Endothelial committed oral stem cells as modelling in the relationship between periodontal and cardiovascular disease
Abstract
In the present study we have mimicked, in vitro, an inflammatory process using Lipopolysaccharide derived from Porphyromonas Gingivalis (LPS-G) and human Periodontal Ligament Stem Cells induced to endothelial differentiation (e-hPDLSCs). The research project has been organized into the three following steps: i) induction of hPDLSCs toward endothelial differentiation; ii) evaluation of the molecular signaling pathway involved in the response to the LPS-G, and iii) functional response evaluation of the living construct constituted by porcine decellularized valve/e-hPDLSCs treated with LPS-G. Obtained results showed that 5 μg/ml LPS-G stimulus provokes: a slowdown of cell growth starting from 24 hr and the release of IL6, IL8, and MCP1 molecules. Signaling network analyzed showed the activation of TLR4/ NFkB/ERK1/2/p-ERK1/2 signaling mediated by MyD88 in LPS-G stimulated e-hPDLSCs, moreover a time course put in evidence a nuclear traslocation of ERK1/2 and p-ERK1/2 in differentiated samples. Following, the ability of e-hPDLSCs to expand and colonize the decellularized porcine heart valves was appraised at ultrastructural level. Considering that, the Reactive Oxygen Species (ROS) play an important role in the progression and development of cardiovascular disease (CVD), in LPS-G living construct model e-hPDLSCs/decellularized porcine heart valves (dPHV), ROS production was assessed. Time lapse experiments evidenced that LPS-G provokes in e-hPDLSCs a rapid and sustained increase in ROS generation, negligible on undifferentiated cells. From obtained data, by multiparametric analyses, a reasonable conclusion may be that the inflammation process activated by LPS-G can affect endothelial cells and could represent in vivo a possible pathological and predictor state of CVD.
1 INTRODUCTION
Regenerative medicine represents a mishmash of biomedical science and engineering to restore cells, tissues, and organs. It includes multidisciplinary approach toward the development of biological materials capable to support normal functions in diseased or injured tissues, and includes three components: cells, biologically active factors, and biomaterials.
Stem cells research offers original occasions in developing new medical therapies aimed at debilitating diseases and in cell biology machinery.
In the oral tissues, six different human adult dental stem cells have been described in literature until now: dental pulp stem cells (DPSCs) (Gronthos, Mankani, Brahim, Robey, & Shi, 2000), exfoliated deciduous teeth stem cells (SHED) (Miura et al., 2003), periodontal ligament stem cells (PDLSCs) (Diomede, Rajan, et al., 2017), apical papilla stem cells (SCAP) (Zhang et al., 2015), dental follicle stem cells (DFSCs) (Morsczeck et al., 2010), and gingiva stem cells (Rajan et al., 2017). Oral stem cells have been investigated clinically for treatment of a wide variety of different disorders comprising bone defects, graft versus host disease, cardiovascular diseases, autoimmune diseases, diabetes, neurological, and periodontal diseases (Diomede et al., 2016; Polymeri, Giannobile, & Kaigler, 2016; Trubiani, Giacoppo, et al., 2016) that represent one of the most widespread infectious diseases destroying tooth-supporting structures.
Periodontal disease is associated to the presence of bacterial plate and its evolution is related to the immune response of the guest. The bacterial species located in the periodontal sites are anaerobic (90%) and gram negative (75%). In particular, the principal bacteria discovered at high levels in chronic periodontitis are P. Gingivalis, T. Forsythia, P. Intermedia, C. Rectus, E. Corrodens, F. Nucleatum, A. Actinomycetemcomitans, P. Micros, Treponema spp., and Eubacterium spp. (Haffajee, Teles, & Socransky, 2006).
Atherosclerosis is a multifactorial disease which represents the most common cause of coronary heart disease. In the atherosclerosis genesis in addition to strong genetic component several anatomical, physiological, and behavioral risk factors, including changes in serum lipid profile, arterial hypertension, smoking, diabetes, obesity, sedentary lifestyle, age, and genderare implicated. In recent years it has been hypothesized that the atherogenesis can be due to the infection and inflammation processes, in fact low-grade of inflammation can be related to slow progression of disease and acute systemic inflammatory response with increased risk of severe cardiovascular disease (CVD). In particular between pathogens, Helicobacter pylori and Chlamydia pneumoniae have been considered responsible of the submentioned disease (Cook et al., 1998; Martin-de-Argila, Boixeda, Canton, Gisbert, & Fuertes, 1995; Patel et al., 1995). Actually, the recent literature put in evidence that also the dental infections can represent a favorable background for atherosclerosis and CVD (Beck, Pankow, Tyroler, & Offenbacher, 1999; Niedzielska, Janic, Cierpka, & Swietochowska, 2008). Uncontrolled periodontal infections may potentially affect macro- and micro-vascular endothelial cells with up-regulation of systemic levels of inflammation, and subsequently contribute to endothelial dysfunction and the onset of atherosclerosis (Vapaatalo & Mervaala, 2001). The object of this report is synthesized into three major topics: i) the evaluation of the ability of periodontal ligament stem cells to undergo versus endothelial commitment, ii) their behavior onto decellularized porcine heart valves (dPHV), and iii) the analysis of the molecular signalling involved during the infection process induced by LPS-G.
