HMGB1 down-regulation mediates terameprocol vascular anti-proliferative effect in experimental pulmonary hypertension
Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease with a poor prognosis. Pulmonary artery smooth muscle cells (PASMCs) play a crucial role in PAH pathophysiology, displaying a hyperproliferative, and apoptotic-resistant phenotype. In the present study, we evaluated the potential therapeutic role of terameprocol (TMP), an inhibitor of cellular proliferation and promoter of apoptosis, in a well-established pre-clinical model of PAH induced by monocrotaline (MCT) and studied the biological pathways modulated by TMP in PASMCs. Wistar rats injected with MCT or saline (SHAM group) were treated with TMP or vehicle. On day 21 after injection, we assessed bi-ventricular hemodynamics and cardiac and pulmonary morphometry. The effects of TMP on PASMCs were studied in a primary culture isolated from SHAM and MCT-treated rats, using an iTRAQ-based proteomic approach to investigate the molecular pathways modulated by this drug. In vivo, TMP significantly reduced pulmonary and cardiac remodeling and improved cardiac function in PAH. In vitro, TMP inhibited proliferation and induced apoptosis of PASMCs. A total of 65 proteins were differentially expressed in PASMCs from MCT rats treated with TMP, some of which involved in the modulation of transforming growth factor beta pathway and DNA transcription. Anti-proliferative effect of TMP seems to be explained, at least in part, by the down-regulation of the transcription factor HMGB1. Our findings support the beneficial role of TMP in PAH and suggest that it may be an effective therapeutic option to be considered in the clinical management of PAH.
1 INTRODUCTION
Vascular remodeling is a central process in the pulmonary arterial hypertension (PAH) pathobiology. This process is associated with structural and functional alterations in the pulmonary artery wall, leading to muscularization of previously nonmuscular arteries, neointima formation, plexiform lesions development, which results in pulmonary artery obliteration. Hypertrophy, proliferation, migration, and resistance to apoptosis are features of medial smooth muscle cells associated to the development of these changes (Crosswhite & Sun, 2014; Nogueira-Ferreira, Ferreira, & Henriques-Coelho, 2014; Tajsic & Morrell, 2011). Despite the increasing knowledge of PAH pathophysiology, this disease is characterized by a late diagnosis and a high mortality rate (McGoon et al., 2013), justifying the urgent need for new and effective therapeutic strategies. Currently approved drugs do not revert nor halt the progression of the disease, have variable efficiency, and frequent systemic side effects. Despite some of them exert slight anti-proliferative effects, their main mechanism of action is through the re-establishment of the balance between vasoconstrictors and vasodilators (Gurtu & Michelakis, 2015; Provencher & Granton, 2015). Since proliferative vascular remodeling is a main feature in PAH, there is a growing interest in targeting proliferative and apoptotic pathways (Guignabert et al., 2013; Gurtu & Michelakis, 2015; Huang et al., 2010). These features are shared by cancer cells (Rai et al., 2008; Voelkel et al., 1998), therefore supporting the potential utility of anti-cancer agents with anti-proliferative and/or pro-apoptotic proprieties in the management of PAH (Guignabert et al., 2013).
Terameprocol (meso-tetra-O-methyl nordihydroguaiaretic acid, TMP) is reported to block cell cycle progression and promote apoptosis by inhibiting the transcription of genes that are dependent on the transcription factor Sp1, such as Cdc2 and survivin (Chang, Heller, Kuo, & Huang, 2004). Previous studies demonstrated that TMP exhibits in vitro and in vivo anti-tumoral activity without relevant systemic toxicity (Fulciniti et al., 2011; Heller, Kuo, Wu, Kast, & Huang, 2001; Lambert, Meyers, Timmermann, & Dorr, 2001; Lopez, Goodman, Rhodes, Blomberg, & Heller, 2007; Park et al., 2005). Clinical trials are currently being conducted to assess the potential of TMP in several cancer types (Church & Talbot, 2012; Lu et al., 2010; Ryan, O'Donovan, & Duffy, 2009). Given its described properties, we hypothesized that TMP might also be of value in the management of PAH. So, the present work explores the effects of TMP in an experimental model of PAH. We studied the in vivo effects of TMP in the monocrotaline (MCT) model and characterized the biological processes modulated by this drug in primary cultures of pulmonary artery smooth muscle cells (PASMCs).
