energy metabolism was presented by Plata-Salaman and Borkoski (1994). It remarkably suggested the roles of MCP-1/MCAF and RANTES in decreasing the short-term (2-hr) food intake; it showed that CXCL8 and CXCL4 could decrease the total daily food intake, and these were, hence, implicated in lipid metabolism and obesity through neuromodulation. A detailed account of chemokines on hypothalamic inflammation and energy balance can be read elsewhere (Le Thuc et al., 2017). The importance of subcutaneous (SC) fat in systemic lipid metabolism is a well-established fact (Tran, Yamamoto, Gesta, & Kahn, 2008). SC fat is intrinsically different from visceral fat, and several studies have positively associated it with weight reduction, diabetes, and obesity (Shimizu, Yoshida, & Minamino, 2015). A recent study connected UV-induced adipochemokines with impairment of fat homeostasis. It was revealed that CXCL5, CCL20, and CCL5/RANTES are induced by UV light in SC fat, which then impair triglyceride synthesis via downregulation of lipogenic enzymes (E. J. Kim et al., 2018). Similar findings were previously derived by Huber et al. (2008), showing increased expression of CC chemokines and their respective receptors in SC and visceral adipose tissue of obese patients, which coexisted with increased systemic inflammation. Another study recently pointed out the role of the Duffy antigen receptor for chemokines in promoting insulin resistance and adipose tissue inflammation (Benson et al., 2018). Evidence has also established obesity-induced prostate cancers, depending on the proinflammatory CXCL12-CXCR4/CXCR7 Signaling Axis (Saha et al., 2017). Subtle changes in the behavior of chemokines was observed recently by Barry, Simtchouk, Durrer, Jung, and Little (2017) in a clinical study that included 37 inactive obese adults, subjected to different modules of exercise for 2 weeks, involving 10 sessions in total. It was proved that moderate-intensity continuous training reduced the percentage of monocytes positive for C-C chemokine receptor type 2 (CCR2) and decreased surface protein expression of CXCR2 on monocytes. However, the surface expression of CCR5 was increased. Other than this, there are a few scientific reports that showed the participation of chemokines in lipid accumulated states in liver (Kanda et al., 2006; Weisberg et al., 2006).
The role of chemokines in obesity is well reported, but the data lacks coherence and analysis. Hence, this review aims to present a brief update about chemokines or adipochemokines and their reported involvement in obesity, with significant upgraded data available. Cytokines and adipokines, despite having a great impact on lipid metabolism—according to a growing body of evidence—are beyond the scope of this review.
3.1 CCL2
CCL2, commonly known as MCP-1 (monocytes chemoattractant protein 1), is one of the widely studied chemokines that act on CCR2 that are present on, but not restricted to, monocytes, macrophages, (T helper) cells, basophil, and natural-killer cells (Huma et al., 2017). MCP-1 was discovered in conditioned media of human myelomonocytic cell line and initially named monocyte chemotactic factor (Matsushima, Larsen, DuBois, & Oppenheim, 1989). Interestingly, CCL2 has significant effects in nonalcoholic fatty liver disease (NAFLD; Townsend & Newsome, 2017), obesity (Xia & Sui, 2009), and other lipid overload states (Chistiakov, Melnichenko, Grechko, Myasoedova, & Orekhov, 2018; Franca et al., 2017). MCP-1 is found to be higher in obese patients as compared with lean individuals in plasma, both in children (Breslin et al., 2012) and adults (Catalan et al., 2007). A clinical study by Cox et al., (2011) consisting of older overweight/obese men and women showed worsening of MCP-1 levels when fructose was administered for 10 weeks. On the contrary, a low-glycemic diet reduced the MCP-1 levels in older, obese prediabetic patients (Kelly et al., 2011). However, greater details into the mode of action of MCP-1 in obesity were provided by Sukumar, Partridge, Wang, and Shapses (2011), who showed that in obese eligible women, adiposity alone could not explain the higher levels of MCP-1 in obesity, but the presence of raised parathyroid hormone levels has direct effects on MCP-1 in plasma. Another clinical report presented by Gokulakrishnan et