MiR-145-5p inhibits proliferation and inflammatory responses of RMC through regulating AKT/GSK pathway by targeting CXCL16
Abstract
The main pathological characteristics of chronic glomerulonephritis (CGN) are diffuse mesangial cells proliferation and inflammatory responses. Our previous studies have confirmed that miR-145-5p was abnormally elevated in CGN rats, but its mechanism remains unclear. Therefore, this study aimed to elucidate the mechanism of miR-145-5p in regulation of renal mesangial cells proliferation and inflammatory responses. In vivo study, the cationic bovine serum albumin (C-BSA)-induced CGN rat model was established, and the content of miR-145-5p in renal was examined by qRT-PCR, meanwhile, we also determined the renal function and inflammatory infiltrate. In vitro, the cell proliferation rate, cell cycle and inflammatory changes of rat mesangial cells (RMCs) were measured. Our results suggested that miR-145-5p extended the G0-G1 phase, shortened S phase, inhibited cell proliferation and suppressed inflammatory responses in RMCs. Moreover, miR-145-5p inhibited CXCL16 protein expression through binding the 3′-UTR of CXCL16, suppressed AKT/GSK signaling pathway, and decreased expression of inflammation related mRNAs, such as IL-1α, IL-2, IL-6, and TNF-α mRNAs. Further, locking CXCL16 alleviated inflammatory reactions and down-regulated AKT/GSK pathway in RMCs. Above all, we concluded that miR-145-5p inhibited proliferation and inflammatory responses of RMCs through regulation of AKT/GSK pathway by targeting CXCL16.
1 INTRODUCTION
Chronic glomerulonephritis (CGN), mainly associated with glomerular damage, shows abnormal proliferation and inflammatory responses of glomerular mesangial cells. Thus, inhibition of mesangial cells proliferation and inflammatory responses are important in the treatment of proliferating glomerular diseases (Kang & Shin, 2015; Kurogi, 2003).
Cationic bovine serum albumin (C-BSA) induced nephritis is a classical model of mesangial proliferative glomerulonephritis, and in which macromolecular immune complexes are formed in situ and deposited in mesangial area, leading to mesangial cells proliferation and inflammation (Wu et al., 2008). In addition, inflammatory mediators stimulate activation, shrinkage, and proliferation of mesangial cells. Those conditions directly aggravate inflammation and impair renal function including injury to the molecular barrier and charge barrier of the filtration membrane, and proteinuria (Qin et al., 2013).
MicroRNAs (miRNAs) are a kind of endogenous, about 22-nucleotide (nt)-long, single-stranded non-coding RNA molecules that influence cells in a variety of biological functions such as differentiation, metabolism, aging, autophagy, proliferation, and apoptosis (Huang, Pinto, & Pandey, 2013). Moreover, it is reported that miRNAs are closely associated with different kinds of kidney diseases, including polycystic, diabetic and other chronic kidney diseases (Hajarnis, Lakhia, & Patel, 2015; Li, Chung, Yu, & Lan, 2014; Trionfini, Benigni, & Remuzzi, 2015). Thus, miRNAs may represent an important potential target in the treatment of CGN.
In our previous study, in order to explore the changes of miRNAs in the CGN disease, we performed a miRNA array assay to identify novel miRNAs in CGN rats and found that miR-145-5p and other two miRNAs were significantly up-regulated (fold change >2.0 vs. normal rats, which showed in the supplementary material). Are the up-regulated miRNAs involved in the progressive development of CGN? Harvey et al. (2008) identified that miR-145 is a “signature” for mesangial cells. Some scholars demonstrated that decreased miR-145 was involved in the vascular remodeling abnormalities and progressive loss of eGFR in chronic kidney disease (Chen et al., 2013). MiR-145 also could suppress inflammatory responses by promoting IL-10 expression in TLR4-triggered macrophages (Lin et al., 2013).
However, the underlying mechanisms of miR-145 involving in CGN physiopathologic proceeding remains unclear. It was reported that CXCL16 was not only related to inflammatory responses and immune system, but also closed to several of kidney disease (Ma, Jin, He, & Wang, 2016). In the present study, we investigated whether miR-145-5p inhibited proliferation and inflammatory responses of glomerular mesangial cells by targeting CXCL16 in order to further explore its anti-glomerulonephritis mechanisms.
