PPARα Antagonist AA452 Triggers Metabolic Reprogramming and Increases Sensitivity to Radiation Therapy in Human Glioblastoma Primary Cells
Abstract
Glioblastoma (GB) is the most common cancer in the brain and with an increasing incidence. Despite major advances in the field, there is no curative therapy for GB to date. Many solid tumors, including GB, experienced metabolic reprogramming in order to sustain uncontrolled proliferation, hypoxic conditions, and angiogenesis. PPARs, member of the steroid hormone receptor superfamily, are particularly involved in the control of energetic metabolism, particularly lipid metabolism, which has been reported deregulated in gliomas. PPARα was previously indicated by us as a potential therapeutic target for this neoplasm, due to the malignancy grade dependency of its expression, being particularly abundant in GB. In this work, we used a new PPARα antagonist on patient-derived GB primary cells, with particular focus on the effects on lipid metabolism and response to radiotherapy. The results obtained demonstrated that blocking PPARα results in cell death induction, increase of radiosensitivity, and decrease of migration. Therefore, AA452 is proposed as a new adjuvant for the gold standard therapies for GB, opening the possibility for preclinical and clinical trials for this class of compounds. J. Cell. Physiol. 232: 1458–1466, 2017. © 2016 Wiley Periodicals, Inc.
Glioblastoma is the most common cancer in the brain with high incidence. The survival for GB patients rarely extends beyond 1–2 years due to the therapeutic resistance and recurrence. Despite major advances in the field, there is no curative therapy for GB to date. Current treatment approaches aim at achieving superior long-term outcome (Kleihues and Ohgaki, 1999; Fisher et al., 2007; Furnari et al., 2007; Louis et al., 2007; Haar et al., 2012; Jovcevska et al., 2013). We previously identified peroxisome proliferator-activating receptor-α (PPARα) as a potential therapeutic target for this disease, due to the malignancy grade dependency of its expression, being particularly abundant in GB (Benedetti et al., 2010a; Laurenti et al., 2011). PPARα is a member of the steroid hormone receptor superfamily that, upon ligand activation, heterodimerizes with the retinoid X receptor binding to the specific promoter sequence and triggering the expression of a variety of target genes, including those involved in glucose, lipid, and amino acid metabolism (Kota et al., 2005; Feige et al., 2006). The PPAR receptors have an important, although likely pleiotropic, role in malignancy due to their multiple roles. Whether they act as tumor suppressors or inducers is still not clear, since their functions may be related to cancer type and/or specific microenvironment of the tumor. Increased PPARα activation has been found to result in tumor progression and growth in many cancers, including renal cancer, hepatocellular carcinoma (Peters et al., 2005; Aboud et al., 2013), and breast cancer (Suchanek et al., 2002; Chang et al., 2013).
Many solid tumors, including GB, experienced metabolic reprogramming in order to sustain uncontrolled proliferation, hypoxic conditions, and angiogenesis (Kroemer and Pouyssegur, 2008; Tennant et al., 2009).
Surprisingly, little attention has been given to targeting metabolic enzymes in GB. While the traditional view has been that cancer cells are fueled by glucose (Warburg, 1956a,b), recent studies demonstrate the involvement of fatty acid oxidation (FAO) and also of mevalonate pathway (Laezza et al., 2015) in cancer cell viability. Some solid tumors, including prostate, ovarian, and renal cell carcinoma, rely on fatty acids to satisfy their metabolic needs (Collett et al., 2000; Liu, 2006; Menendez and Lupu, 2007; Carracedo et al., 2013). Solid tumors that are initially dependent on glucose can undergo a metabolic switch upon detachment from the extracellular matrix and start depending on FAO for survival (Carracedo et al., 2012).
It was recently shown that compared with normal B cells, CLL cells overexpress PPARα, rendering them dependent on β-oxidation for energy (Spaner et al., 2013; Tung et al., 2013; Messmer et al., 2015). The same was reported by us in glioma post-surgical specimens, describing a malignancy grade dependency of PPARα expression, with the GB displaying the higher PPARα levels (Benedetti et al., 2010b). These reports indicated PPARα as a promising molecular target for the treatment of this cancer. However, to date, no clinically relevant, selective PPARα antagonists have been available to address this idea in human trials.
We report herein on the effects of a new PPARα antagonist (N-(methylsulfonyl) amides, AA452, (Ammazzalorso et al., 2013) on the energetic metabolism of different human GB primary cells obtained from patient's post-surgical specimens. The treatment affected cell death and radiation response. In summary, these preclinical data substantiate the idea that PPARα is a key regulator of metabolic reprogramming in tumor cells and that its inactivation induces cell death and increases radiation sensitivity in patients-derived GB cultures.