al., (2017) showed improvement in diabetes and weight gain after a 4-months-long life-style improvement plan, demonstrating a corresponding MCP-1 decrease. It was also found to be higher in plasma in children with a waist-to-height ratio more than 0.5 (Mendes et al., 2017). Similar results were obtained in rodents where raised levels of MCP-1 were found in obese individuals as compared with leaner individuals (Higa, Liu, Berry, & Panee, 2011; Sartipy & Loskutoff, 2003). Interestingly, CCX872, a blocker of CCR2, has shown improvement in hepatic inflammation and fibrosis in murine models of NAFLD (Parker et al., 2018). The finding was validated by Weisberg et al., (2003), who showed that CCR2-deficient mice were protected from deposition of visceral fat and insulin resistance even under HFD (high-fat diet) states. The raised levels of CCL2 in obese women of reproductive age as compared with nonobese counterparts are another example of its implications (Crisosto et al., 2017). Pharmacological studies also approve the above notion as Freeze-dried strawberry and blueberry supplementation showed lower levels of MCP-1 corresponding to decreased insulin resistance and obesity (Aranaz et al., 2017). Similar pharmacological evidence was provided in a report representing the anti-adiposity effects of caffeine in HFD-induced rodents. This investigation also presented lower MCP-1 along with reduced adiposity (C. W. Liu et al., 2017). Likewise, other pharmacological studies on vitamin D (Farhangi, Mesgari-Abbasi, Hajiluian, Nameni, & Shahabi, 2017), Thymoquinone (Karandrea, Yin, Liang, Slitt, & Heart, 2017), Nobiletin (Namkoong et al., 2017), Green pea (Bibi, de Sousa Moraes, Lebow, & Zhu, 2017), Grape seed proanthocyanidin extract (W. Liu et al., 2017), Chondroitin Sulfate (Stabler, Montell, Verges, Huebner, & Kraus, 2017), Alantolactone (M. Kim, Song, & Kim, 2017), Rosmarinic Acid (Rui et al., 2017), Tartary Buckwheat Extracts (Lee et al., 2017), and Quercetin (Yang et al., 2017) also drew parallels between reduced MCP-1 levels with obesity/adiposity protection. Reports also confirmed the role of MCP-1 in other lipid metabolic disorders than obesity, which is beyond the scope of this review but can be read elsewhere (Panee, 2012).
3.2 Regulated on activation normal T cell expressed and secreted
RANTES (regulated on activation normal T cell expressed and secreted) was initially considered to be secreted by T cells, but later reports proved that it is expressed by many other cell types, including epithelial cells and platelets, and acts as a major chemoattractant for cells such as monocytes, NK cells (Loetscher, Seitz, Clark-Lewis, Baggiolini, & Moser, 1996), memory T cells (Schall, Bacon, Toy, & Goeddel, 1990), eosinophils (Rot et al., 1992), and dendritic cells (DCs) (Dieu et al., 1998). CCL5 belongs to the C-C chemokine family, along with CCL3 and CCL4, which attaches to its receptor known as CCR5. CCR5 is a promiscuous seven-transmembrane G-protein-coupled receptor, modulating multiple signaling cascades in response to its ligands (Oppermann, 2004; Soria & Ben-Baruch, 2008). The signal transduction of RANTES is beyond the scope of this review, but it can be read in detail in Aldinucci & Colombatti, 2014; de Oliveira et al., 2014; Oppermann, 2004. The role of RANTES in obesity is still obscure, but there is a group of studies that have continuously reported it (Yao, Herlea-Pana, Heuser-Baker, Chen, & Barlic-Dicen, 2014). Though the involvement is progressing, there is no review collecting relevant data at one place. We have made an effort to gather such data for scientific convenience. For instance, Pisano et al. (2017) reported higher levels of RANTES in antipsychotic-induced weight gain. Just recently, another reported linked UV light-induced impaired fat metabolism with a parallel rise in RANTES levels (E. J. Kim et al., 2018). An important study in this direction was put forward by Keophiphath, Rouault, Divoux, Clement, and Lacasa (2010), showing participation of CCL5 in inflammation of obese WAT by recruiting blood monocytes and exerting antiapoptotic properties on WAT macrophages. A much clearer study was performed by Kitade et al. (2012), which not only indicated the presence and upregulation of CCR5 and its ligands in WAT of DIO and ob/ob mice but also examined it in