2 MATERIALS AND METHODS
2.1 C-BSA induced CGN rat model
C-BSA was prepared as described in our previous studies according to the method of Border, Ward, Kamil, and Cohen (1982). A total of 20 adult male Sprague–Dawley rats (special pathogen-free level, Certificate No. SCXK 2013-0020), weighing approximately 200 ± 20 g, supplied by The Experimental Animal Centre of Guangzhou University of Chinese Medicine, were used in this study. All animal experimental programs were authorized by the Animal Ethics Committee of Guangzhou University of Chinese Medicine submitting to the guidelines of the European Community and the National Institute of Health of the USA. Our researchers have received permission from the institutional Animal Care and Use Committee of Guangzhou University of Chinese Medicine. All rats were housed in an air-conditioned room at 25 ± 2°C, with 65% humidity and 12 hr light/12 hr dark cycle. One week of acclimatization later, the rats were randomly divided into two groups including normal and CGN-model groups. Rats in model group were tail vein injected with C-BSA for 4 weeks to establish the CGN rat model according to our previous study (Wu, Liu et al., 2016). The 24 hr proteinuria was detected to ensure that the model was established successfully.
2.2 Blood sampling and renal tissue removal
On day 28, all animals were euthanatized to collect their blood and obtain renal tissue samples, as our previously described (Liang et al., 2014). Five milliliters of blood from the abdominal aorta was collected after anesthetization, and then centrifuged at 3,500 rpm at 4°C for 15 min to obtain the serum for renal function analysis. Both kidneys from each rat were divided into two parts. One was fixed in 10% neutral formalin phosphate buffer for hematoxylin and eosin (HE) and periodic acid-Schiff (PAS) staining, and the other was quickly frozen in liquid nitrogen and stored at −80°C for the subsequent detection as a backup.
2.3 CBA analyses for inflammatory factor
The levels of IL-1α and IL-2 in the serum were detected with a Cytometric Bead array kit (BD Biosciences, Franklin Lakes, NJ; cat. No.558267) according to the manufacturer's instructions and detected with flow cytometry analysis.
2.4 Immunocytochemistry analyses
The protocol of immunocytochemistry was based on our previous study (Wu, Zhou et al., 2017). Briefly, histologic paraffin sections were cut to 5-µm thickness. The paraffin sections were dewaxed with dimethylbenzene and a graded series of alcohol. Endogenous peroxidase was blocked with 3% hydrogen peroxide. The sections were heated with a microwave oven twice for 5 min, and incubated with 1:100 diluted primary rabbit anti-CD68 antibody (Aobosen, Beijing, China). For a negative control, the sections were incubated with PBS. After removal of unbound primary antibody, the sections were incubated with reagent 1 (Polymer Hepler) and reagent 2 (polyperoxidase-anti-mouse/rabbit IgG) for 20 min and 30 min at 37°C sequentially, then washed with PBS and counterstained with 3′-diaminobenzidine substrate as a substrate chromogen solution to produce a brown color. Finally, sections were counterstained with hematoxylin and evaluated under high-power light microscopy (×400) in a blinded fashion.
2.5 Cell culture and transfection
Rat mesangial cells (RMCs) were purchased from the China Center for Type Culture Collection in Wuhan University (CCTCC, Resource No.142C0001000000124), cultured in DMEM(1X) + GlutaMAXTM-I (Gibco, Grand Island, NY; LOT: 1596764) with 10% fetal bovine serum (Gibco; LOT: 41F3043K) and maintained in a 37°C incubator with 5% CO2. Subsequently, RMCs (∼50% confluence) were transfected with miR-145-5p mimics, negative control (RiboBio Co., Ltd, GuangZhou, China, Cat:2215FAM, LOT:6031), siCXCL16 (Ambion, Waltham, MA, Cat.#AM16708), and siRNA control (Ambion, Cat.#AM4611) by using Lipofectamine RNAiMAX (Life technology, Carlsbad, CA, LOT:13778-150) for 24 hr, followed by determination of protein and RNA levels.