2 MATERIALS AND METHODS
2.1 Ethic statement
The present study was approved by the Medical Ethics Committee at the Medical School, “G. d'Annunzio” University, Chieti, Italy (n°266/17.04.14). Every patients enrolled in a study signed the informative consent form. The Department of Medical, Oral and Biotechnological Sciences and the Laboratory of Stem Cells and Regenerative Medicine are certified according to the quality standard ISO 9001:2008 (certificate n° 32031/15/S).
2.2 hPDLSCs isolation and culture
Human periodontal ligament biopsies were carried out from human premolar teeth, scheduled to be removed for orthodontic purposes on healthy volunteers. Each patient was prepared for surgery by pre-treatment for 1 week with professional dental hygiene and during the surgery by decontamination of the oral cavity with chlorhexidine. The periodontal ligament tissue was collected after tooth extraction and the explants were obtained from alveolar crest and horizontal fibers of the periodontal ligament by scraping the roots of non-carious molar teeth with a Gracey's curette (Trubiani, Giacoppo, et al., 2016). Periodontal tissue fragments were crumbled and washed with PBS (Lonza, Basel, Switzerland) and placed in a TheraPEAK™MSCGM-CD™ Bullet Kit serum free, chemically defined (MSCGM-CD) medium for the growth of human Mesenchymal Stem Cells (Lonza) at 37 °C. The medium was changed twice a week, and cells spontaneously migrating from the explants. After reaching about 80% of confluence, were trypsinized (LiStar Fish, Milan, Italy), and subsequently subcultured until passage 2 (P2). Cells at P2 have been used for the experiments in the present work.
hPDLSCs and e-hPDLSCs were treated with 5 µg/ml LPS-G (InvivoGen, San Diego, CA) or left untreated for 24 hr.
2.3 Morphological analysis and mesengenic differentiation of hPDLSCs
hPDLSCs at P2 were fixed with paraformaldehyde, stained with toluidine blue and observed with a Zeiss Axiophot apparatus. Images captured using a Nikon digital camera Digital Sight. For osteogenic and adipogenic differentiation, hPDLSCs were incubated in MSCGM-CD (Lonza) medium with osteogenic supplements and in adipogenesis induction/maintenance medium (Lonza), respectively. Both the mesengenic differentiation were confirmed by means of colorimetric assay as previously described by Trubiani, Giacoppo, et al., (2016). Osteogenic and adipogenic markers were evaluated by real-time polymerase chain reaction (PCR) according to Trubiani, Giacoppo, et al., (2016) expression of Runt-related transcription factor-2 (RUNX-2) and alkaline phosphatase (ALP) was evaluated after 7 days in osteogenic differentiated culture. The expression of fatty acid binding protein 4 (FABP4) and peroxisome proliferator-activated receptor γ (PPARγ) were analyzed after 28 days of adipogenic differentiation culture. Commercially available TaqMan Gene Expression Assays (RUNX-2 Hs00231692_m1; ALP Hs01029144_m1; FABP4 Hs01086177_m1; PPARγ Hs01115513_m1; ACAN Hs00153936_m1) and the TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) were used according to standard protocols. Beta-2 microglobulin (B2M Hs99999907_m1) (Applied Biosystems, Foster City, CA) was used for template normalization.
2.4 hPDLSCendothelial differentiation
Differentiation of hPDLSCs to e-hPDLSCs started at 50–60% confluency of cells. The cells culture was stimulated with endothelial growth medium (EGM-2) composed of Endothelial Basal Medium-2, growth supplements (containing hydrocortisone, human Fibroblast Growth Factor (hFGF-b), R3-Insulin-like Growth Factor-1 (R3-IGF-1), ascorbic acid, human Epithelial Growth Factor (hEGF), GA-1000, heparin), 5% FBS and 50 ng/ml of Vascular Endothelial Growth Factor-165 (VEGF-165) (EGM-2 Bullet Kit; Lonza). The cells were maintained at 37 °C with 5% CO2 and the medium was changed every 3 days. The cells were detached from the monolayer with 0.25% Trypsin–EDTA (LiStar Fish), and analyzed after 10 days of stimulation for endothelial differentiation. Human PDLSCs used as control were maintained in DMEM (Lonza).
2.5 Tube formation test
A tube formation assay was performed in 12-well culture plates pretreated with Cultrex® Basement Membrane Extract (Trevigen Inc., Gaithersburg, MD) (300 µl/well). The matrix solidified at 37 °C for 30 min, then hPDLSCs and e-hPDLSCs at a density of 2 × 105 cells per well were seeded onto pretreated wells. Capillary-like tubes structure have been observed by means inverted light microscope at phase contrast after 4 hr of culture.
2.6 Immunophenotypic analysis
The phenotypical analysis was performed on cells detached from passages 2 as previously described by Rajan, Giacoppo, Trubiani et al. 2016. hPDLSCs were stained using monoclonal antibody for CD31 PE-conjugated (Miltenyi Biotech), the experiments were performed in triplicate.
2.7 Immunofluorescence analysis
hPDLSCs and e-hPDLSCs were processed as previously reported by Diomede, Zingariello, et al. (2017). Primary monoclonal antibodies anti-human CD31 (1:300, mouse) (Dako, Agilent Technologies, Santa Clara, CA) was used, followed by Alexa Fluor 568 conjugated goat anti-mouse as secondary antibodies (1:200; Thermofisher, Life Tech., Monza, MB, Italy) for 1 hr at 37 °C.
hPDLSCs and e-hPDLSCs treated or not with 5 μg/ml of LPS-G for different time points were incubated for 1 hr at 37 °C with primary monoclonal antibodies anti-human TLR4 (1:100, rabbit), NFkB (1:250, rabbit) (OriGene), MYD88 (1:100, rabbit) (OriGene Technologies, Inc., Rockville, MD), ERK1/2 (1:100, rabbit) (Thermofisher) and p-ERK1/2 (1:100, rabbit) (OriGene), followed by Alexa Fluor 568 red fluorescence conjugated goat anti-rabbit as secondary antibodies (1:200) (Thermofisher).