2 MATERIALS AND METHODS
2.1 Experimental design
Housing and experimental treatment were in accordance with the Guide for the Care and Use of Laboratory Animals from the Institute for Laboratory Animal Research (ILAR, NIH Pub. No. 85-23, Revised 2011) and with the European Parliament Directive 2010/63/EU. Adult male Wistar rats (Charles River Laboratories; Barcelona, Spain) weighting 180–200 g were housed in a controlled environment under a 12:12 hr light-dark cycle at room temperature of 22°C, with a free supply of food and water. Rats randomly received a subcutaneous injection of MCT (60 mg/kg body weight (BW), Sigma–Aldrich) (MCT groups) or an equal volume of saline solution (2 ml/kg BW) (SHAM groups). In the first protocol, we treated MCT and SHAM animals with TMP (166 mg/kg BW, ip, Cayman Chemical) or an equal volume of vehicle (DMSO, Sigma–Aldrich) on days 7, 12, and 17 after MCT/saline injection, thus creating four groups: SHAM + Vehicle, SHAM + TMP, MCT + Vehicle, MCT + TMP (n = 9–14 rats per group). At day 21 after MCT/saline injection, invasive hemodynamic evaluation was performed. In a second protocol, rats injected with MCT or saline solution (n = 5 rats per group) were sacrificed on day 21 in order to isolate and establish a primary culture of PASMCs for in vitro evaluation of TMP effects.
2.2 Hemodynamic evaluation
Animals were anesthetized by inhalation of a mixture of sevoflurane (8% for induction and 2.5–3% for maintenance) and oxygen, endotracheally intubated for mechanical ventilation (150 min−1, 100% O2, 14–16 cm H2O inspiratory pressure, with tidal volume adjusted to animal weight, and 5 cm H2O end-expiratory pressure; TOPO Small Animal Ventilator − Kent Scientific, Dual Mode) and placed over a heating pad. Under binocular surgical microscopy (Leica, Wild 384000), the right jugular vein was cannulated for fluid administration (prewarmed 0.9 % NaCl solution, 32 ml/kg/hr) to compensate for perioperative losses. The heart was exposed through a median sternotomy. Conductance catheters were placed in the right ventricle (RV) and left ventricle (LV) (PVR-1045 and PVR-1035, respectively, Millar Instruments). After complete instrumentation, the animal preparation was allowed to stabilize for 15 min. Hemodynamic recordings were made with respiration suspended at end-expiration under basal conditions. Data were continuously acquired (MPVS 300, Millar Instruments), digitally recorded at 1,000 Hz (ML880 PowerLab 16/30, Millar TM Instruments), and analyzed (LabChart, ADInstruments, RRID:SCR_001620).
2.3 Morphometric and histological analysis
After complete hemodynamic assessment, animals were euthanized by exsanguination under anesthesia. The heart, lungs, and gastrocnemius muscle were excised and weighted. The right tibia was also excised and its length was measured with a millimetric ruler. The RV free wall was dissected from the LV + septum (S), under binocular magnification (3.5×) and weighted separately. Heart, lungs, RV, and LV + S weights were normalized to BW and gastrocnemius weight was normalized to tibial length. RV weight was also normalized to that of LV + S. For histological analysis, RV and lung samples were immersion fixed in 4% paraformaldehyde and embedded in paraffin. Sections 4 µm thick were cut and stained with hematoxylin and eosin. RV free wall specimens were obtained from each heart at midway between the apex and base. Studied samples were observed at microscope, photographed with a digital camera, and measured with a digital image analyzer (cell^B life science basic imaging software, Olympus). All the measurements were made directly at 400× magnification and only round to ovoid nucleated myocytes were considered for analysis. RV samples were divided into five sections and the area of fifty cardiomyocytes per sample was measured and averaged. On the pulmonary specimens, external diameter and medial wall thickness in small muscular arteries (diameter <50 µm, 12–18 arteries/lung) were analyzed at 400× magnification. Orthogonal intercepts were used to generate eight random measurements of external diameter (distance between the external lamina) and sixteen random measurements of medial thickness (distance between the internal and external lamina). For each artery, medial hypertrophy was expressed as follows: % wall thickness = [(medial thickness × 2)/(external diameter)] × 100.
2.4 Isolation and primary culture of PASMCs
A primary culture of PASMCs was established using an enzymatic dissociation process adapted from a previously described protocol (Ducret et al., 2010). Animals were euthanized with an intraperitoneal injection of pentobarbital (120 mg/kg BW). The left upper lung lobe was removed and placed in a calcium enriched Hank's Buffered Salt Solution (HBSS, Invitrogen) (5.4 mM KCl, 137 mM NaCl, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 0.25 mM NaH2PO4, 1 mM d-glucose, 0.2 mM phenol red, 2 mM CaCl2, and 1 mM MgCl2). First order intrapulmonary artery was dissected free of connective tissue under a stereomicroscope (Leica EZ4). After extraction from the lung, the adventitia was removed, the vessel was opened longitudinally, and endothelium was removed by gently rubbing the inner surface with forceps tips. In order to release smooth muscle cells, the artery, mainly consisting of medial layer, was submitted to an enzymatic dissociation process with papain (Sigma–Aldrich) and collagenase type 1 (Worthington Biochemical Corp.), in a calcium-poor HBSS solution (25 µM CaCl2 and 1 mg/ml BSA). Next, a mechanical dissociation process was performed with a fire polished, silicone coated glass pipette, in order to release the cells. The cell-free tissue was removed and the solution was centrifuged (250g, 5 min, room temperature) in order to pellet the cells. Finally, cells were seeded in DMEM culture medium (PAN Biotech), supplemented with 1% penicillin-streptomycin-amphotericin B (Invitrogen), 1% sodium pyruvate (Sigma–Aldrich), and 1% nonessential aminoacids (Sigma–Aldrich) containing 10% FBS (fetal bovine serum, Sigma–Aldrich), in a 24-well culture plate (500 µl/well) and placed in an incubator (37°C, 5% CO2). The medium was changed after 24 hr and then every 48 hr. Cell passage was performed when approximately 80% confluence was achieved and they were then detached with trypsin (PAN Biotech), passaged and cultured continuously. Cells between passages 2 and 4 were used for all experiments. Smooth muscle origin was confirmed by typical morphology (fusiform shape with hills and valleys) and detection of smooth muscle α-actin expression by immunocytochemistry (data not shown).