2.6 Cell proliferation assays
RMCs were seeded in 96-well plates at a density of 3,000/well, incubated in DMEM without FBS for 12 hr to synchronize, transfected with miR-145-5p mimics or a miRNA control for 24, 48, and 72 hr and then incubated with 5 mg/ml methyl thiazolyl tetrazolium (MTT) (Biosharp, GuangZhou, China, LOT:0793) for 4 hr at 37°C. The formazan crystals were dissolved in dimethylsulfoxide (Germany, Merck Millipore, LOT: 0219605580). Optical density was determined at 490 nm with a plate reader (Thermo Fisher Scientific, Multiskan GO 1510, Waltham). Cells were incubated with 5 mg/ml CCK8 (Japan, Dojindo, Cat:FN687) for 2 hr at 37°C and optical density was determined at 450 nm with a plate reader.
2.7 Cell cycle analyses
RMCs were seeded in 6-well plates at a density of 1.0 × 105/well, incubated in DMEM without fetal bovine serum for 12 hr to synchronize and transfected for 48 hr. After then, cells were digested with pancreatin, washed with PBS for twice, suspended and fixed in 75% ethanol at −20°C overnight. Fixed cells were centrifugated, washed with PBS for three times, and stained by propidium iodide (50 µg/ml; Sigma, Darmstadt, Germany) with RNaseA for 3 min away from light. A BD flow cytometer (BD) was used to determine cellular DNA contents. The percentage of cells in G0-G1, S, and G2-M phases was determined by using the Cell FIT Cell Cycle Analysis Software (version 2.01.2; BD).
2.8 Quantitative reverse-transcriptase polymerase chain reactions (qRT-PCR)
RNA extraction from the renal cortex and the qPCR assays were performed as previously described (Wu et al., 2014). The cycling program was set at 1 cycle of pre-denaturation at 95°C for 30 s, and then 45 cycles at 95°C for 15 s and 60°C for 1 min (ABI 7500, Carlsbad, CA). The relative levels of the miR-145-5p, CXCL16, IL-1α, IL-2, IL-6, and TNF-α mRNAs were normalized to GAPDH and calculated according to the 2−ΔΔCt formula. The primer sequences presented in Table 1 were designed by the Primer Premier 5.0 software (Premier Bio soft, Palo Alto, CA).
| Genes | Sense primers (5’ to 3′) | Antisense primers (5’ to 3′) | Product size (bp) |
|---|---|---|---|
| CXCL16 | ACCCTTGGCCCTTACACTCT | GGGTGCCAGAAGGAATTGTA | 119 |
| IL-1α | AGCCTGTGTTGCTGAAGGAG | GAAAGCTGCGGATGTGAAGT | 119 |
| IL-2 | CAGCGTGTGTTGGATTTGAC | TGATGCTTTGACAGATGGCTA | 190 |
| IL-6 | GTCAACTCCATCTGCCCTTC | TGTGGGTGGTATCCTCTGTG | 154 |
| TNF-α | GCTCCCTCTCATCAGTTCCA | GCTTGGTGGTTTGCTACGAC | 102 |
| GAPDH | AAACCCATCACCATCTTCCA | GTGGTTCACACCCATCACAA | 198 |
2.9 Western-blot analyses
The western blot analyses were performed as described in our previous study (Wu et al., 2014). The following primary antibodies: rabbit anti-CXCL16 (1:1,000), anti-phospho-Akt (1:800), anti-phospho-GSK-3α/β (1:1,000), anti-p65 (1:500), anti-phospho-p65 (1:500), and GAPDH (1:500) mAbs and the secondary antibody biotinylated goat anti-rabbit IgG (1:5,000) were used. The immunoreactive protein bands were visualized by a chemiluminescence reagent using a multi-functional imaging system (Tanon 5200). Then, the Image pro Plus 6.0 Software was used to measure the intensity of the CXCL16, p-AKT, p-GSK-3α/β, and p-p65 protein level bands with respect to the GAPDH reference band for calculating the levels of each protein.
2.10 Plasmids
CXCL16 cDNA was amplified from RMCs by PCR. Gene-specific primers (Sense, 5′-GGGTACCCGGCTCCATCAACGAACTGA-3′; Antisense, 5′-GGCTAGCCGAATTTGTCCAGTTTATTGTTA-3′, product size = 391 pb) of wild-type or muted miR-145-5p binding site predicted on CXCL16 were synthesized by RIB&BIO (Guangzhou, China). The 1,521-bp fragment of the CXCL16 3′-UTR containing the miR-145-5p targeting sequence, was cloned into the hRluc luciferase expressing vector (pmiR-RB-REPORT™) at the Nhel and Kpnl site to produce pmiR-RB-REPORT™-WT-CXCL16. Sequence-specific primers for the indicated genes were designed using the Primer Premier software, version 5.0. The sequences of all constructs were verified.