Subsequently cells were incubated with Alexa Fluor 488 phalloidin green fluorescence conjugate (1:200; Thermofisher), to mark cytoskeleton actin. Cell nuclei were stained with TOPRO (1:200; Thermofisher) for 1 hr at 37 °C. Glass coverslips were placed face down on glass slides and mounted with Prolong antifade (Thermofisher) (Biamonte et al., 2014).
Samples were observed by means Zeiss LSM510META confocal system (Zeiss, Jena, Germany), connected to an inverted Zeiss Axiovert 200 microscope equipped with a Plan Neofluar oil-immersion objective. Images were collected using an argon laser beam with excitation lines at 488 nm and a helium-neon source at 543 and 633 nm. Post-acquisition image analyses were carried out with a Zeiss ZEN software (ver.2.3) (Orciani, Trubiani, Guarnieri, Ferrero, & Di Primio, 2008). For each images deriving from ERK1/2 or p-ERK1/2 immunolabeled samples were evaluated regions of interest including nuclei in distinct cells. Their fluorescence were analyzed comparing to that of nuclei stained with TOPRO. The data were calculated using the ZEN software colocalizations tool and quantified by Pearson's correlation coefficient: were values of zero indicated no colocalization, while values of one indicated complete colocalization (Guarnieri, Fano, Rathbone, & Mariggio, 2004; Salvatorelli et al., 2006).
2.8 Quantitative RT-PCR
hPDLSCs and e-hPDLSCs from three independent samples were processed for gene expression analysis. Total RNA was extracted using RNeasy Mini Kit (Qiagen GmbH,Hilden, Germany), as indicated by manufacturer's protocol. Primers from PECAM1 and ACTB genes (Forward GAACCTGTCCTGCTCCAT; Reverse TCAAACTGGGCATCATAAGAAAT) were designed from coding sequences published on GenBank database with the help of Bea-con Designer v.7 Software (Premier Biosoft International, Palo Alto,CA). Each sample was run as a duplicate. Real-time data were analyzed by DataAssist software (Applied Biosystems). A global normalization analysis was used GAPDH as internal controls.
2.9 Viability assay
The effect on the viability of the treatment with LPS-G on hPDLSCs and e-hPDLSCs were determined using the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazoliumbromide (MTT) method. hPDLSCs and e-hPDLSCs seeded at 1 × 104 in a 96-well tissue culture plate in 200 µl medium were treated or not with LPS-G cultured and incubated at 37 °C for 24, 48, and 72 hr. At each time point, MTT solution (20 μl) (Promega, Milan, Italy) was added and plates transferrred in incubator for 3 hr at 37 °C. After incubation the plates were read at 650 nm wavelength using a microplate reader (Synergy HT, BioTek Instruments, Winooski, VT).
2.10 Porcine heart valves and decellularization procedure
Porcine heart valves were obtained from local slaughterhouse (Chieti, Italy), under veterinary supervision; materials were exempt from cardiac damages. Semilunar porcine heart valves were obtained under sterile conditions.
Valves were washed with a cleaning solution comprising Dulbecco's phosphate-buffered saline (DPBS, Lonza, Basel, Switzerland) and antibiotics (1% penicillin/streptomycin, Lonza). To decellularize valves was used 1% sodium dodecyl sulfate 1% Triton X–100 (Sigma-Aldrich, Milan, Italy) in DPBS (Lanuti et al., 2015). The solution was changed every 9 hr during the procedure (total time procedure 48 hr), keeping tissues in a continuous flutter. A post decellularization cleaning procedure (DPBS + 1% penicillin/streptomycin) was performed for the following 48 hr (solution were changed every 9 hr).
2.11 Western blot analysis
Proteins from hPDLSCs and e-hPDLSCs treated or not with 5 µg/ml of LPS-G for 24 hr were separated on SDS–PAGE and subsequently transferred to nitrocellulose sheets using a semidry blotting apparatus. Sheets were saturated for 120 min at room temperature in blocking buffer (1xTBS, 5% milk, 0.1% Tween-20), then incubated overnight at 4 °C in blocking buffer containing primary antibodies to TLR4 (1:2000, rabbit) (OriGene), NFkB (1:2000, rabbit) (OriGene), MYD88 (1:1000, rabbit) (OriGene), ERK1/2 (1:1000, rabbit) (Thermofisher), p-ERK1/2 (1:1000, rabbit) (OriGene) and β-Actin (1:750, mouse) (Santa Cruz Biotechnology, Santa Cruz, CA). After four washes in TBS containing 0.1% Tween-20, samples were incubated for 60 min at room temperature with peroxidase-conjugated secondary antibody diluted 1:1000 in 1× TBS, 2,5% milk, 0.1% Tween-20 (Verrucci et al., 2010). Bands were visualized and quantized by the ECL method with Alliance 2.7 (UVItec Limited, Cambridge, UK).