2.5 Cell proliferation assay
The effect of TMP in cell proliferation was evaluated using the 5′-bromodeoxyuridine (BrdU) immunoassay (Roche Diagnostics). BrdU is a thymidine analogue and its incorporation in the DNA is a measure of cell proliferation. For BrdU assay, cells were seeded in 24-well plates at a density of 1.5 × 104 cells⁄ml. After 72 hr, medium was removed and cells were incubated with different concentrations of TMP prepared in DMSO (0, 0.1, 1, 10, and 20 µM) for 24 hr in medium without FBS. The BrdU assay was conducted following the 24 hr incubation with TMP/vehicle. All protocol was followed according to manufacturer's instructions. Each condition (concentration) was tested in triplicate and in cells from three animals per group.
2.6 Apoptosis assay
For apoptosis evaluation, we performed the TUNEL (TdT-mediated dUTP Nick End Labeling) assay using the In Situ Cell Death Detection kit (Roche Diagnostics), according to the manufacturer's instructions. For TUNEL assay, cells were seeded in 24-well plates, with glass coverslips, at a density of 1.5 × 104 cells/ml. After 72 hr, medium was removed and cells were incubated with different concentrations of TMP prepared in DMSO (0, 0.1, 1, 10, and 20 µM) for 24 hr in medium without FBS. After 24 hr incubation with TMP/vehicle, cells were stained with TUNEL and DAPI (4′,6-diamidino-2-phenylindole, Roche Diagnostics). The percentage of apoptotic cells was calculated by dividing the number of cells stained with TUNEL (apoptotic cells) by the total number of nuclei stained with DAPI, in at least 10 different fields at 200× magnification. Each condition (concentration) was tested in triplicate and in cells from three animals per group.
2.7 Preparation of protein extracts and peptide labeling with iTRAQ
For iTRAQ (isobaric tags for relative and absolute quantification) analysis, cells were seeded in 6-well plates at a concentration of 1.5 × 104 cells⁄mL. Medium was changed after 24 hr and after 48 hr cells were incubated for 24 hr in medium without FBS, containing TMP 10 µM or DMSO (CONT). This TMP concentration was chosen based on data from proliferative and apoptotic assays. Indeed, at this concentration, the proliferation of PASMCs was significantly inhibited whereas signs of apoptosis were not evident. So, a sufficient number of cells was obtained for proteomics at an effective anti-proliferative concentration of TMP. The medium was removed from the culture plates and after a wash in ice cold 1× PBS, cells were lysed in 1× RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25% sodium deoxycholate, 1% NP-40, 1 mM EDTA) supplemented with Phosphatase Inhibitor Cocktail (Sigma–Aldrich) and protease inhibitor (200 mM PMSF) and detached from the plates using cell scrapers. Samples were vortexed (5 min), sonicated, and then centrifuged (12,000g, 5 min, 4°C). The supernatant was collected and the protein content of the cell homogenate was assayed with the RC-DC method (Bio-Rad), following the instructions of the manufacturer, using bovine serum albumin (BSA) as standard.