2.11 Dual-luciferase reporter assay
A total of 293 T cells were cultured to approximately 80% confluence in a 24-well plates and then co-transfected with a dual-luciferase reporter plasmid (pmiR-RB-REPORT™-WT-CXCL16 or pmiR-RB-REPORT™-MUT-CXCL16) and miR-145-5p mimics or negative control, using Lipofectamine 2000 (Invitrogen, #11668027) for 48 hr. The activities of firefly and renilla luciferases were determined using the Dual-Luciferase Reporter Assay System (Promega, Fitchburg, WI, cat. No. E1910), and firefly luciferase activity was normalized to that of Renilla luciferase.
2.12 Statistical analyses
Measurement data were expressed as mean ± SD, unless otherwise stated. Statistical analyses were performed using the SPSS 16.0 software package (SPSS, Inc., IBM, NY). Comparisons among groups were conducted with ANOVA. Statistical differences were evaluated with analysis of variance. A p-value <0.05 denoted a statistically significant difference. All the artworks were created by SigmaPlot 12.5.
3 RESULTS
3.1 Pathological and renal function changes in CGN rat model
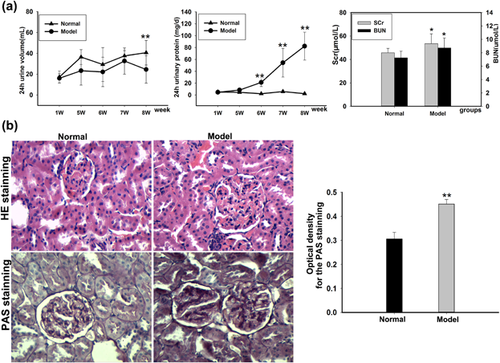
The levels of 24 hr urine volumes and urine proteins as well as the contents of serum creatinine (Scr) and blood urea nitrogen (BUN) in serum are shown in Figure 1a. The data showed decreased 24 hr urine volumes but significantly increased 24 hr urine proteins in C-BSA-induced glomerulonephritis rats when compared with normal rats. Furthermore, the C-BSA-induced glomerulonephritis rats exhibited the high levels of Scr and BUN. The results of the histopathology examinations are shown in Figure 1b. Using HE staining, the normal rat shows clear capsule of the glomerulus and kidney tubule. While in C-BSA-induced glomerulonephritis rat, the representative photomicrograph showed obvious inflammatory cells infiltration in the juxtaglomerular apparatus and the substrate. In addition, there were granular degeneration in part of renal tubular epithelial cells, swelling in glomerulus and obviously hyperplasia of collagen fiber in the matrix. Meanwhile, the optical density levels of periodic acid-Schiff (PAS) staining in model rat's kidney tissues increased markedly when compared with normal one, which testified a proliferation in glomerular mesangial matrix of model rats.
3.2 Inflammatory changes in CGN rat model
To measure the inflammatory changes in rats with glomerulonephritis, our study performed immunohistochemistry analysis to detect the expression of CD68 and cytometric bead array to determine the inflammatory factors. The positive expression of CD68 which developed with the clay bank coloration was the reflection of macrophage infection. According to the results of immunohistochemistry analysis in Figure 2a, CD68 was mainly expressed in the cytoplasm of glomerular and renal tubular interstitial. Compared with the normal group, macrophages infection obviously enlarged in kidney tissue of model rats, which suggested a notable inflammation. Results in Figure 2b showed that the contents of interleukin-1α (IL-1α) and interleukin-2 (IL-2) were raised conspicuously in model rats.
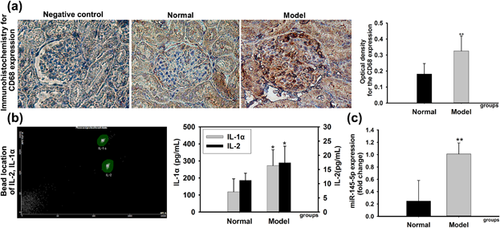
As mentioned in introduction, miRNA-145-5p was confirmed highly expressed in mesangial cells and play an important role in inflammatory responses. Thus, we performed real-time PCR to determine the expression of miRNA-145-5p in renal tissue. Collectively, the results showed that the expression of miRNA-145-5p was upregulated in model rats obviously (Figure 2c).