2.12 Cytokines evaluation
For detection of IL6, IL8 and MCP1, the Quantikine ELISA Kit (R&D Systems, RLB00, Minneapolis, MN) was used according to the manufacturer's instructions. Supernatants were collected from hPDLSCs and e-hDPSCs treated or not with 5 µg/ml of LPS-G for 24 hr.
2.13 Reendothelialization
dPHVs were placed in incubator for 24 hr at 37 °C at 5% CO2, using two types of culture media: DMEM (Lonza) culture medium and EGM-2 BulletKit culture medium added by 5% Foetal Bovine Serum (FBS) (Lonza). Heart valves were recellularized for 15 days by hPDLSCs or ehPDLSCs previously differentiated for 15 days in EGM-2; for both, cells were seeded at the concentration of 1 × 106 cells/ml. The culture medium was changed every 48 hr (Lanuti et al., 2015).
2.14 Scanning electron microscopy (SEM) investigation
dPHVs cultured with hPDLSCs and e-hPDLSCs were fixed for 4 hr at 4 °C in 4% glutaraldehyde in 0.05 M phosphate buffer (pH 7.4), dehydrated in increasing ethanol concentrations and then critical point dried. They were then mounted on alluminium stubs and gold-coated in an Emitech K550 (Emitech Ltd. Ashford, UK) sputter-coater before imaging by means Zeiss SEM EVO 50 (Zeiss, Jena, Germany).
2.15 Live cells ROS measurements
Reendothelialized dPHVs were washed twice with Normal External Solution (NES) containing (in mM): 125 NaCl, 5 KCl, 1 MgSO4, 1 KH2PO4, 5.5 glucose, 1 CaCl2, 20 HEPES, pH 7.4. After, the cells were incubated for 30 min with 10 µm DCFH-DA (Thermo Fisher Scientific) at 37 °C in humidified incubator. At the end of loading, the valve leaflets were washed with NES ad observed using a Zeiss LSM 7 MP multiphoton microscope (Carl Zeiss, Jena, Germany), equipped with upright microscope Axio-Examiner.Z1 and an objective W-Plan-Apo 20X/1.0 NA VIS-IR DIC. The two photon excitation was obtained using a Ti:Sapphire Laser CoherenCamaleon Vision II in mode locked (Coherent, Inc., Santa Clara, CA). Excitation was fixed at 850 nm and emission collected by BiG detector in reflection mode equipped with bandpass filter set over 505–530 nm. Off-line image analyses were performed with a Zeiss ZEN 2011 software. Regions of interest were identified in individual cells, and their average fluorescence intensities expressed as F/F0 ratios, where F0 indicate the fluorescence of the first acquired image and F the fluorescence of the same cell acquired during a time lapse.
2.16 Statistical analysis
All experiments were performed in triplicate. The data are presented as means ± standard error and were analyzed using SPSS (SPSS Inc., Chicago, IL) or Prism 5.0 (GraphPad Software, Inc.). One-way repeated-measurement analysis of variance (ANOVA), followed by the post hoc Holm-Sidak test (when appropriate) or One-way measurement analysis of variance (ANOVA) “Bonferroni's Multiple Comparison Test” (colocalization test) and the Student paired t-test were used for statistical analysis. A p-value of less than 0.001 (**) or less than 0.0001 (***) is considered statistically significant.
3 RESULTS
3.1 hPDLSC characterization and mesenchymal differentiation
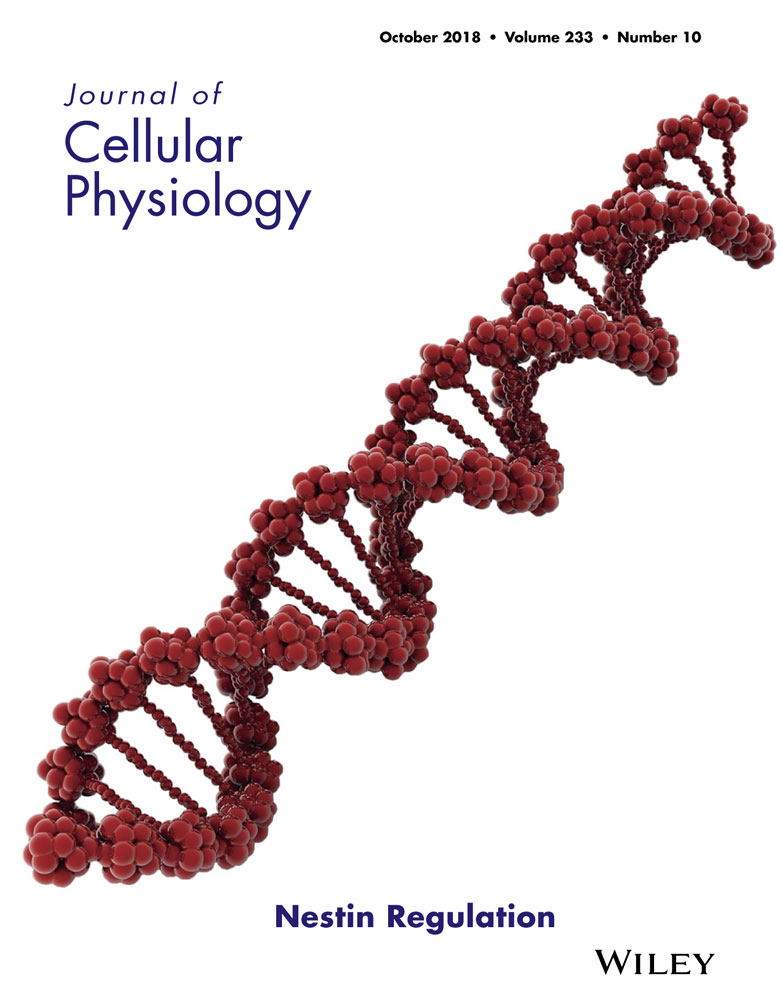
Morphological investigations at light microscopy showed hPDLSCs with spindle shaped morphology with elongated cytoplasmic processes (Figure 1a). Alizarin Red S stained confluent cells showed after 3 weeks of osteogenic induction process a characteristic arrangement; in fact hPDLSCs appear detached from the bottom well forming a bone nodule higly mineralized (Figure 1b). The analysis of transcripts RUNX2 and ALP confirmed the ability of the hPDLSCs committed to osteogenic differentiation (Figure 1d). To estimate the adipogenic differentiation of hPDLSCs, the cellular monolayer was stained with Oil Red O and observed by light microscopy. Adipogenic-induced cells showed the fine evident intracellular lipid droplets as single or grouped (Figure 1c). RT-PCR confirmed the adipogenic induction evidencing the upregulation of the transcripts FABP4 and PPARγ, master molecules of adipogenesis (Figure 1e).