Ccr5 (−/−) mice. The study concluded that CCR5 plays a vital role in adipose tissues macrophage (ATM) recruitment and polarization. However, Kennedy and colleagues showed contrasting results (Kennedy et al., 2013), leading to suspicious functional dichotomy. CCL5/RANTES also functions as a neuroendocrine element that modulates food intake and body temperature in the hypothalamus through unidentified receptors (Chou et al., 2016; Guzman, Guevara-Martinez, Montes-Rodriguez, & Prospero-Garcia, 2006; Tavares & Minano, 2000). A cohort study involving 100 healthy volunteers fasted overnight, undergoing an oral lipid tolerance, and showed increase in RANTES levels, suggesting that dietary lipids affect immune function (Schmid et al., 2016). A clinical study, with participants including 24 hypertensive obese, 27 healthy, and 22 non-hypertensive obese children, presented that RANTES was significantly raised in pretreatment hypertensive and non-hypertensive obese groups than in the controls (Ovunc Hacihamdioglu, Zeybek, Gok, Pekel, & Musabak, 2015). In 2015, Montecucco et al. (2015) reported the presence of CCL5 in morbid obese patients before gastric bypass surgery. Similarly, Gao et al. (2015) noted lower levels of RANTES in phosphatidylethanolamine N-methyltransferase (Pemt (−/−) mice), which are mice resistant to HFD-induced obesity (DIO). More recently, E. J. Kim et al. (2018) showed that UV-induced CCL5 can impair TG synthesis in human adipocytes, while knockdown of the same receptor can reverse these changes, highlighting a critical role of CCL5 in lipid synthesis in general. Another important study revealed that CCL5 is one of the altered chemokines in HFD-induced obesity, which extends into prostate cancer (Hu et al., 2018). RANTES was found to have higher levels in adipose tissues (AT) of middle-aged females, which was in turn associated with cardiovascular disease (Ahnstedt et al., 2018). Besides, RANTES and its CCR5 receptor are well-established regulators of insulin resistance in obese individuals (Ota, 2013). Baturcam et al. (2014) investigated the effects of physical exercise on the expression of RANTES and CCR5 in obese humans. RANTES and CCR5 expression was higher in the SC adipose tissue of obese individuals compared with lean, which could be brough back to normal through physical exercise. The results were earlier verified by Wasinski et al. (2013).
3.3 Growth-regulated pep-tide alpha (GROα)-CXCL1
The primary source of proinflammatory cytokines in obesity is adipose tissue. Both resident immune cells in adipose tissue and adipocytes themselves can contribute to the increase in circulating cytokines. CXCL1, an adipochemokine also known as fractalkine, is scarcely implicated in obesity though it was clearly reported by Sindhu et al. (2017) recently. Currently, an interesting notion establishes linking CXCL1 with obesity-induced cancers. For example Zhang et al. (2016) observed that chemokines CXCL1 and CXCL8 chemoattract adipose stromal cells (ASC) by signaling through their receptors, CXCR1 and CXCR2, in cell culture models. It was later proposed by the same group that obesity-associated adipocyte death and the resulting recruitment of leukocytes trigger the IL-22 signaling cascade, which induces CXCL1 secretion by cancer cells responsible for ASC trafficking to tumors (Zhang & Kolonin, 2016). Important evidence was presented by Chang et al. (2015) implicating the role of hepatic CXCL1 expression in HFD-induced obesity combined with acute ethanol consumption. A land mark study was reported by Nunemaker et al. (2014) documenting raised serum levels of CXCL1 and CXCL5 in obesity, hyperglycemia, and impaired islet function. Intriguingly, contrasting results were presented in muscle-derived expression of CXCL1. Pedersen, Olsen, Pedersen, and Hojman (2012) found that overexpression of CXCL1 in muscles diminishes diet-induced obesity through a CXCL1-induced improvement of fatty acid oxidation and oxidative capacity in skeletal muscle tissue. A speculative conclusion can only be drawn out of these results that the CXCL1 origin (muscle or AT) controls the dichotomy of these dual effects. Prospectively, it can be a fruitful area for future research to not only know the exact role of CXCL1 in obesity but also ponder over its effects through origin differentiation.