3.3 Effect of miR-145-5p on mesangial cell proliferation
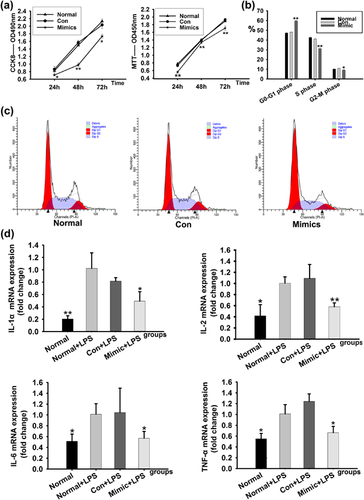
After RMCs were transfected with miR-145-5p mimics or a miRNA-negative control for 24, 48, and 72 hr, the proliferation changes of miR-145-5p mimics-transfected cells were determined with the Cell Counting Kit-8 and MTT. Proliferation rate of RMCs was reduced differentially at 24, 48n and 72 hr in miR-145-5p mimics-transfected group when compared with the normal group and Con group, which indicated that RMCs proliferation could be inhibited by miR-145-5p mimics (Figure 3a). Cell cycle was further assessed by flow cytometry. The results showed that miR-145-5p mimics markedly extended the G0-G1 phase and reduced the S phase, compared with the normal group and Con group (Figures 3b and 3c).
3.4 Effect of miR-145-5p on mesangial cells inflammatory responses
To evaluate the inflammatory responses, the expressions of IL-1α, IL-2, interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-a) mRNA were detected in RMCs. Data showed that after stimulated by lipopolysaccharide(LPS), the expressions of IL-1α, IL-2, IL-6, and TNF-a mRNA in RMCs were increased in both normal plus LPS and miRNA negative control plus LPS. In comparison, the mRNAs expressions were decreased in RMCs treated with LPS plus miR-145-5p mimics (Figure 3d). These data demonstrated that miR-145-5p had an inhibiting effect on inflammation related mRNAs.
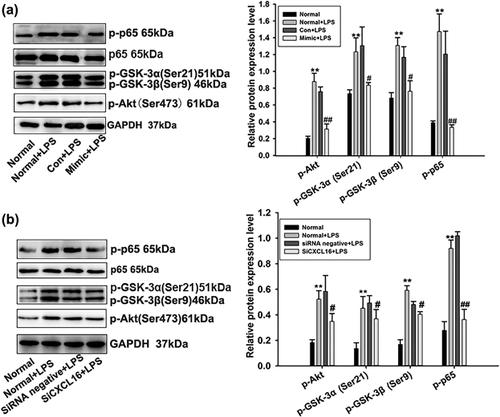
3.5 Influence of miR-145-5p on the proteins potentially involved in AKT/GSK signaling pathway
The expression levels of proteins including p-AKT, p-GSK-3a,3β in the AKT/GSK signaling pathway were detected. In addition, NF-κB was indicated to play a key role in glomerular injury (Hussain et al., 2009). Hence, the expression of p-65 and p-p65 proteins were also detected. Expression levels of p-AKT, p-GSK-3a,3β, p-65 were significantly increased in RMCs after stimulated by LPS with or without miRNA negative control transfection. In comparison, the expressions of proteins were decreased in RMCs treated by LPS plus miR-145-5p mimics (Figure 4a). Our results suggested that the AKT/GSK signaling pathway could be down-regulated by miR-145-5p.
3.6 Influence of miR-145-5p on possible target gene, CXCL16
By using online predicted and validated microRNA targets database (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/micrornapredictedtarget.html), we hypothesized that CXCL16 may be a target gene of miR-145-5p (p = 0.0027, Figure 5a). To investigate whether miR-145-5p could interact with the 3′-UTR of CXCL16 and regulate target gene expression at a post transcriptional level, the 3′-UTR fragment of CXCL16 was cloned into the dual-luciferase reporter vector plasmid, which was co-transfected into RMCs together with miR-145-5p mimics or negative control. The observations reveal that miR-145-5p mimics significantly down-regulated firefly luciferase activity (Figure 5b).