Two independent assays were performed, one for SHAM cells and other for MCT cells, using three animals per group. An in-solution digestion was performed for iTRAQ labeling, as previously described (Carvalhais, Cerca, Vilanova, & Vitorino, 2015). Briefly, 100 µg of protein was precipitated with nine volumes of cold acetone at −20°C for 3 hr. After samples centrifugation (20,000g, 30 min, 4°C) and acetone removal, pellets were resuspended with 0.1 M TEAB (triethylammonium bicarbonate buffer, Sigma–Aldrich), pH 8.5, and 2% SDS to achieve a final concentration of 0.05%. Samples were then reduced with 50 mM TCEP (tris(2-carboxyethyl)phosphine, Sigma–Aldrich) for 1 hr at 60 °C with agitation. Then, samples were alkylated with 10 mM MMTS (S-methyl methanethiosulfonate, Sigma-Aldrich) for 10 min at room temperature with agitation. Trypsin was added to each sample and the digestion was performed for 18 hr at 37°C. Digested sample peptides were subsequently labeled with the iTRAQ® reagent 8-plex (ABSciex) following the protocol provided by the manufacturer. Briefly, labels were reconstituted in 60% isopropanol, added to each sample peptides and incubated for 2 hr at room temperature with agitation. Labeled samples were then combined and dried in a SpeedVac. Then, labeled peptides were separated by a multidimensional LC approach, as described by Carvalhais et al. (2015). Sample loading was performed at 200 μl/min with buffers (A) 2% ammonium hydroxide and 0.014% formic acid in water, pH 10; and (B) 2% ammonium hydroxide and 90% ACN (acetonitrile) in water, pH 10. After 5 min of sample loading and washing, peptide fractionation was performed with linear gradient to 70% B over 85 min. Sixty fractions were collected, dried in a SpeedVac and resuspended in 5% ACN and 0.1% TFA (trifluoroacetic acid). Collected fractions were then separated by LC. Briefly, peptides loaded onto a C18 pre-column (5-mm particle size, 5 mm; Dionex) connected to an RP column PepMap100 C18 (150 mm × 75-mm i.d., 3-mm particle size). The flow rate was set at 300 nl/min. The mobile phases A and B were 2% ACN and 0.05% TFA in water, and 90% ACN with 0.045% TFA in water, respectively. The gradient started at 10 min and ramped to 60% B till 50 min and 100% B at 55 min and retained at 100% B till 65 min. The separation was monitored at 214 nm using a UV detector (Dionex/LC Packings). Using the micro-collector Probot (Dionex/LC Packings) and, after a lag time of 5 min, peptides eluting from the capillary column were mixed with a continuous flow of α-CHCA matrix solution (in internal standard Glu-Fib) and were directly deposited onto the LC-MALDI plates. The spectra were generated and processed with 4800 MALDI-TOF/TOF. Protein identification based on MS/MS data were performed with ProteinPilot™ software (v.4.04, ABSciex) using Paragon search method. SwissProt from Rattus norvegicus (release date October 21, 2014) was used as protein database. Default search parameters used were as follows: trypsin as the digestion enzyme with two missed cleavages, 40-ppm tolerance, carbamidomethyl modification on cysteine residue, iTRAQ 8-plex modification of N-terminal, and lysine peptide residues as fixed modification. Additionally, biological modifications with emphasis on methionine oxidation, deamidation and iTRAQ 8-plex modification of tyrosine residue and deamidation were considered variable modifications. Bias correction was applied, and proteins were identified with an unused ProtScore >1.3 with at least one peptide at a confidence level of 95%. Proteins identified with one peptide were manually validated when MS/MS spectra presented at least five successive amino acids covered by b or y fragmentations. Only proteins identified with a minimum of two peptides at a confidence level of 95% were included in the quantification. The quantification results were reviewed manually for all proteins found to be differentially expressed (p < 0.05).
2.8 Immunohistochemical analysis
Cubic pieces from lung tissue were fixed (4% [v/v] buffered paraformaldehyde) by diffusion during 24 hr and subsequently dehydrated with graded ethanol and included in paraffin blocks. Serial sections (5 µm of thickness) of paraffin blocks were cut by a microtome and mounted on silane-coated slides. The slides were dewaxed in xylene and hydrated through graded alcohols finishing in PBS solution. Deparaffinized sections of lung tissue were stained for immunohistochemical staining of rabbit anti-HMGB1 (1:400 dilution; Abcam, Cat# ab79823, RRID:AB_1603373), using the secondary anti-body goat biotinylated anti-rabbit (1:200 dilution; Vector Laboratories, Cat# BA-1000, RRID:AB_2313606) and counterstained with hematoxylin-eosin, as previously described (Moreira-Goncalves et al., 2011).
2.9 Western blotting analysis
Equivalent amounts of PASMC protein from each experimental group were electrophoresed on a 15% SDS–PAGE as described by Laemmli (1970). Gels were blotted onto nitrocellulose membranes (Whatman®, Protan®) in transfer buffer (25 mM Tris, 192 mM glycine, pH 8.3, and 20% methanol) for 2 hr (200 mA). Then, nonspecific binding was blocked with 5% (w/v) nonfat dry milk in TBS-T (100 mM Tris, 1.5 mM NaCl, pH 8.0, and 0.5 % Tween 20). Membranes were incubated with primary anti-body solution diluted 1:10,000 in 5% (w/v) nonfat dry milk in TBS-T (rabbit anti-HMGB1, ab79823, Abcam). After 2 hr incubation at room temperature with agitation, membranes were washed with TBS-T and incubated, with agitation, with anti-rabbit IgG peroxidase secondary antibody (Amersham, GE Healthcare) diluted 1:1,000 in 5% (w/v) nonfat dry milk in TBS-T. Immunoreactive bands were detected with enhanced chemiluminescence reagents (ECL, Amersham Pharmacia Biotech) according to the manufacturer's procedure and images were recorded using X-ray films (Amersham Hyperfilm ECL, GE Healthcare). The films were scanned in Molecular Imager Gel Doc XR + System (Bio-Rad) and analyzed with Image Lab software (v4.1, Bio-Rad). Protein loading was confirmed by staining the membranes with Ponceau S and immunoblotting with a mouse anti-alpha tubulin antibody (ab7291, Abcam, Cat# ab7291, RRID:AB_2241126).