3.4 plateletfactor-4 (PF4)-CXCR4
The chemokines system, consisting of chemokines and their respective chemokine receptors, directs inflammatory leukocytes into adipose tissue and is therefore thought to be an important promoter of obesity-induced adipose tissue inflammation (Surmi & Hasty, 2010). The chemokine receptor CXCR4 present in adipose tissue is expressed on adipocytes and ATMs (Hazan et al., 2002); however, its role in fat tissue remains unknown. CXCR4 signaling is connected to its cognate chemokine CXCL12. This chemokine receptor is unique because it is expressed in a wide variety of cell types, not only on leukocytes, as is the case with most other chemokine receptors. CXCR4 coordinates homing and cell trafficking, which are essential during embryonic cerebellar and cardiac and development and for homeostasis and regulation of the immune and stem cell systems (Bachelerie et al., 2014). The adipose tissue CXCR4 expression pattern suggests that CXCR4 controls obesity-induced leukocyte recruitment, homeostasis, adipose tissue inflammation, and functional responses of adipocytes (Cannon & Nedergaard, 2010). Studies have evidenced that the ablation of adipocyte, not myeloid leukocyte, CXCR4 exacerbated HFD-induced obesity, which was not a result of hyperphagia but was associated with increased adiposity and WAT and BAT hypertrophy. A study also showed that the obese phenotype in mice lacking CXCR4, specifically in adipocytes (adipocyte-specific CXCR4-knockout [AdCXCR4ko] mice), resulted in increased proinflammatory leukocyte content in WAT. In contrast, no significant difference in the number and immunophenotype of ATMs and lymphocytes was observed in the WAT of obesogenic diet-fed mice lacking CXCR4 in myeloid leukocytes (myeloid leukocyte-specific CXCR4-knockout mice) and wild-type C57BL/6 controls. Interestingly, HFD-fed AdCXCR4ko mice had significantly lower metabolic rates and strikingly different BAT (Enerback, 2010; Vosselman, van Marken Lichtenbelt, & Schrauwen, 2013). Notably, inactivation of adipocyte CXCR4 prevented the HFD-induced increase in the expression of UCP1, which acts as the main regulator of cold and diet-induced metabolic and adaptive nonshivering thermogenesis that, during exposure to cold or chronic overeating, increases energy expenditure to maintain body temperature or protect from obesity (Cannon & Nedergaard, 2010; Enerback et al., 1997; Matthias et al., 2000). The lack of UCP1 upregulation in the BAT of HFD-fed AdCXCR4ko mice was associated with the inability of these mice to maintain body temperature when exposed to cold. Thus, study suggests that contrary to other chemokine receptors, which promote obesity by supporting adipose tissue inflammation, CXCR4 limits it by preventing excessive inflammatory leukocyte influx into WAT and by supporting an increase in the thermogenic response of BAT (Matthias et al., 2000; Yao, Heuser-Baker, Herlea-Pana, et al., 2014). Alternatively, Peng, Zhang, and Zhu (2016) reported upregulated CXCR7 expression in adipose tissue and ATMs during obesity. CXCR7 neutralizing therapy expectedly attenuated inflammation and improved insulin sensitivity in obesity.
3.5 IL-8-CXCL8
Interleukin-8 (IL-8) is one of the proinflammatory cytokines, which might also have atherogenic properties (Straczkowski et al., 2003). Through its multiple actions, IL-8 might promote intimal thickening and atherosclerosis. These actions include recruitment of neutrophils and T lymphocytes into the subendothelial space, monocyte adhesion to endothelium (Gerszten et al., 1999) and migration of vascular smooth muscle cells (Tian-Li Yue, Mckenna, Gu, & Feuerstein, 1993). Macrophage-derived human foam cells contain high amounts of IL-8 (Liu, Hultén, & Wiklund, 1997). IL-8 is produced and secreted in vitro by human adipocytes (Bruun, Pedersen, & Richelsen, 2000). It has also been demonstrated that plasma IL-8 concentrations are increased in obese subjects with normal glucose tolerance and are related to body mass index, waist-to-hip ratio, percentage body fat, fat mass, and tumor necrosis factor-α system (Straczkowski et al., 2003). Recently, a remote report implicated CXCL8 in obesity-induced asthma, which may lead to severe tissue inflammation (Rutting et al., 2018). Earlier, Tse et al. (2015) marked the upregulated expression of CXCL8 after Huh-7 cells were induced with free fatty acid (FFA), which emulates in vitro steatosis. An important scientific report linking fatty liver with diabetes confirmed increased expression of CXCL8 in isolated primary pancreatic preadipocytes and differentiated adipocytes (Gerst et al., 2017).