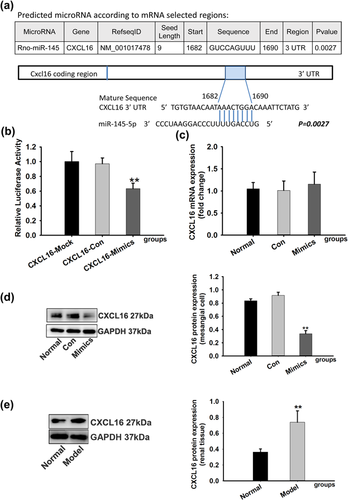
In the further study, we determined the levels of CXCL16 mRNA and protein expression in the miR-145-5p mimics-transfected cells to confirm the direct action of miR-145-5p. There was no significant difference between miR-145-5p mimics-transfected cells and control cells according to CXCL16 mRNA expression level (Figure 5c). Western blotting showed that expression of CXCL16 was significantly decreased in the miR-145-5p mimics-transfected cells when compared with the control cells (Figure 5d). These results indicated that the level of CXCL16 protein could be regulated by miR-145-5p. At the same time, we detected expression of CXCL16 protein in CGN rats. Compared with normal rats, the protein expression of CXCL16 was significantly increased in model rats (Figure 5e), which indicated that CXCL16 may play a regulative role in the progression of CGN.
3.7 Effects of siCXCL16 on RMC cell proliferation, cell inflammation, and the AKT/GSK signaling pathway
To further investigate whether CXCL16 participate in cell proliferation and cell inflammation of RMCs, we evaluated the influences of siCXCL16 on the cell proliferation, cell inflammation, and the AKT/GSK signaling pathway molecules in LPS-induced RMCs. After 24 hr transfection with siCXCL16 (Transfection efficiency is 82.5%), proliferation of RMCs was measured using CCK8 and MTT analysis. CCK8 and MTT analysis showed that the proliferation was enhanced after RMCs induced with LPS which could not be reversed by siCXCL16 (Figure 6a). Taken together, these data indicated that the suppressing effect of miR-145-5p in abnormal proliferation of RMCs might be beside the mark of CXCL16.
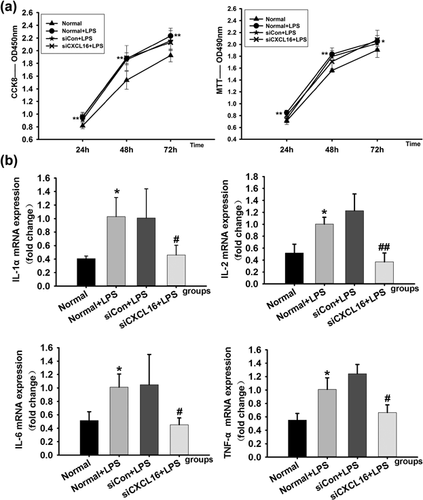
Cell inflammation was measured by the expression levels of IL-1α, IL-2, IL-6, and TNF-α mRNA in RMCs after treated with LPS and transfection with siCXCL16 or siRNA negative control meanwhile. The real-time qPCR analysis showed that the expressions of IL-1α, IL-2, IL-6, and TNF-a mRNA in RMCs were increased after being induced by LPS with or without siRNA negative control transfection. In comparison, the expression of mRNAs was decreased in RMCs induced by LPS together with siCXCL16 (Figure 6b). These data demonstrated that CXCL16 had a promoting effect on inflammation related mRNAs.
The expression levels of proteins in the AKT/GSK signaling pathway of RMCs were detected. Protein expression levels of p-AKT, p-GSK-3a,3β, p-65 were significantly increased in RMCs after being treated by LPS with or without siRNA negative control transfection. In comparison, the proteins expressions were decreased in RMCs treated by LPS together with siCXCL16 (Figure 4b). The above results suggested that the AKT/GSK signaling pathway could be down-regulated by siCXCL16.
4 DISCUSSION
Abnormal proliferation and inflammatory responses are two main pathological changes of chronic glomerulonephritis (CGN). A study suggests that chronic kidney disease could be such prevalent in the next 20 years that the proportion of people with chronic kidney disease among adults will rise from the current 13.2–14.1% in 2020 and 16.7% in 2030 (Hoerger et al., 2015). The CGN model induced by C-BSA was exactly similar to human CGN, and regarded as a classical immunocomplex induced glomerulonephritis model (Andersson, Nilsson, Hjalmarsson, Haraldsson, & Nystrom, 2007; Border et al., 1982). And mesangial cells are one of the most active cells in glomerular inherent cells. In general, the number, shape and location of mesangial cells in glomeruli are relatively stable. However, it can be activated by a series of factors such as immunoglobulins, complements and cell factors. The activated mesangial cells show the abnormal proliferation as well as the secretion of cytokines which cause the variety of glomerular disease (Kurogi, 2003). LPS, endotoxin of gram-negative bacteria, which can induce metabolic and gene expression changes of almost all eukaryotic cells and make them release various of cytokines, have been found activating mesangial cells as well (Shen et al., 2017). In this study, a C-BSA-induced CGN model in rats and LPS-induced inflammation in RMCs were established to research abnormal proliferation and inflammatory reactions related to CGN.