2.10 Statistical analysis
Statistical analysis was performed using Prism GraphPad v6 software (RRID:SCR_002798). Group data are presented as means ± SE (standard error) and were compared using an unpaired t-test (data sets with two variables) or a two-way ANOVA (data sets with four variables). When the normality test failed, the two-way ANOVA was preceded by a logarithmic transform to obtain a normal distribution. When treatments were significantly different, the Holm–Sidak test was selected to perform pairwise multiple comparisons. Statistical significance was set at p < 0.05.
3 RESULTS
3.1 Effects of terameprocol on cardiac hemodynamics
The effects of TMP treatment in hemodynamics are summarized in Table 1. Compared to the SHAM group, animals from the MCT + Vehicle group presented increased RV peak systolic pressure, dP/dtmax and absolute value of dP/dtmin, as well as a significant reduction in RV stroke volume, ejection fraction, and cardiac output. TMP treatment improved cardiac function in MCT-induced PAH, normalizing RV peak systolic pressure, dP/dtmax, RV ejection fraction, stroke volume, and cardiac output and attenuating the increase in the absolute value of dP/dtmin. Regarding LV, we found significant reduction in stroke volume and cardiac output in MCT + Vehicle, while these changes were prevented in MCT + TMP group.
| SHAM + Vehicle | SHAM + TMP | MCT + Vehicle | MCT + TMP | |
|---|---|---|---|---|
| RV function | ||||
| HR (bpm) | 381 ± 12 | 377 ± 12 | 365 ± 8 | 393 ± 10 |
| Stroke volume (µl) | 154 ± 9 | 151 ± 7 | 71 ± 18* | 134 ± 15‡ |
| Output (ml/min) | 59 ± 4 | 57 ± 4 | 26 ± 6* | 52 ± 5‡ |
| EF (%) | 65 ± 6 | 70 ± 4 | 29 ± 7* | 71 ± 5‡ |
| PSP (mmHg) | 26.1 ± 0.7 | 26.4 ± 0.7 | 58.2 ± 8.9* | 35.2 ± 1.9‡ |
| dP/dtmax (mmHg/s) | 1,419 ± 56 | 1,567 ± 81 | 2,979 ± 365* | 2,107 ± 83‡ |
| EDP (mmHg) | 4.5 ± 0.8 | 4.7 ± 0.7 | 5.1 ± 0.9 | 4.2 ± 0.7 |
| dP/dtmin (mmHg/s) | −1,316 ± 152 | −1,217 ± 71 | −2,747 ± 212* | −2,000 ± 97‡,† |
| LV function | ||||
| HR (bpm) | 378 ± 15 | 383 ± 17 | 367 ± 8 | 392 ± 10 |
| Stroke volume (µl) | 243 ± 25 | 212 ± 18 | 131 ± 16* | 177 ± 23 |
| Output (ml/min) | 92 ± 8 | 81 ± 6 | 48 ± 6* | 69 ± 8 |
| EF (%) | 74 ± 4 | 73 ± 4 | 71 ± 3 | 69 ± 4 |
| PSP (mmHg) | 88.4 ± 6.0 | 98.1 ± 1.5 | 94.9 ± 4.9 | 94.4 ± 4.0 |
| dP/dtmax (mmHg/s) | 5,582 ± 461 | 6,871 ± 293 | 5,724 ± 218 | 6,047 ± 428 |
| EDP (mmHg) | 5.4 ± 1.5 | 4.4 ± 1.3 | 4.5 ± 0.7 | 6.6 ± 1.9 |
| dP/dtmin (mmHg/s) | −6,040 ± 828 | −7,691 ± 501 | −5,242 ± 458 | −6,831 ± 392 |
- SHAM + Vehicle, saline-injected, vehicle-treated group; SHAM + TMP, saline-injected, terameprocol-treated group; MCT + Vehicle, monocrotaline-injected, vehicle-treated group; MCT + TMP, monocrotaline-injected, terameprocol-treated group; RV, right ventricle; LV, left ventricle; HR, heart rate; EF, ejection fraction; PSP, peak systolic pressure; dP/dtmax, peak rate of pressure rise; EDP, end-diastolic pressure; dP/dtmin, peak rate of pressure fall. Data are presented as mean ± SE. *p < 0.05 versus SHAM + Vehicle; †p < 0.05 versus SHAM + TMP; ‡p < 0.05 versus MCT + Vehicle.