3.2 hPDLSC endothelial differentiation
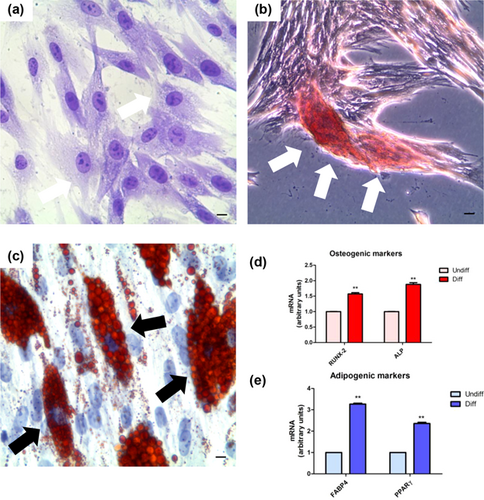
Undifferentiated hPDLSCs showed a characteristic fibroblast like shape (Figure 2a). After treatment with differentiation media EGM-2 in presence of VEGF for 2 weeks, cells changed their appearence. In fact, a characteristic morphological arrangement were observed in differentiated cells, the endothelial differentiated hPDLSCs (e-hPDLSCs) showed a star shape morphology with long cytoplasmic processes taking contact with neibouring cells (Figure 2b). e-hPDLSCs seeded onto Cultrex changed their morphology from elongated to stellate shape and started to take contact with neibouring cells forming tubular structures (Figure 2c). Gene expression analysis of PECAM1(CD31) mRNAs confirming the commitment of endothelial differentiation (Figure 2d).
The ability to differentiate in endothelial phenotype, after 2 weeks of endothelial commitment, was evaluated analyzing CD31 surface specific endothelial marker. Immunohistochemistry carried out at CLSM evidenced a positivity of CD31 in e-hPDLSCs (Figure 2f). Immunophenotype analysis also showed the positivity for CD31 in e-hPDLSCs (Figure 2h).
3.3 Proliferation ability of e-hPDLSCs
After 24 hr of treatment in presence of 5 µg/ml LPS-G, both hPDLSCs and e-hPDLSCs revealed a reduction in cell density related to control condition (Figures3a–c and 3b–d, respectively). The proliferation rate of hPDLSCs and e-hPDLSCs was analyzed trough the MTT assay starting from 24 to 72 hr of treatment. The comparison of the data obtained, showed a significant inhibition of the proliferation rate at time points examinated in hPDLSCs and e-hPDLSCs treated with LPS-G (Figure 3e).

3.4 Signaling pathway analyses
A time course and quantitative analysis of nuclear traslocation of ERK1/2 or p-ERK1/2 were performed in immunofluorescence labeled sample of hPDLSCs or e-hPDLSCs and expressed as Pearson's colocalization coefficient. No detectable colocalizations were observed in control condition both in ERK1/2 and p-ERK1/2 (Figures 4e and 4f, 0 min respectively). While, after 5, 15, or 60 min of LPS-G treatment an increasing value of colocalization were detected in hPDLSCs or e-hPDLSCs cells. Interenstingly, in e-hPDLSCs cells the rise in both ERK1/2 or p-ERK1/2 nuclear translocation were significant more high than hPDLSCs counterpart (Figures 4e and 4f). The same results were detected by means immunofluorescence experiments for ERK1/2 and p-ERK1/2 in hPDLSCs and e-hPDLSCs (Figure 4a1–4d4). To validate the immunofluorescence data, the expression of ERK1/2 and p-ERK1/2 proteins was performed by Western blotting using anti ERK1/2 and p-ERK1/2 antibodies, respectively. As shown in Figure 4, bands of ERK1/2 and p-ERK1/2 were increased in hPDLSCs and e-hPDLSCs stimulated with LPS-G (Figure 4g).
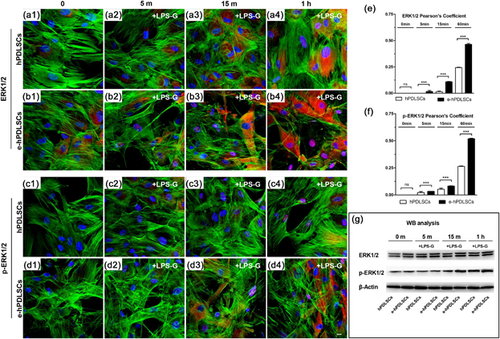
Moreover, in order to validate signaling network induced from LPS-G treatment in hPDLSCs and e-hPDLSCs the expression of TLR4, NFkB, MyD88, ERK1/2, and p-ERK1/2 were analyzed after 24 hr (Figure 5).