MiRNA is a series of small molecules involved in all kinds of kidney disease, including renal cell carcinoma (Lv et al., 2013; Yi, Fu, Zhao, Zhang, & Ma, 2010), nephrotic syndrome (Sui et al., 2014; Wang et al., 2013), diabetic nephropathy (Wang et al., 2014; Zhang et al., 2012), IgA nephropathy (Xing et al., 2014), polycystic kidney disease (Patel et al., 2013; Sun et al., 2010), lupus nephritis (Lu et al., 2012), acute rejection (Liu & Xu, 2011) etc. In our previous study, to determine the role of miRNAs in CGN, we performed a miRNA array assay on RNAs extracted from CGN rats. A total of 26 miRNAs including 3 upregulated miRNAs and 23 downregulated miRNAs were found differently expressed in normal rats and rats with CGN (supplement file). Of those differently expressed miRNAs, miR-145-5p was especially interesting because it was confirmed to highly express in mesangial cells (Harvey et al., 2008) and make a difference in inflammatory responses and cell proliferation (Lin et al., 2013), Brigant et al. (2017) found that serum level of miR-145 was elevated in patients with CKD compared with healthy controls, which was correlated with estimated glomerular filtration rate. However, the mechanism of how miR-145-5p regulates CGN remained unclear. Therefore, the biological function of miR-145-5p in the progress of CGN was elucidated in the present study.
In this study, we detected renal pathological changes, kidney function and inflammatory changes and further confirmed the differential miR-145-5p expression in model rats contrasted with normal rats. We found obvious pathological changes, reduction of kidney function and inflammatory responses. Then, we detected miR-145-5p expression in kidney tissues and found that the expression of miRNA-145 was upregulated markedly in rats with CGN.
But the protective or opposite response of up-regulated miRNA-145 in CGN model remains unclearly. Our study found that cell proliferation rate was reduced in the miR-145-5p-transfected RMCs group compared with the control group, which indicate that miR-145-5p could inhibit RMCs proliferation. The flow cytometry analysis showed that miR-145-5p inhibited RMCs proliferation and extended the G1 phase but shortened the S phase. And the mRNA expression levels of IL-1α, IL-2, IL-6, and TNF-a mRNA in RMCs were increased after treated by LPS but decreased after transfected with miR-145-5p mimics. These data demonstrated that miR-145-5p had a protective effect via inhibiting effects on proliferation and inflammation in RMCs.
AKT/GSK signaling pathway is widely involved in cell proliferation, differentiation, metabolism, which is closely related to various kidney diseases (Liu, 2004; Winbanks et al., 2007). To further define whether miR-145-5p regulates RMCs proliferation and inflammatory responses through the AKT/GSK signaling pathway, we used miR-145-5p mimics to transfect RMCs and AKT/GSK signaling pathway related proteins, such as phosphorylated-Akt, -GSK-3α,-3β were detected. Our results showed that proteins expression levels of p-AKT, p-GSK-3a,3β were significantly increased in RMCs after treated by LPS but decreased after transfected with miR-145-5p mimics which suggested that the AKT/GSK signaling pathway could be down-regulated by miR-145-5p in inflammation-induced RMCs. In addition, the expression of p-65 protein was also carried out to evaluated the anti-inflammatory of miR-145-5p. P65 is a subunit of NF-kB, and NF-κB is an important transcription factor that exists in animal cells which take part in the body's immune responses. It is closely related to abnormal immune responses such as inflammation, autoimmune diseases, and viral infections (Meffert, Chang, Wiltgen, Fanselow, & Baltimore, 2003). Research has confirmed that a variety of cytokines, such as TNF, IL- 8, IL-6, IL-2, IL-1, and G-CSF can be activated by NF-κB and affect the inflammatory responses (Siebenlist, Franzoso, & Brown, 1994). In this study, we also detected the protein expression of p-65 in RMCs transfected with miR-145-5p mimics and inhibitor.