3.2 Effects of terameprocol on morphometric and histological characteristics
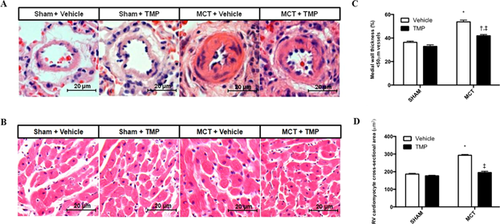
MCT-induced RV hypertrophy was normalized by TMP treatment, as shown by RV/(LV + S) and RV cardiomyocyte cross-sectional area (Table 2 and Fig. 1). In the MCT group, small pulmonary arteries presented significant medial hypertrophy, which was significantly reduced by TMP. TMP treatment also normalized the lung/body weight ratio. No evidence of hepatic or renal histological damage was found in TMP treated animals (data not shown).
| SHAM + Vehicle | SHAM + TMP | MCT + Vehicle | MCT + TMP | |
|---|---|---|---|---|
| BW (g) | 295 ± 8 | 265 ± 8* | 254 ± 7* | 247 ± 6 |
| HW/BW (g/Kg) | 2.89 ± 0.05 | 2.68 ± 0.08 | 3.59 ± 0.24* | 2.92 ± 0.11‡ |
| RV/(LV + S) (g/g) | 0.30 ± 0.01 | 0.31 ± 0.02 | 0.49 ± 0.03* | 0.35 ± 0.02‡ |
| RV/BW (g/Kg) | 0.57 ± 0.02 | 0.59 ± 0.04 | 0.94 ± 0.09* | 0.68 ± 0.04‡ |
| (LV + S)/BW (g/Kg) | 1.33 ± 0.05 | 1.20 ± 0.05 | 1.41 ± 0.08 | 1.31 ± 0.05 |
| LuW/BW (g/Kg) | 4.59 ± 0.30 | 4.34 ± 0.08 | 9.56 ± 1.01* | 5.88 ± 0.35‡ |
| GW/tib (g/cm) | 0.52 ± 0.01 | 0.49 ± 0.01 | 0.50 ± 0.01 | 0.47 ± 0.01 |
- SHAM + Vehicle, saline-injected, vehicle-treated group; SHAM + TMP, saline-injected, terameprocol-treated group; MCT + Vehicle, monocrotaline-injected, vehicle-treated group; MCT + TMP, monocrotaline-injected, terameprocol-treated group; BW, body weight; HW, heart weight; RV, right ventricle weight; LV + S, left ventricle plus septum weight; LuW, lung weight; GW, gastrocnemius weight; tib, tibial length. Data are presented as mean ± SE. *p < 0.05 versus SHAM + Vehicle; ‡p < 0.05 versus MCT + Vehicle.
3.3 Effects of terameprocol on pulmonary artery smooth muscle cell proliferation and apoptosis
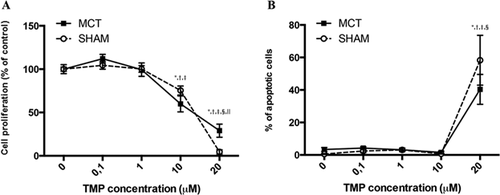
TMP treatment (10 and 20 µM) significantly inhibited the proliferation of PASMCs from SHAM and MCT-injected rats, in a dose-dependent manner (Fig. 2A). At a 20 µM concentration, the inhibitory effects of TMP in the proliferation of cells from the MCT group were less marked. TMP treatment (20 µM) induced apoptosis of PASMCs from both SHAM and MCT-treated rats (Fig. 2B).
3.4 Analysis of terameprocol impact on the proteomic profile of pulmonary artery smooth muscle cells
NanoLC-MS/MS analysis of PASMCs isolated from SHAM and MCT animals allowed the identification of a total of 560 distinct proteins (Supplementary Table S1). In order to detect variations in protein abundance induced by TMP treatment, iTRAQ-based quantification was performed. With this methodology, 18 and 65 proteins were found to be differentially expressed (p < 0.05) between PASMCs treated with TMP (compared with control), in cells from SHAM and MCT rats, respectively (Supplementary Tables S2 and S3 and Fig. 3A and 3B). Nine proteins were found to be common to SHAM and MCT groups, namely brain acid soluble protein 1, moesin, non-muscle caldesmon, nucleoside diphosphate kinase B, protein disulfide-isomerase A1, protein S100-A6, tropomyosin alpha-1 chain, tropomyosin alpha-4 chain, and tropomyosin beta chain. The majority of these proteins exert a role at the level of the cell's structure.