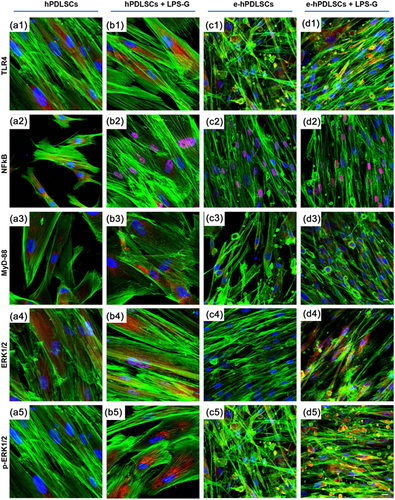
Immunofluorescence study showed an increase of TLR4 protein in stimulated hPDLSCs (Figure 5b1) and e-hPDLSCs (Figure 5d1), respect to the hPDLSCs (Figure 5a1) and e-hPDLSCs (Figure 5c1),indicating that LPS-G acts on the receptor and start a molecular cascade up-regulating MyD88 (Figures 5b3 and 5d3) and inducing NFkB to traslocated at nuclear level (Figures 5b2 and 5d2).
ERK1/2 and p-ERK1/2 were over expressed in LPS-G treated samples (Figures 5b4, 5d4, 5b5, and 5d5) compared to untreated culture conditions (Figures 5a4, 5a5, 5c4, and 5c5) and their colocalization at nuclear level was analyzed and reported in Supplementary material.
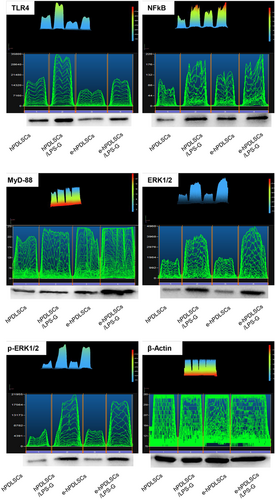
3.5 Western blot analysis
The protein level of cellular signaling network was evaluated in differentiated and undifferentiated samples after 24 hr of culture in presence of LPS-G. The image analysis of the western blot specific bands showed an overexpression of TLR4 receptor after LPS-G treatment. Furthermore the signaling cascade network molecules constituted by NFkB, MyD88, ERK1/2, and p-ERK1/2 displayed an evident overexpression indicating their involvement (Figure 6).
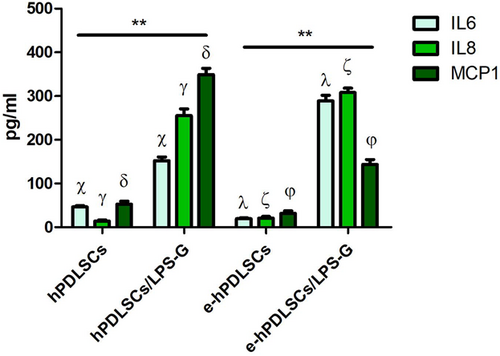
3.6 Cytokines release assessment
The analysis of released cytokines in the culture medium of the hPDLSCs and e-hPDLSCs treated with LPS-G evaluated by RayBiotech quantitative method showed an increase of the IL6, IL8, and MCP1 inflammatory molecules after 24 hr of LPS-G treatment. The data confirm that inflammatory stimulus is capable to induce changes in the pro-inflammatory cytokines secretion profile in both hPDLSCs and e-hPDLSCs (Figure 7).
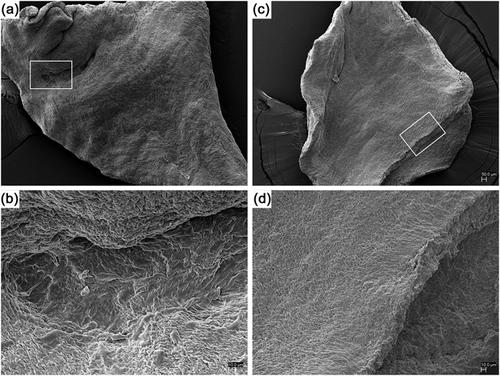
3.7 Scanning electron microscopy analysis
Ultrastrucutral analysis of hPDLSCs (Figure 8c) and e-hPDLSCs (Figure 8a) seeded on dPHV after 48 hr of culture put in evidence, at different magnification, the ability of cells to grow, adhere, and recolonize the decellularized natural scaffold. In section b and d higher magnification of e-hPDLSCs and hPDLSCs, respectively, evidenced the full covering of the dPHV surface.
3.8 ROS measurements
The ROS generation on hPDLSCs or e-hPDLSCs seeded on dPHV, were verified using two photon microscopy on DCFH-DA loaded cells. In Figures 9a and 9b are shown the tridimensional reconstruction of Z-stack acquisition of hPDLSCs or e-hPDLSCs. It is interesting to note that while undifferentiated cells seems to maintain a round shaped profile, the differentiated one acquired a typical endothelial cells morphology.