Materials and Methods
Materials
DMEM, fetal bovine serum (FBS), penicillin/streptomycin, glutamine, formaldehyde, paraformaldehyde, triton X-100, phosphate buffered saline (PBS), bovine serum albumin (BSA), poly-l-lysine, DAPI, trypan blue, ethanol, propidium iodide, Nonidet-P40, sodium deoxycholate, sodium dodecyl sulphate (SDS), Tween 20, Igepal CA 630, phosphatase inhibitor cocktail 2, protease inhibitor cocktail, ethylenediamine tetraacetic acid (EDTA), acrylamide/bis-acrylamide, Tris(hydroxymethyl)aminomethane (Tris), hydrogen chloride (HCl), sodium chloride (NaCl), xylazine hydrochloride were purchased from Sigma Chemical Co. (St. Louis, MO). 4,4-difluoro-1,3,5,7,8-pentamethyl 4-bora-3a,4a-diaza-s-indacene (BODIPY 493/503) and CellTrace CFSE Cell Priliferation kit were from Molecular Probes, Life Technologies (Lofer, Salzburg, Austria Life Technologies). Cell Titer One Solution Cell Proliferation Assay was purchased from Promega (Lyon, France). 5′-Bromo-2′-deoxyuridine (BrdU) labeling and detection kit was purchased from Roche (Penzberg, Germany). Primary antibodies: anti-COX2, anti-actin, anti-perilipin, anti-cyclin D1, antibodies were purchased from Sigma–Aldrich (St. Louis, MO); anti-cMyc and anti-pERK1,2 were from Santa Cruz (Dallas, TX); βIII-tubulin (mouse) was from Promega (Madison, WI); anti-GFAP was from Immunological Sciences (Rome, Italy), anti- PPARα (rabbit) was from Thermo Scientific (Rockford, IL). Anti-mouse/rabbit IgG Alexa Fluor 488/546 conjugated secondary antibodies were purchased from Molecular Probes (Life Technology, Carlsbad, CA) and peroxidase conjugated anti-mouse or anti-rabbit IgG secondary antibodies were from KPL (Gaithersburg, MD). Bicinchoninic acid (BCA) protein assay kit was from Pierce Biotechnology (Rockford, IL). Vectashield mounting medium was required to Vector Laboratories (Burlingame, CA), non-fat dry milk was from Bio-Rad Laboratories (Hercules, CA), SuperSignal West Pico Chemiluminescent Substrate was purchased from Thermo Scientific. Immobilon-P Transfer Membrane (PVDF) was required to Millipore Corporation (Billerica, MA) and O.C.T. was from Sakura (St. Torrance, CA). PPARα antagonist (N-(methylsulfonyl) amides) (AA452) was from Dr. Alessandra Azzalororso Department of Pharmacy, G. d'Annunzio University, Chieti, Italy. All chemicals were of the highest analytical grade.
Patient population
This study was ethically approved (Hospital Ethics Committee, n 3729), and all patients voluntary signed an informed consent. Newly diagnosed GB patients (41–70 years old, mean age of 60 years) were surgically resected at the Department of Neurosurgery, San Salvatore Hospital, L'Aquila, Italy (Table 1). Patients followed complete clinical and neurological evaluation at the admission in order to evaluate clinical conditions and Karnofsky Performance Status, including neuro-radiological investigation by CT scan without contrast enhancement, MRI with and without gadolinium, technetium 99 MIBI brain APECT. Tumor biopsies used in this study were from patients whose lesions were suitable for gross total removal. Surgical removal starts from the edematous brain surrounding the tumor and moves from the borders avoiding initial lesion debulking. Surgery was performed by image-guided surgery.
| Age | Name | Gender | Tumor localization |
|---|---|---|---|
| 68 | B.S. | Male | Frontal-parietal lobes |
| 70 | D.G. | Male | Occipital lobe |
| 70 | L.B. | Male | Temporal lobe |
| 50 | P.A. | Male | Temporal-parietal-occipital lobes |
| 41 | C.L. | Male | Temporal lobe |
Cell culture
Individual tumor biopsies excluding necrotic fragments were maintained in culture medium and addressed in ice to our laboratory. The fragments were then rinsed with Hank's balanced salt solution (HBSS). The necrotic areas and red endothelial parts were moved aside. Primary cell culture was established as previously described (Laurenti et al., 2011).