3.6 Monocyte interferon gamma inducing factor (MIG)-CXCL9
The effects of CXCL9 on obesity or other lipid metabolic disorders are largely unknown, as scarce data are available to support the notion. However, a few studies have highlighted the preliminary results. For instance, a genetic study by Faucher et al. (2012) showed no observed association between single nucleotide polymorphisms (SNPs) and C-reactive protein (CRP) level in context of obesity. Similar noncontribution is reported in adipocytes (Vielma, Klein, Levingston, & Young, 2013). But its findings are more obvious in diabetes as CXC receptor 3, 9, and 10, which represents a novel target for therapeutic interference early in type 1 diabetes (Frigerio et al., 2002).
3.7 Interferon-gamma-inducible protein 9 (I-TAC)-CXCL11
Study reveals that circulating levels of Gro (CXCL1, -2, -3), CXCL9,-10, -11 (CXCR3 ligands), and sgp130, key players in the maintenance and amplification of autoimmunity-related inflammatory processes (Lacotte, Brun, Muller, & Dumortier, 2009), are elevated in obese subjects, whereas antiproliferative tumor growth factor concentrations are lower (Mayi et al., 2012). ATM overexpressed both (onco statin M) OSM family members (IL-6, LIF, OSM, and IL-11) and their receptors. Indirect coculture experiments on monocyte derived macrophages indicated that the phenotype of ATM is related specifically to the factors released by the preadipocytes, whereas the lipids released from mature adipocytes do not induce these effects. These data are in line with previous observations, suggesting the high proinflammatory potential of preadipocytes (Mack et al., 2009; Mayi et al., 2012).
3.8 BCA-1-CXCL13
CXCL13 (chemoattractant of B lymphocyte) is a fundamental chemokine responsible for the formation and maintenance of B lymphocyte follicles and ganglionic cells in the spleen and lymph nodes (Ansel et al., 2000) and has also been recognized in inflamed autoimmune-associated tertiary lymphoid organs (TLOs; Amft et al., 2001; Magliozzi, Columba-Cabezas, Serafini, & Aloisi, 2004). Transgenic CXCL13 expression in normal mouse islets is sufficient for full formation of ectopic lymphoid aggregates, a process that is lymphotoxin dependent (Luther, Lopez, Bai, Hanahan, & Cyster, 2000). Lymphotoxin blockade stops the development of diabetes in nonobese diabetic mice (Ettinger et al., 2001; Wu et al., 2001) and has been shown to reverse the insulitis (Wu et al., 2001). Therefore, the blockade of CXCL13 could reasonably be expected to disrupt this process in a similar fashion. In these studies, the data show the CXCL13 blockade basically disrupted B lymphocyte organizational morphology without altering their recruitment to islets. Additional questions yet to be explored include: the ways in which cellular organization affects dendritic cell and T-cell pathogenic function in islet TLOs, and whether these cellular clusters may have other functions not explored in this study, such as downregulation or modulation of the autoimmune response (Henry & Kendall, 2010). Hence, further studies are required to explore the mechanism that link CXCL13 with diabetes mellitus.
4 ADIPOKINES AND ADIPOSE TISSUE ANGIOGENESIS
AT angiogenesis is a developing concept of the formation of newer blood vessels in AT to accommodate the raised trafficking as a direct result of AT expansion. Greater expansion of ATs brings along the need for angiogenesis, which involves participation of adipokines, blood vessels, stromal cells, immune cells, and adipocytes themselves (Corvera & Gealekman, 2014; Nishimura et al., 2007). The secretary profile of several of the adipokines changes during AT expansion phase and angiogenesis. In addition to locally and systemically produced growth factors like vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and hepatocyte growth factor (HGF), locally produced adipokines (adiponectin, leptin, IL-6, angiogenesin, vaspin, visfatin, chemerin, omentin, resistin, etc.) play a critical role in the angiogenesis process (Adya, Tan, Punn, Chen, & Randeva, 2008; Cohen, Nahari, Cerem, Neufeld, & Levi, 1996; Kaur, Adya, Tan, Chen, & Randeva, 2010; Mu et al., 2006). The subject is so vast that it warrants a separate review to draw scientific attention. It is given here to remotely introduce a topic for future endeavors, and its prospective relationship with chemokines and lipid metabolism (Table 1).