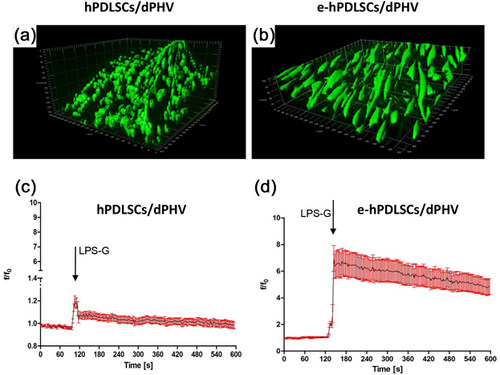
Results of time lapse experiments revealed that after treatment with 5 μg/ml LPS-G a rapid and sustained increase in ROS generation was observed mainly in e-hPDLSCs. Comparing the average response of undifferentiated and differentiated hPDLSCs is clearly evident that the latter showed an six fold increase in ROS production after LPS-G exposure, while on undifferentiated appeared to be negligible (Figures 9c and 9d).
4 DISCUSSION
The discover of stem cells with a mesenchymal origin owning a multilineage differentiation potential represents an innovative contest to explore togheter the cell biological machinery and the progression of several diseases. For their specific features as, the ability to self-renew and to generate specialized cells, stem cells could represent a possible solution for regenerative therapy. Recently, oral tissues have been recognized as readily available sources of adult stem cells (Diomede, Rajan, et al., 2017).
In particular, our earlier findings provided evidence that in the periodontium are present stem cells expressing stemness markers as: Nanog, Oct4, SSEA1, and SSEA4 (Trubiani et al., 2015), competent to undergo toward differentiation into osteogenic, chondrogenic, neurogenic and adipogenic phenotype (Trubiani, Giacoppo, et al., 2016). Human PDLSCs show proteins associated to the growth, regulation, and genesis of neuronal cells, in agreement with their neural crest origin (Trubiani, Guarnieri, et al., 2016). The transplantation of hPDLSCs may represent a putative novel and helpful tool for multiple sclerosis treatment (Rajan, Giacoppo, Diomede, et al., 2016; Trubiani, Giacoppo, et al., 2016). The periodontal ligament biopsy, obtained by scraping the alveolar crest and the horizontal fibers of the root, is apt to find an elevated number of autologous stem cells with genomic stability and capacity to differentiate into ecto-mesenchymal lineages (Trubiani et al., 2015). hPDLSCs have anti-thrombogenic and immunosuppressive properties (Cianci et al., 2016), and can induce homing and differentiation of autologous host cells through paracrine signaling, involving an array of cytokines and growth factors (Diomede et al., 2016).
Human PDLSCs are anatomically found in highly vascular areas, situated in close proximity to blood vessels surrounding the tooth. They possesses the capacity to produce proangiogenic cytokines as VEGF and b-FGF, essential in angiogenesis genesis and in physiological mechanisms activated in response to changes in the local microenvironment (i.e., injury). Moreover, hPDLSCs expressed perivascular cell markers such as NG2, alpha-smooth muscle actin, platelet-derived growth factor receptor ß, and CD146 (Bae et al., 2017).
Recently, the angiogenic potential of stem cells derived from dental pulp, gingival fibroblasts, and tooth germ has been demonstrated, putting in evidence that the oral neural crest derived stem cells may be appropriate for generating endothelial tissues (Dogan, Demirci, & Sahin, 2015; Iohara et al., 2008; Szepesi et al., 2016). In our study, we have induced xeno-free hPDLSCs to endothelial commitment and their differentiation ability was appraised trough CD31 expression evaluated using multiparametric analyses. Tubule formation is one of the hallmarks of angiogenesis, along with cell proliferation and migration (Barachini et al., 2014). After seeding on Cultrex, hPDLSCs formed a well developed capillary-like tubes network. The extensive tubular network formed by hPDLSCs mostly consisted of long and thin tubes, resembling capillaries, indicating that also in xeno-free media hPDLSCs can be committed versus endothelial phenotype (Weber et al., 2016). Moreover, our study aims to establish an in vitro model to study the signaling pathway involved in inflammation sustained by LPS-G in endothelial committed hPDLSCs through an approach of “in situ heart valve tissue engineering” method to establish a possible link between the periodontal and cardiovascular disease. At this purpose we have tested LPS-G on e-hPDLSCs seeded onto decellularized porcine valves. Scanning electron microscopy study put in evidence e-hPDLSCs adhering and proliferating in presence of decellularized valves recostituting a cellular monolayer. Endothelial stem cells derived from periodontal tissues can be an easy, autologous, alternative, and valid stem cells platform that can be used in terms of applicability towards to endothelium investigation and regeneration in heart valve surgery. Anaerobic gram negative bacterium, Porphyromonas gingivalis, has been shown to be involved in chronic and aggressive periodontitis but also in cardiovascular diseases. It has been demonstrated that the infection induces the acceleration of atheroma formation, the invasion of endothelium tissue and vascular smooth muscle cells with subsequent endothelial dysfunction. The periodontal disease, triggered by bacterial antigens, which directly engage the innate immune system at the site of the infection. One of these antigens, LPS, is a well-characterized ligand specific to innate immune receptor, Toll-like receptor 4 (TLR4). LPS can potentiate different activities on TLR4 signaling (Berezow et al., 2009; Coats et al., 2011), and several reports have demonstrated that the binding of LPS-G to TLR4 on human gingival fibroblasts triggers various second messenger pathways. In particular the common signaling feature among all TLRs is the activation of the transcription