Treatments
AA452: PPARα antagonist (N-(methylsulfonyl) amides) was from Dr. Alessandra Ammazzalorso Department of Pharmacy, G. d'Annunzio University, Chieti, Italy. AA452 was synthesized according to Ammazzalorso et al. (2013) and is reported in Supplementary Figure S1. Briefly, 5-chloro-2-mercaptobenzothiazole (1 eq) and ethyl 2-bromophenylacetate (1 eq), both dissolved in absolute EtOH (10 mL), were added to a solution of sodium (1 eq) in absolute EtOH (10 mL), under nitrogen atmosphere. After stirring for 2–5 h at refluxing temperature, the solvent was removed under reduced pressure. The residue was poured into water (20 mL) and extracted with diethyl ether (3 × 20 mL). The organic layer was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (eluent cyclohexane/ethyl acetate 95:5) to give desired ester. It was then hydrolyzed by 1N NaOH (10 eq) and the mixture was stirred at r.t. for 10–15 h. After this time, the reaction was treated with 6N HCl. The aqueous layer was extracted with CH2Cl2 (3 × 20 ml), and the organic layer was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography dichloromethane/methanol (9:1) affording desired acid. To a cooled mixture (0°C) of synthesized acid (1 eq) in dry CH2Cl2 (15 ml), 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC, 1 eq) and 4-dimethylaminopyridine (DMAP, 1 eq) were added, under stirring in a nitrogen atmosphere. After 15 mins, benzenesulfonamide (1.1 eq) was added, and the mixture was allowed to warm to r.t. After stirring overnight, the reaction was diluted with CH2Cl2 (15 ml), washed with 2N HCl (3 × 30 ml), and dried over Na2SO4. After evaporation of solvent under reduced pressure, crude product was purified on silica gel (eluent dichloromethane/methanol 9:1), affording AA452 in good purity. It was found to be a selective PPARα antagonist in a cell-based assay (IC50 6.5 µM) and showed a repressive dose-dependent effect on CPT1A expression (Ammazzalorso et al., 2013) Melting points were determined on a Büchi B-540 apparatus and are uncorrected. Flash chromatography was performed on silica gel 60 (Merk, Merk Millipore, Italy) and TLC on silica gel 60, F254. Infrared spectra were recorded on a FT-IR 1600 Perkin-Elmer spectrometer. NMR spectra were run at 300 MHz on a Varian instrument; chemical shifts (δ) are reported in ppm. Elemental analyses were carried out with an Eurovector Euro EA 3000 model analyzer; results were within ±0.4% of the theoretical values.
Grade IV primary GB cell cultures were treated with AA452 (stock solution 20 mM) in DMSO at the final concentration of 6 μM for 24, 48, 72 h for MTS assay; 24 and 48 h for quantitative PCR; and 48 h for the others experiments. All experiments were performed in quintuplicate.
Cell viability assay
Cells were seeded (2,500 cells/well) in a 96-well plate, the day after the cells were treated with AA452 and the control cells received only DMSO, every treatment was performed in quintuplicate. The cells were incubated for 24, 48 and 72 h; at expiration of incubation period, cell viability was determined using Cell Titer One Solution Cell Proliferation Assay reading the absorbance at 492 nm, in a spectrophotometric microplate reader Infinite F200 (Tecan, Männedorf, Switzerland). The results were expressed as absorbance at 492 nm.
Cell proliferation assay
Cells were seeded (2,500 cells/well) in a 96-well plate, the day after the cells were treated with AA452 and the control cells received only DMSO, every treatment was performed in quintuplicate. Cells were incubated for 48 h with AA452 after that cell proliferation was determined using 5′-Bromo-2′-deoxyuridine (BrdU) labeling detection kit reading the absorbance at 405 nm, measured in a spectrophotometric microplate reader (Infinite F200 Tecan). The results are expressed as ratio between the absorbance of treated cells compared with absorbance of control untreated cells.
Cell count
Cells were seeded 5,000 cells/cm2 in 100 mm petri dishes, the day after cells were treated with AA452 and while control cells received only DMSO, every treatment was performed in quintuplicate. Cells were incubated for 48 h with AA452 after that were harvested by exposure to trypsin, incubated with Trypan blue and counted with Burker chamber. The results were expressed as ratio between the absorbance of treated cells compared with absorbance of control untreated cells.
Immunofluorescence
Cells plated on poly-L-lysine coated coverslip were washed twice with PBS, fixed for 10 min at RT in 4% paraformaldehyde in PBS, and permeabilized in PBS containing 0.1% Triton X-100 for 10 min at RT. Nonspecific binding sites were blocked for 30 min with 3% BSA in PBS (incubation buffer). According to the experiment, cells were then incubated with either anti-GFAP (1:500), anti-βIIITubulin (1:500), anti-PPARα (1:200) in incubation buffer overnight at 4°C. After extensive washings with PBS, the cells were incubated with AlexaFluor 488 or 546 secondary antibodies 30 min at RT. Cells were then washed and mounted with Vectashield mounting medium from Vector Laboratories containing DAPI. Cells were photographed at fluorescence microscope AXIOPHOT (Zeiss microscope, Jena, Germany).