| Chemokine name | Primary source | Receptor | Main effects | Main references |
|---|---|---|---|---|
| CCL2 (MCP-1) |
|
CCR2 | Recruits monocytes, memory T cells, and dendritic cells, chemotactic activity for monocytes and basophils, augments monocyte anti-tumor activity, autocrine loop in human osteoclast differentiation |
|
| CCL5 (RANTES) | T-cells, epithelial cells and platelets | CCR1, CCR3, CCR5 | Recruiting leukocytes, proliferation and activation of certain natural-killer (NK) cells, HIV-suppressive factor, autocrine loop in human osteoclast differentiation, T cell–DC interactions |
|
| CXCL1 (GROα) |
|
CXCR1, CXCR2 | Neutrophil trafficking, spinal cord development, angiogenesis, Inflammation, Wound healing, Tumorigenesis |
|
| CXCR4- PF4 | Present on newly generated neurons, adipose tissue | Receptor for CXCL12 | Potential HIV inection site on CD4+T cells, |
|
| CXCL8 | Macrophages, epithelial cells, airway smooth muscle cells | CXCR1, CXCR2 |
|
Bruun et al. (2000)Straczkowski et al. (2003) |
| CXCL9 | Macrophages | CXCR3 | Th1 response; Th1, CD8, NK trafficking |
|
| CXCL11 |
|
CXCR3 | Th1 response; Th1, CD8, NK trafficking | Mack et al. (2009); Mayi et al. (2012) |
| CXCL13 | Liver, spleen, lymph nodes, and gut of humans | CXCR5 | B cell and Tfh positioning LN, selectively chemotactic for B cells |
|
- Note. DC, dendritic cells; MCP-1: monocytes chemoattractant protein 1; NK: natural killer; RANTES: regulated on activation normal T cell expressed and secreted.
5 CONCLUSION
The role of chemokines in obesity is a subject of growing interest, and scientific data are still developing to reach any conclusive narrative. However, the existing data support the pleiotropic nature of chemokines, where most of the chemokines contribute to the obesity, development, or worsening of it. The mechanisms of the action by which they act remain largely obscure or misunderstood, and their pleiotropic nature adds to the scientific mystery. Among chemokines, a few have a more established role in obesity (MCP-1 and RANTES), while other require more extensive scientific investigation to reach a conclusion. A plethora of questions remain to be answered, including, how macrophage polarization is regulated by chemokines, the specific roles of 50 chemokines and 23 chemokines receptors in metabolic disorders, how chemokines expression is regulated during obesity, and so on. Further research is required to explore the molecular mechanism of chemokines and their linkage to metabolic disorders that can ultimately contribute to the development of novel drugs. Conclusively, chemokines present important therapeutic targets that could control immune system-mediated disorders and help combat obesity. (figure 1).
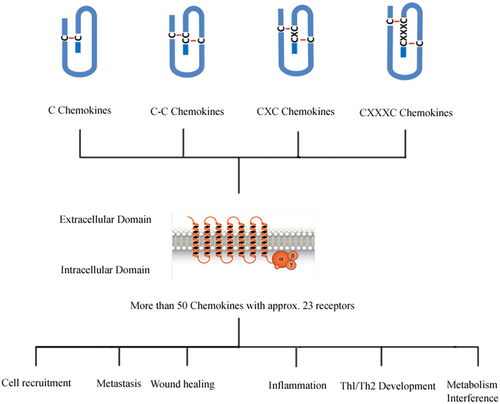
The scheme depicts the general structure and some of the common functions chemokines [Color figure can be viewed at wileyonlinelibrary.com]
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (Grant no. 31670895, 71673254), the National Key Research and Development Program of China—The construction and promotion of the demonstration system of based on telemedicine/Mhealth network (Grant no. 2017YFC0909900), Program of Science & Technology of Henan Province (Grant no. 201602037); special funds from the central government for the guidance of local science and technology development: Special funds of major science and technology project in Henan province (2016): Construction and demonstration application of medical and health big data analysis system based on telemedicine cloud platform (Grant no. 151100310800); Doctor research team fund from the First Afliated Hospital of Zhengzhou University for the In-Hospital Interdisciplinary Collaboration Research: The impact of air pollution exposure levels on lung cancer and its potential molecular mechanism, based on satellite remote sense big data (Grant No. 2016-BSTDJJ-15).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.