factor NFkB which has been implicated in controlling the expression of inflammatory cytokines and cell maturation molecules (Nishikawa et al., 2016). hPDLSCs and e-hPDLSCs showed on their surface TLR4 receptor, and LPS-G stimulus upregulates the TLR4 expression. Both cellular populations release high level of several pro-inflammatory cytokines and chemokines, such as interleukins (ILs) IL6, IL8, and MCP1. In vivo, the increased secretion of IL6 promotes monocyte differentiation into mobile andactive macrophages that secrete MCP1 and MMPs, which facilitate the invasion from the adventitia into the media (Kagan & Medzhitov, 2006). All TLRs, with the exception of TLR3, use MyD88 (Brown, Wang, Hajishengallis, & Martin, 2011) and TLR4/MyD88 signaling pathway, also known as inductors of the expression of pro-inflammatory cytokines (Horng & Medzhitov, 2001). LPS-G response may be mediated by both MyD88-dependent and −independent pathways, each of which leads to the activation of MAP kinases and NFkB. Nevertheless, the MyD88-dependent pathway is essential for the inflammatory response mediated by LPS-G. NFkB is a ubiquitously expressed transcription factor that has been reported to translocate into the nucleus and initiate the transcription of inflammatory cytokines (Diomede, Zingariello, et al., 2017; Ghosh, May, & Kopp, 1998). Stem cells express low levels of ERK1/2 and its phosphorylated isoforms, while on the contrary cells induced to the differentiation exhibit an increased p-ERK1/2 (Arino et al., 2008). It has been shown that the MAPK pathway is critical for endothelial differentiation of bone marrow stem cells (Xu, Han, Ito, Deng, & Chai, 2008). Our experiments demonstrated, trough confocal microscopy and western blotting analysis, an increase of MyD88 level in hPDLSCs and in e-hPDLSCs upon treatment with LPS-G and a nuclear translocation of the NFkB trascription factor after 24 hr of treatment. Unstimulated hPDLSCs and e-hPDLSCs express low level of ERK1/2 and p-ERK1/2 and these levels are up-regulated upon LPS-G treatment. Starting from 1 hr after LPS-G treatment a nuclear translocation of ERK1/2 and p-ERK1/2 occur, indicating a pivotal role in the regulation of the cell differentiation and inflammation process. In fact, it has been demonstrated that the ERK signaling pathway is critical for VEGF-A/VEGFR2-induced differentiation of adipose mesenchymal stem cells into Endothelial Cells (ECs) and provide new insights into the role of the ERK signaling pathway in AMSCs differentiation to ECs forpotential clinical use in cardiovascular diseases (Almalki & Agrawal, 2017).
ROS, including hydrogen peroxide (H2O2), superoxide radicals (O2−), and hydroxyl radicals (OH−), are important signaling molecules that regulate multiple biological responses, including angiogenesis (Nita & Grzybowski, 2016). ROS produced by normal metabolism are important biological mediators in the cellular signaling cascades (Irani, 2000). Appropriate amounts of ROS have beneficial effects on several physiological processes including protection from pathogens, wound healing and tissue regeneration (Bhattacharyya, Chattopadhyay, Mitra, & Crowe, 2014). On the other hand, harmful levels of ROS could be generated in response to ultraviolet radiation (Jia et al., 2010), some drugs (Deavall, Martin, Horner, & Roberts, 2012), ischemia–reperfusion injury (Mallick, Yang, Winslet, & Seifalian, 2004) and inflammatory disorders (Roessner, Kuester, Malfertheiner, & Schneider-Stock, 2008). Disproportionate levels of ROS give rise to further disease development such as cardiovascular disease, neurodegeneration, cancer, and chronic inflammation (Halliwell & Cross, 1994). Recently a functional link between ROS and TAK1-MAPKs/NFkB-Cox2-PGE2 has been demonstrated (Onodera, Teramura, Takehara, Shigi, & Fukuda, 2015). Considering that LPS-G induces an inflammatory response with pro-inflammatory cytokines release and NFkB activation, the level of ROS production has been analysed in hPDLSCs and e-hPDLSCs seeded on decellularized porcine hearth valves.
Results of time lapse experiments revealed that after treatment with LPS-G a rapid and sustained increase in ROS generation was observed mainly in e-hPDLSCs seeded onto valve leaftelets, clearly showingthat endothelial differentiation of the hPDLSCs lead the cell able to respond to LPS-G activating ROS production, while on undifferentiated appeared to be negligible.
Our data demonstrate that LPS-G induces vascular the release of IL6 that can coordinate inflammation via MCP1 upregulation. Furthermore, the functional link between ROS, ERK1/2, p-ERK1/2 and NFkB acquire meaning and could be considered strategic for establish in vitro a model relationship between oral and cardiovascular disease.
5 CONCLUSIONS
Taking into account the limitation of the in vitro study, the identification and the characterization of the study cell model that lead the knowledgement of the intracellular signaling pathways regulating the the inflammatory response of the endothelial differentiated hPDLSCs treated with LPS-Pophyromonas Gingivalis. Starting from our results, further studies could establish a possible in vivo correlation between oral and cardiovascular disease.
ACKNOWLEDGMENTS
This study was supported by OT ex 60%. Authors would like to thank Prof. Mariggiò M.A. (University “G. d'Annunzio” of Chieti-Pescara) for helpful suggestions and textual revision.
CONFLICTS OF INTEREST
All authors disclose no potential conflicts of interest.