Carboxyfluorescein succinimidyl ester staining (CFSE)
Cells were seeded at 5,000 cells/cm2 on coverslips inside a tetrawells plates filled with the appropriate culture medium. The day after the medium was removed from the plate and PBS containing the CFSE [2.5 µM] was added and cells were incubated for 15 min at 37°C. The loading solution was replaced with fresh, prewarmed medium and cells were incubated for another 30 min at 37°C. During this time, CFSE undergoes to acetate hydrolysis. After that, cells were treated with AA452, while control cells received only DMSO; every treatment was performed in quintuplicate. Cells were washed with PBS and were fixed for 15 min at room temperature using 3.7% formaldehyde, cells were then washed in PBS and mounted with Vectashield mounting medium, and were photographed at florescence microscope AXIOPHOT (Zeiss microscope).
Protein assay
Proteins were assayed by the micro-BCA kit. Briefly, this assay is a detergent-compatible formulation based on bicinchoninic acid (BCA) for the colorimetric detection and quantitation of total protein. The method combines the reduction of Cu2 to Cu1 by protein in alkaline medium with the high-sensitive and selective colorimetric detection of the cuprous cation, using a reagent containing BCA. The purple-colored reaction product of this assay is formed by the chelation of two molecules of BCA with one cuprous ion. This complex exhibits a strong absorbance at 562 nm.
Western blotting
For Western blotting, cell lysates in ice-cold RIPA buffer were centrifuged and the supernatants were assayed for protein content. Thirty micrograms of proteins were fractionated on 10% polyacrylamide gel and transferred onto PVDF membrane. Nonspecific binding sites were blocked for 1 h at RT in 20 mM Tris–HCl (pH 7.4) buffer, 55 mM NaCl, and 0.1% Tween 20 containing 5% non-fat dry milk (blocking buffer). Membranes were then incubated overnight at 4°C with primary antibody diluted in blocking buffer. Primary antibodies used are anti-actin (1:2,000), anti-cyclin D1 (1:3,000), anti-cMyc (1:100), anti-Perilipin (1:1000), anti-pERK1,2 (1:1,000), pFak(1:500), and COX2 (1:200). All these antibodies were dissolved in blocking solution. After extensive washings and incubation with the respective horseradish peroxidase-labeled secondary antibodies, protein presence was visualized by enhanced chemiluminescence reaction from Pierce Biotechnology. Band relative densities obtained using Alliance 4.7 UVITEC (Cambridge, UK) were normalized to actin and values were given as relative units
In vitro irradiation and colony formation assay
Radiation was delivered at room temperature using an x-6 MV photon linear accelerator, as previously described (Marampon et al., 2016). The total single dose of 4 Gy was delivered with a dose rate of 2 Gy/min using a source-to-surface distance (SSD) of 100 cm. A plate of Perspex thick 1.2 cm was positioned below the cell culture flasks in order to compensate for the build-up effect. Tumor cells were then irradiated placing the gantry angle at 180°. Non-irradiated controls were handled identically to the irradiated cells with the exception of the radiation exposure. For clonogenic survival assays, exponentially growing cells in 25-cm2 flasks were harvested by exposure to trypsin and counted. They were diluted serially to appropriate densities and plated in triplicate in six multi-well plates with 2 ml of complete medium/each well. After incubation for 24 h, the cells were exposed at room temperature to radiation treatment as already described. The cells were then washed with PBS, cultured in growth medium for 14 days, fixed with methanol:acetic acid (10:1, v/v), and stained with crystal violet. Colonies containing >50 cells were counted. After count, the staining was released by 10 ml of a solution containing 50% methanol and 1% SDS overnight at RT. The absorbance was determined at 550 nm in a microplate reader (Infinite F200 Tecan).
Quantitative real-time PCR
For AOX1 gene expression analysis, the following protocol was used: total RNA was extracted by Trizol reagent, according to the manufacturer's instructions. The total RNA concentration was determined spectrophotometrically in RNAase-free water and 1 μg aliquots of total RNA were reverse transcribed into cDNA using ProtoScript First Strand cDNA Syntesis Kit. RT-PCR was carried out on ABI 7300HT sequence detection system (ABI), in a total volume of 20 µl containing EagleTaq Universal Master Mix (ROX), DEPC water, 2 µl of cDNA and the following the Prime Time qPCR Assay (IDT) for human AOX1 (Hs.PT.56A.38879910). Quadruplicate samples were run for each gene. The reference gene TBP was used as an internal control to normalize the expression of target genes. For MVA genes expression analysis, the following protocol was used: Total RNA was isolated using the NucleoSpin RNA II kit. Complementary DNA (cDNA) was transcribed using SuperScript II Reverse Transcriptase (Invtrogen Thermofisher Scientific, Italy), starting from 1 µg of high pure RNA. MVA genes expression profiles were evaluated with specific primer sets (Table 2) and using SsoFast EvaGreen reagents (Bio-Rad), b2-microglobulin was used as housekeeping gene. RT-PCR protocol was: a pre-heating step for 3 min at 95°C, 40 cycles at 95°C for 10 sec and 60° for 30 sec and last end-step at 65°C for 10 sec. Relative expression levels were calculated for each sample after normalization against reference gene, using the ΔΔCt method for comparing relative fold expression differences, as previously described (Livak and Schmittgen, 2001).