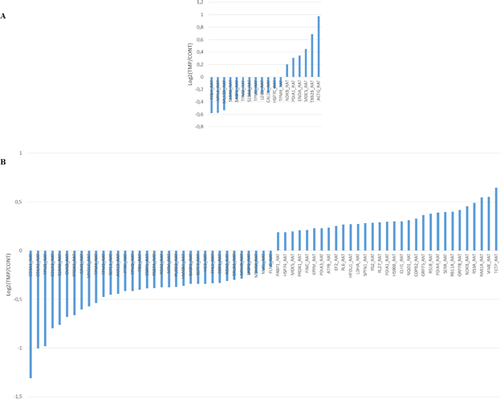
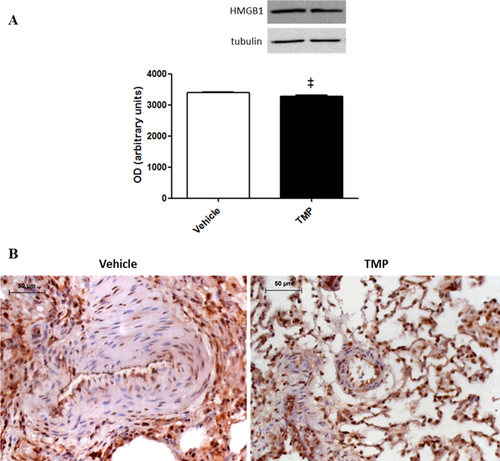
Among the 18 proteins that we found to be differentially expressed in cells from SHAM animals, six were up-regulated and 12 were down-regulated. According to PANTHER (RRID:SCR_004869) (Mi, Muruganujan, & Thomas, 2013), 42.9% of the proteins are involved in structural integrity of the cell. Cellular (21.1%) and developmental (17.5%) processes are the biological processes associated with the majority of the proteins. Furthermore, 47.4% of the proteins belong to the cytoskeleton (Supplementary Fig. S1). Between the 65 proteins differentially expressed in cells from MCT rats, 31 proteins were found to be up-regulated and 34 were down-regulated. According to PANTHER analysis (Mi et al., 2013), structural activity (32.9%), catalytic activity (24.7%), and binding (23.5%) were the main molecular functions associated with the differentially expressed proteins. Metabolic (20.8%) and cellular (18.5%) processes were the most prevalent biological processes. Regarding the protein class, the majority of the proteins differentially expressed were cytoskeletal proteins (21.4%), followed by nucleic acid binding proteins (13.1%) (Supplementary Fig. S2). Although not being a major class, the protein class of transcription factors (3.6%) was also highlighted from PANTHER analysis of PASMCs from the MCT group (but not in cells from SHAM animals). This cluster included the protein HMGB1, whose levels decreased with TMP treatment. Western blotting analysis confirmed the decreased expression of this protein in PASMCs from MCT rats treated with TMP (Fig. 4A). Additionally, immunohistochemical analysis of HMGB1 in lung tissue from MCT + Vehicle and MCT + TMP groups evidenced the staining of this protein in PASMC nuclei in both groups (Fig. 4B).
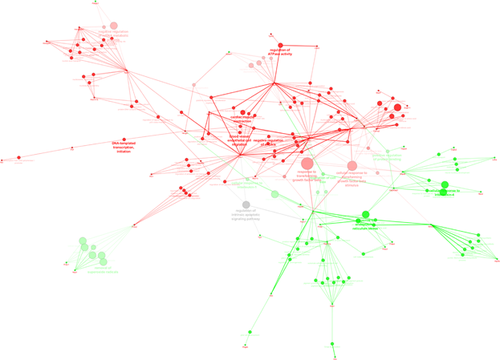
An integrated analysis of proteins that were found to be differentially expressed in cells from MCT rats treated with TMP was performed with Cytoscape (v3.1.1.) (RRID:SCR_003032) (Shannon et al., 2003), plugins ClueGO + CluePedia (Bindea et al., 2009; Bindea, Galon, & Mlecnik, 2013). This analysis retrieved “positive regulation of protein binding,” “regulation of cell size,” “cellular response to IL-4,” “cellular response to IL-1,” “response to endoplasmic reticulum (ER) stress,” and “removal of superoxide radicals” as the biological processes up-regulated in PASMCs treated with TMP. The biological processes “response to transforming growth factor beta (TGF-beta),” “regulation of ATPase activity,” “negative regulation of anoikis,” “negative regulation of mRNA metabolic process,” and “DNA-templated transcription” were shown to be down-regulated by TMP (Fig. 5).
Finally, comparison of proteins identified experimentally with those predicted by SwissTargetPrediction tool (Gfeller et al., 2014) as TMP targets (Supplementary Figs. S3 and S4), evidenced common processes, such as regulation of cytoskeleton organization, cell mobility, migration, and growth. So, our data suggest that the regulation of processes retrieved from proteomic analysis, such as DNA-templated transcription and response to TGF-beta, might be the specific of PASMCs treated with TMP in experimental PAH.