CXCL16 is a chemotactic factor associated with immune inflammation which is mainly found in immune cells such as dendritic cells, B cells, T cells, and macrophage. There are several studies confirmed that the activation of CXCL16 participate in the progression of a variety of kidney disease progress (Norlander, Saleh, & Madhur, 2013). Compared with healthy people, CXCL16 levels significantly increased in serum of patients with diabetic nephropathy (Elewa et al., 2016), nephrotic syndrome (Zhen et al., 2014), and lupus nephritis (Reyes-Thomas, Blanco, & Putterman, 2011). These researches suggest that CXCL16 is likely to be an effective indicator of kidney damage. By using online Predicted and Validated micro RNA Targets database, we hypothesized that CXCL16 may be a direct target gene of miR-145-5p. Our study confirmed that CXCL16 is a direct target gene of miR-145-5p using a dual-luciferase reporter plasmids assay in which miR-145-5p mimics significantly down-regulated firefly luciferase activity. Moreover, the CXCL16 protein level was markedly reduced in the miR-145-5p-transfected RMCs, while the mRNA level did not change, which accounting for that miR-145-5p directly regulating translation process of CXCL16 protein rather than transcription process. It seemed strange that the protein expression of CXCL16 was significantly increased in model rats compared with normal rats, which was the opposite of what might be expected, given that miR145-5p levels increase in model rats. However, researchers have found that inflammatory mediators, such as IFN-γ, TNF-α, IL-1α, IL-1β, IL-6, and IL-18, increased CXCL16 expression (Izquierdo et al., 2014). This contradiction of CXCL16 protein expression in model animals and miRNA-145-5p-transfecred RMCs would be one of the direction of our future research.
Finally, to demonstrate that miR-145-5p modulates cell cycle proliferation, inflammatory responses and the AKT/GSK signaling pathway molecules in LPS-induced RMCs by directly inhibiting CXCL16, we used siCXCL16 to inhibit the expression of CXCL16. The results showed that the proliferation was enhanced after RMCs treated with LPS but could not be reversed by siCXCL16 which indicated that the suppressing effect of miR-145-5p in abnormal proliferation of RMCs might be beside the role of CXCL16. And the expression of IL-1α, IL-2, IL-6, and TNF-a mRNAs in RMCs were increased after being treated with LPS and reversed after transfected by siCXCL16 which demonstrated that CXCL16 had an inhibiting effect on inflammation related mRNAs. Then, we found that protein expression levels of p-AKT, p-GSK-3a,3β, and p-65 were significantly increased in RMCs after treated by LPS and reversed after transfected by siCXCL16. The above results suggested that the AKT/GSK signaling pathway could be down-regulated by siCXCL16.
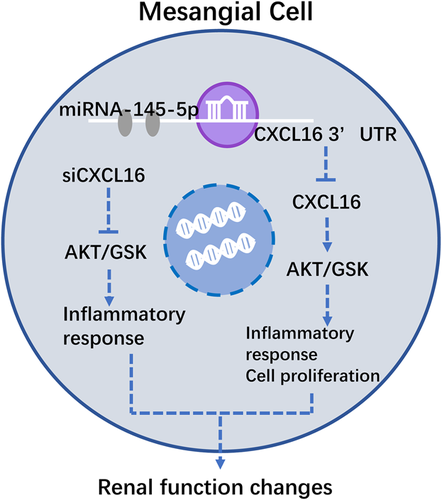
In conclusion, this study demonstrated that miR-145-5p inhibited inflammatory responses of RMCs through regulating AKT/GSK pathway by targeting CXCL16. And miR-145-5p may be also a directly factor affecting cell proliferation (Figure 7).
ACKNOWLEDGMENTS
This research was supported by grants (81603371, 81673874) from the National Natural Science Foundation of China, grants (2016A030310292, 2014A020210024) from the National Natural Science Foundation of Guangdong Province, grants from China Postdoctoral Science Foundation to Dr. J. B. Wu (2017M612645) and The Specific Research Fund for TCM Science and Technology of Guangdong Provincial Hospital of Chinese Medicine (No. YN2016YX04). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICTS OF INTEREST
All the authors declared no competing interests.