| RT-PCR | ||
|---|---|---|
| Gene symbol | Forward primer | Reverse primer |
| HMGCR | taccatgtcaggggtacgtc | ccagtcctaatgaaaccttagaag |
| MVK | gctcaagttcccagagatcg | atggtgctggttcatgtcaa |
| FDPS | agcaggatttcgttcagcac | tcccggaatgctactaccac |
| FDFT1 | ggtcccgctgttacacaact | aaaactctgccatcccaatg |
| RABGGTA | gaccccctgctgtatgagaa | cacctcggcatactccatct |
| LDLR | gaatttggccagacacaggt | caccgtacccagctgatttt |
| β2M | cctggattgctatgtgtctgg | ggagcaacctgctcagataca |
Bodipy staining
Cells grown on coverslips after 48 h of treatment were washed twice with PBS, fixed for 10 min at RT in 4% paraformaldehyde in PBS, and permeabilized in PBS containing 0.1% Triton X-100 for 10 min at RT. A stock solution 1 mg/ml of BODIPY 493/503 dissolved in ethanol was prepared and then stored at −20°C in the dark until required. Before use, BODIPY stock solution was diluted 1:1,000 in PBS and used for incubate coverslips in the dark for 10 min. After incubation, the cells were washed with PBS, mounted with Vectashield mounting medium with DAPI, and photographed at fluorescence microscope AXIOPHOT.
Lipids extraction
Cell pellets were dissolved in a buffer containing Tris–HCl 20 mM, pH 7.4, 1 μM PMSF, 10 μM leupeptin, 10 μM pepstatin, 1 μM aprotinin. After the incubation time (5 min at 4°C), samples were sonicated (5 W, 80% output, 1 min and 50 sec, alternating 10 sec sonication and 10 sec pause) with a Vibracell sonicator (Sonic and Materials, Inc., Danbury, CT). Protein concentration of cell lysates was determined through the BioRad Protein Assay (Hercules, CA), with BSA standards. Lipids were extracted by the sequential addition of 400 μl methanol, 500 μl chloroform, and 200 μl water. Samples were stirred for 2 min on a vortex-mixer and centrifuged at 10,800g for 10 min. The extraction and centrifugation steps were repeated twice. The organic phases, obtained from different extraction steps, were collected, dried under nitrogen, and then analyzed by TLC.
Thin layer chromatography
TLC was performed on 20 cm × 20 cm aluminum silica plates. Eluent mixture (hexane/diethyl ether/acetic acid, 80:20:2 v/v) (100 ml) was introduced into an elution tank to separate neutral lipids. Lipids were applied on silica plates as thin rows at 2 cm distance above the bottom of the silica plate, air dried, and placed immediately in the elution tank. The solvent was allowed to ascend to 1 cm from the top of the plate, and then the plate was removed, air-dried, and stained. Cholesteryl-ester (CE) was used as standards. TLC staining was obtained by vaporizing 10% phosphomolybdic acid solution on plates. Phosphomolybdic acid solution was prepared by dissolving 10 g in 100 ml ethanol. The plates were dried for 10 min at 80°C.
Cell migration assay
Cell migration assay was carried out with RTCA DP Instrument (ACEA Biosciences, San Diego, CA) using 16-well plates which has an upper chamber, sealed at the bottom with a microporous (8 µm of diameter) PET membrane coated with 0.1% gelatin using FBS as chemoattractant.
Statistical analysis
For statistical analysis, samples were processed by SPSS software. Statistical analysis of two population means was performed by the unpaired Student's t-test, while statistical differences comparing multiple means were analyzed by the analysis of variance followed by Scheffe's post-hoc test analysis. *P < 0.05; **P < 0.005, **P < 0.0005. Data were expressed as mean ± SE of five separate experiments.