4 DISCUSSION
The hyperproliferative and apoptotic-resistant phenotype of PASMCs constitutes an attractive target in the treatment of PAH. In order to study the potential beneficial effects of TMP in PAH, we evaluated its in vivo effects in MCT model of PAH and searched for the molecular mechanisms regulated by this drug in primary cultures of PASMCs using a quantitative proteomic approach. The MCT rat model has been extensively used to study the pathogenesis of PAH, as well as the effects of drug interventions. Although there are some differences between this experimental model and human PAH, MCT induces pulmonary artery medial layer remodeling (Maarman, Lecour, Butrous, Thienemann, & Sliwa, 2013; Ryan, Marsboom, & Arche, 2013; Stenmark, Meyrick, Galie, Mooi, & McMurtry, 2009), which was the focus of this study.
TMP is being studied as an anti-cancer drug, reported to cause growth arrest in several cancer types, both in vitro and in vivo, showing no relevant toxicity (Chang et al., 2004; Lopez et al., 2007; Lu et al., 2010). TMP administration improved the hemodynamic features of MCT-induced PAH, such as RV peak systolic pressure and RV ejection fraction, stroke volume, and cardiac output. Importantly, TMP did not change hemodynamic parameters in the SHAM animals, suggesting it has no adverse effects on cardiac function. Additionally, cardiac hypertrophy and pulmonary vascular remodeling decreased with TMP administration in MCT-induced PAH. TMP exerted anti-proliferative and pro-apoptotic effects in PASMCs, supported by the down-regulation of proteins involved in mRNA metabolism and DNA-templated transcription. It was reported that TMP reduces the transcription of genes controlled by Sp1 (Huang, Chang, & Mo, 2006). Our data highlighted the modulation of the transcription factors HMGB1, Fhl-1 and Ptrf by TMP in PASMCs from PAH rats. The role of HMGB1 in PAH was already reported and implicated with pulmonary vascular remodeling by enhancing PASMC proliferation and migration (Bauer et al., 2012; Sadamura-Takenaka et al., 2014; Wang et al., 2014). The inhibition of HMGB1 by Glycyrrhizin was previously shown to attenuate the progression of MCT-induced PAH (Yang et al., 2014). Therefore, our results suggest that the anti-proliferative effects of TMP on PAH can be mediated, at least in part, by the down-regulation of HMGB1 in PASMCs. In cells from SHAM animals, TMP had no significant impact on HMGB1 levels or even on overall PASMC proteome, which presented only subtle expression differences, mainly for structural proteins, in opposition to the observed in cells from MCT rats treated with this drug.
In addition to nucleic acid binding proteins, PANTHER analysis highlighted other major cluster of proteins modulated by TMP in PAH. Within this group we found collagen alpha-1 (I) chain, collagen alpha-2 (I) chain and collagen alpha-1 (III) chain. Collagen deposition in pulmonary vessels is one of the main features of vascular remodeling seen in PAH and circulating markers of collagen metabolism have been associated with worse stages of PAH (Safdar et al., 2014). According to our data, TMP decreased the levels of these proteins, which could be mediated by TGF-beta signaling. Dysfunctional signaling of the TGF-beta pathway is associated with PAH pathogenesis, contributing to abnormal SMC proliferation (Davies et al., 2012; Morrell et al., 2001). In addition, TGF-beta signaling inhibition was reported to prevent MCT-induced PAH development and progression, through the inhibition of PASMC proliferation (Long et al., 2009; Thomas et al., 2009; Zaiman et al., 2008). Thus, TMP-related down-regulation of the response to TGF-beta might have contributed to the anti-proliferative effects of this drug on PASMCs from MCT rats.
Interestingly, TMP also up-regulated the biological process “response to ER stress.” This is associated with the increased levels of HSP70 and HSP90 family of chaperones (heat shock protein HSP90-beta, 78 kDa glucose-regulated protein, hypoxia up-regulated protein 1, and stress-70 protein, mitochondrial) involved in the correct folding and degradation of misfolded proteins. ER stress was recently suggested as a new therapeutic target for the management of PAH. The use of some chemical chaperones in experimental PAH was shown to prevent and even reverse the disease, suppressing proliferation and inducing apoptosis of PASMCs (Dromparis et al., 2013).
Taken together, our results suggest that TMP may be seen as an attractive therapeutic strategy for the management of PAH since it ameliorated the hemodynamic features and both cardiac and vascular remodeling. The regulation of DNA transcription and TGF-beta pathway seems to underlie these functional and morphological adaptations.
ACKNOWLEDGMENTS
The authors are thankful to Nádia Gonçalves, Maria José Mendes, Marta Oliveira, Joana Brandão, and Fábio Carneiro for their technical assistance in animal studies and sample processing. We gratefully acknowledge Dr. Delfim Duarte and Professors Raquel Soares, Rita Negrão, and Laura Ribeiro from the Department of Biochemistry, Faculty of Medicine of Porto, for their invaluable technical contribution to the cell culture experiments. We also thank Celeste Resende from Faculty of Sport, University of Porto for skilled assistance in immunohistochemistry experiments.
CONFLICTS OF INTEREST
The authors declare no conflicts of interests, financial, or otherwise.