Results
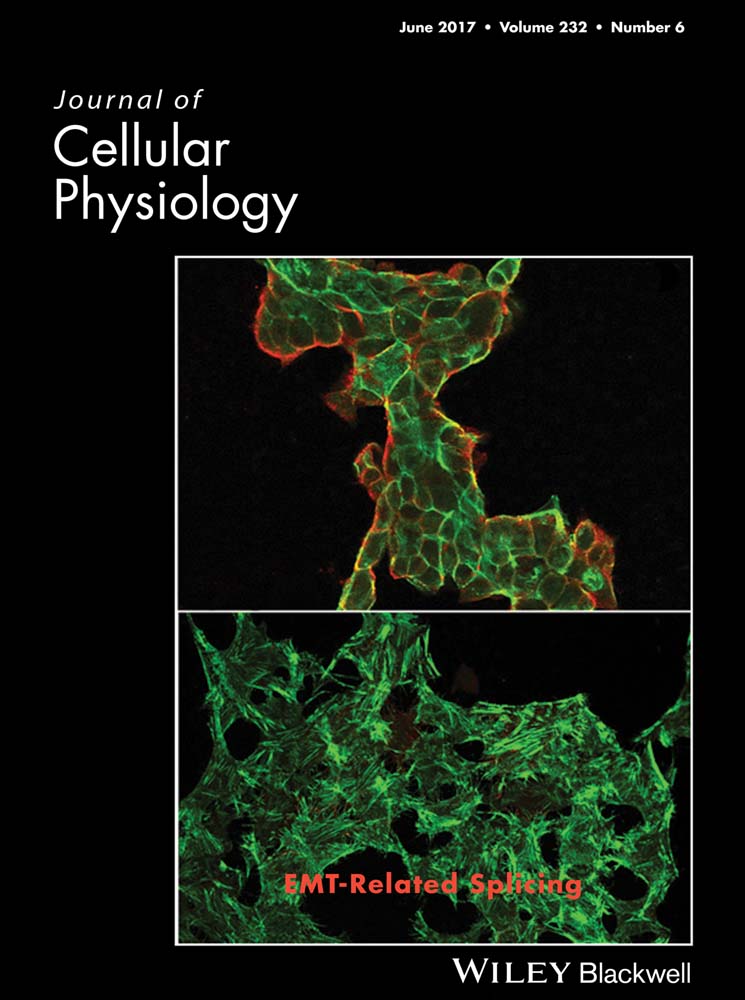
Primary GB cultures were characterized for the presence of the astroglial marker GFAP and for GB malignancy marker β-tubulin III (Katsetose et al., 2009a,b) (Fig. 1A). Preliminary AA452 dose–response curves were performed in order to choose the concentration for the subsequent experiments (Fig. 1B). On this basis, the 6 µM dose was chosen, that is closer to the IC50 value of 6.5 µM. Cell viability (Fig. 1C) at different time-points and cell number (Fig. 1D) and proliferation (Fig. 1E) at 48 h in treated and control cells were then assayed (Fig. 1). PPARα antagonist AA452 significantly decreased cell viability starting from 48 h. At the same time-point, it significantly decreased cell number and cell proliferation.
In Figure 1F, the presence and localization of PPARα in GB cells before and after AA452 treatment is reported. In agreement with previous results, a strong nuclear localization of the transcription factor in control cells is observed. Interestingly, upon antagonist PPARα appeared localized in the cytoplasm, where it is likely inactive. Accordingly, also a decrease of AOX1, a PPARα target gene, was observed upon treatment (Fig. 1G)
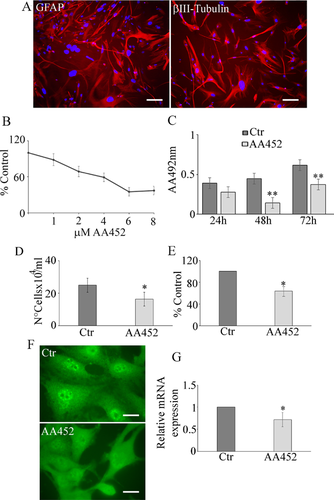
Figure 2 shows CFSE staining (Fig. 2A) and the Western blotting analysis for c-Myc (Fig. 2B) and cyclin D1 (Fig. 2C) in control and treated cells. CFSE is a vital staining that is highly retained in non-dividing cells, while is scarcely accumulated in highly proliferating cells. Contrary to what was seen in controls, CFSE staining was concentrated in the cells upon treatment with AA452, indicating a block of cell proliferation. In agreement, c-Myc (Fig. 2B) and cyclin D1 (Fig. 2C) significantly decreased. The same figure shows the effect of the antagonist in increasing sensitivity to radiotherapy (Fig. 2D). The condition antagonist plus radiotherapy determined a significant decrease of colony number (Fig. 2E) and of the absorbance at 550 nm (Fig. 2F), with respect to cell receiving only with radiotherapy. It is worth noting that the antagonist was able to decrease colony number also when administered alone.
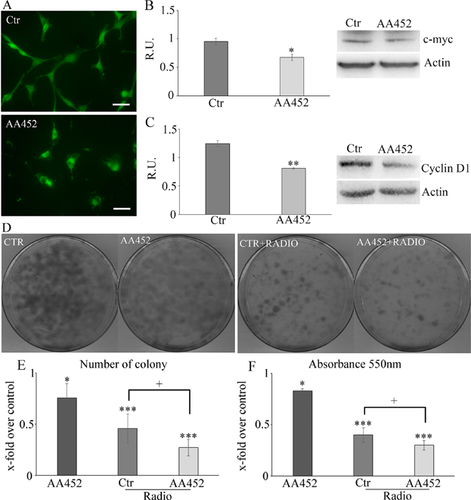
In Figure 3, the bodipy staining for lipid droplets (LDs) (Fig. 3A) and the Western blotting analysis for perilipin A (Fig. 3B), a membrane protein of LDs (Brasaemle, 2007; Fujimoto et al., 2008) that is under indirect PPARα control (Straub et al., 2008), are reported. LDs strongly decreased upon treatment as well as perilipin levels. In the same picture, the TLC analysis for cholesteryl esters in control and treated cells (Fig. 3C) shows thatAA452 strongly decreased the cholesteryl ester levels. The mevalonate pathway was assayed by real-time PCR (Fig. 3D) showing a significant decrease of the enzymes of the mevalonate pathway, in agreement with the observed decrease of bodipy and of cholesteryl esters.
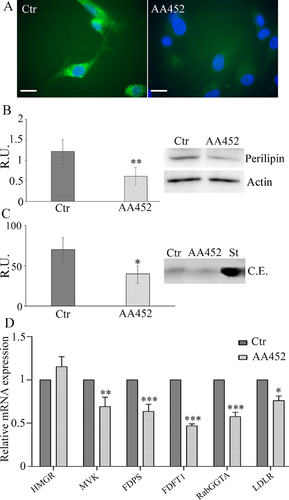
In Figure 4, cell migration (Fig. 4A) in control and agonist-treated cells and the Western blotting analysis for the protein of focal adhesions (p-FAK) (Fig. 4B) are shown, demonstrating a clear correlation between the decrease of migration and the expression of active form of FAK. Moreover, the phalloidin staining (Fig. 4C) further strengthened these results, indicating less focal adhesions upon treatment. Finally, since it is known that COX-2 is overexpressed in grades III and IV astrocytomas (Perdiki et al., 2007) and that GB cell lines treated with COX-2 inhibitors showed reduced cell migration (Joki et al., 2000), in the same figure, the Western blotting analysis for the COX2 protein and pERK1,2, that is involved in its activation (Tanabe and Tohnai, 2002), are shown (Fig. 4D). The antagonist determined a significant decrease of the expression and activation of proteins involved in cell migration.

Discussion
Many cancer cells consume glucose avidly and produce lactic acid rather than catabolizing glucose via the TCA cycle, which is crucial for generating ATP in non-hypoxic normal cells. The shift toward lactate production in cancer, even in the presence of adequate oxygen, is termed the Warburg effect or aerobic glycolysis (Warburg, 1956b). Although not coupled to oxidative phosphorylation, in tumor cells, TCA cycle ensures the efflux of biosynthetic intermediates for lipid and aminoacid synthesis, a process called cataplerosis. Citrate is for instance transferred from the mithocondrial matrix to the cytosol for cleaving by ATP citrate lyase (ACL) into oxaloacetate and acetyl-CoA, a lipogenic precursor giving rise to isoprenoid, cholesterol, and fatty acid. (Feron, 2009). Although the Warburg effect has been recognized since 1920s, less well appreciated are alterations in lipid metabolism and the high rates of de novo fatty acid biosynthesis exhibited by many tumors. The cholesterol synthesis is mediated by mevalonate pathway which uses acetyl-CoA as substrate. Several investigations have shown an abnormally active de novo synthesis of cholesterol from acetate and mevalonate (MVA) in malignant glial cells, compared with their normal counterparts (Goldstein and Brown, 1990; Kawata et al., 1990; Duncan et al., 2004). The mevalonate pathway produces various end products that are crucial for both normal and tumor cells. These products include cholesterol, dolichol, ubiquinone, isopentenyladenine, geranylgeranyl pyrophosphate, and farnesyl pyrophosphate (Goldstein and Brown, 1990). The rate-limiting step of the MVA pathway is the conversion of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) to mevalonate, which is catalyzed by HMG-CoA reductase (Goldstein and Brown, 1990). Blockade of this pathway by HMG-CoA reductase inhibitors determines reduced levels of mevalonate and its downstream products significantly influencing many crucial cellular functions. Malignant cells seem highly dependent on the sustained availability of the products of the mevalonate pathway (Chan et al., 2003; Laezza et al., 2015). Deregulated or elevated activity of HMG-CoA reductase has been reported in a series of different tumors (Chan et al., 2003). The statins, widely used as anti-hypercholesterolemic drugs, are potent inhibitors of HMG-CoA reductase (Corsini, 2005) Previous findings have demonstrated that statin treatment can decrease tumor growth, invasion, and metastatic potential both in vitro and in vivo (Keyomarsi et al., 1991; Chan et al., 2003). The link between cellular replication and lipid metabolism have been under study by us in the last years (Benedetti et al., 2010a; Laurenti et al., 2011; Laezza et al., 2015); we have demonstrated the involvement of PPARs (Benedetti et al., 2010b,2010a; Laurenti et al., 2011) in tumor metabolic reprogramming leading to proliferation and resistance.
PPARα controls the metabolism of fatty acids, that is, the peroxisomal enzymes of β-oxidation (Yoon, 2009). Recent work has highlighted increased mevalonate (MVA) synthesis in malignant cells as a consequence of increased levels and catalytic efficiency of 3′-hydroxy-3′-methylglutaryl-CoA reductase (HMGCR), the rate-limiting enzyme of cholesterol (CHO) biosynthesis that catalyzes the formation of MVA. In our previous study, we have demonstrated that PPARα is strongly upregulated as a function of the glioma malignancy grade and that this increase is paralleled by the significant increase of HMGCR (Benedetti et al., 2010a). These findings indicated that PPARα is involved in the strong lipid metabolism perturbation observed in gliomas. In fact, PPARα regulates enzymes of the ß-oxidation pathway that produces acetyl-CoA, which constitutes the substrate for MVA synthesis (Kovacs et al., 2007). Moreover, in the in vitro model, we observed a significant increase of lipid droplets as a function of the malignancy grade (Laurenti et al., 2011). The hypothesis currently under investigation is that the upregulation of CHO metabolism could then drive tumorigenesis (Laezza et al., 2015). On the basis of these evidences, our results obtained by inhibiting PPARα transcriptional activity appear to be of great interest. In this study, we used a compound, a N-phenylsulfonyl amide derivative, recently described by us in the search for novel compounds able to block PPARα activation (Ammazzalorso et al., 2011; Ammazzalorso et al., 2012). PPARα inhibition by AA452 determined a strong effect on cell viability, proliferation, and migration that may be related to the significant effects on MVA pathway. As a consequence, a strong decrease of cholesteryl esters and of LDs is observed, thus indicating that by blocking PPARα activity, the resulting lipid metabolic reprogramming may lead to the observed cell death and decrease of invasiveness. On the other hand, the decrease of cholesteryl esters, upon antagonist, is really promising since the described roles of cholesteryl esters in malignancy (Tosi and Tugnoli, 2005).
It is worth noting that the radiosensitizing effects of the PPARα antagonist on GB cells are accompanied by a decrease of cyclin D1. It has been previously demonstrated, in prostate cancer cells, that cyclin D1 is a key regulator in controlling the DNA double-strand break (DSB) repair mechanisms mediated by the non-homologous end joining (NHEJ) pathway and that silencing cyclin D1 radiosensitizes PCa cells (Marampon et al., 2016). In this view, the effect of the antagonist in increasing cell sensitivity to radiotherapy may be due to its effects on cyclin D1 levels. Interestingly, c-myc, which is also associated with radioresistance (Marampon et al., 2016), in parallel decreased in cells treated with antagonist. Cyclin D1 also plays an important role in cell migration (Li et al., 2006a), promoting the migratory and invasive capacities of macrophages (Neumeister et al., 2003), fibroblasts (Li et al., 2006b), breast epithelial cells (Li et al., 2006c), and human GB cells (Arato-Ohshima and Sawa, 1999; Wang et al., 2012). Furthermore, cyclin D1 regulates metalloproteinase (MMPs) (Lin et al., 2001; Casimiro et al., 2013), traditionally associated with matrix remodeling, cancer invasion, and angiogenesis. Here, we demonstrate that the inhibition of PPARα activity is accompanied by a significant decrease of cyclin D1, paralleled by a strong decrease of cell migration and of the proteins related to migration, that is, p-FAK, all events accompanied by increase in radiosensitivity of GB cells, as summarized in Figure 5
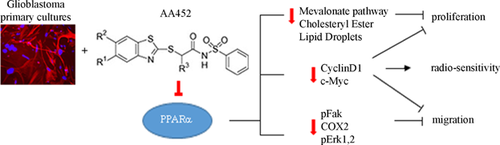
On these bases, the results obtained with the PPARα antagonist AA452 open the possibility for pre-clinical and clinical trials for this class of compounds for GB therapy.
Acknowledgments
This work was supported by the RIA funds (Proff Ippoliti, Di Cesare, and Cimini). Many of the experiments have been performed in the Research Center for Molecular Diagnostics and Advanced Therapies granted by the Abruzzo Earthquake Relief Fund (AERF).